Abstract
Exurban areas are expanding throughout the world, yet their effects on local biodiversity remain poorly understood. Wetlands, in particular, face ongoing and substantial threats from exurban development. We predicted that exurbanization would reduce the diversity of wetland amphibian and invertebrate communities and that more spatially aggregated residential development would leave more undisturbed natural land, thereby promoting greater local diversity. Using structural equation models, we tested a series of predictions about the direct and indirect pathways by which exurbanization extent, spatial pattern, and wetland characteristics might affect diversity patterns in 38 wetlands recorded during a growing season. We used redundancy, indicator species, and nested community analyses to evaluate how exurbanization affected species composition. In contrast to expectations, we found higher diversity in exurban wetlands. We also found that housing aggregation did not significantly affect diversity. Exurbanization affected biodiversity indirectly by increasing roads and development, which promoted permanent wetlands with less canopy cover and more aquatic vegetation. These pond characteristics supported greater diversity. However, exurbanization was associated with fewer temporary wetlands and fewer of the species that depend on these habitats. Moreover, the best indicator species for an exurban wetland was the ram’s head snail, a common disease vector in disturbed ponds. Overall, results suggest that exurbanization is homogenizing wetlands into more permanent water bodies. These more permanent, exurban ponds support higher overall animal diversity, but exclude temporary wetland specialists. Conserving the full assemblage of wetland species in expanding exurban regions throughout the world will require protecting and creating temporary wetlands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A major threat to species in the twenty first century originates not just from concentrated urbanization, but from the diffuse expansion of residential growth in exurban areas (McKinney 2006; Grimm et al. 2008; Seto et al. 2012; Pejchar et al. 2015). Exurbia defines low-density, large-lot residential development located beyond the suburbs of a city, but within commuting range (Daniels 1999; Theobald 2004; Irwin and Bockstael 2007). Exurban development is increasing twice as fast as urban development (Berube et al. 2006), covers eight times more area than cities in the United States including > 25% of the contiguous United States (Brown et al. 2005), and increasingly threatens natural areas of high conservation value (Hansen et al. 2002; Radeloff et al. 2005). Consequently, exurban development’s overall ecological impact might ultimately surpass that of more compact urban and suburban areas.
As exurban development fans out from the city center, habitats and biological communities generally become homogenized toward an “exurban savanna” of open manicured lawns interspersed with ornamental trees and permanent water bodies (Gobster 1994; McKinney 2006; Suarez-Rubio et al. 2011). These exurban ecological impacts remain less studied than other forms of habitat degradation (Brown et al. 2005; Hansen et al. 2005). Moreover, exurban development is distinct in its form and biological impacts from suburban growth (Hansen et al. 2005). Thus, findings about biodiversity patterns in urban or suburban areas might not apply accurately to exurban areas.
The effects of exurbanization on biodiversity are often highly variable, suggesting complex and system-specific relationships between residential development and ecosystem responses (McKinney 2002; Hansen et al. 2005). Some studies suggest a negative effect of exurbanization on species diversity, whereas others demonstrate a peak in diversity in exurban regions, often owing to higher numbers of introduced species or greater habitat heterogeneity (McKinney 2002; Hansen et al. 2005). Understanding how exurbanization alters biodiversity patterns could provide insights in how to design future housing developments that minimize impacts on biodiversity and threatened species.
One of the most widespread forms of habitat degradation over the last several centuries has been the modification and filling of wetlands (Whitney 1996; McCauley and Jenkins 2005). Wetlands support some of the densest concentrations of biodiversity of any habitat on Earth (Millenium Ecosystem Assessment 2005) and provide 40% of all ecosystem services (Costanza et al. 1997; Zedler 2003). Yet, humans have degraded wetlands more than any other ecosystem, eliminating more than half of the wetlands on Earth (Dahl 2000; Zedler and Kercher 2005). As a consequence, obligate wetland species face the highest extinction rates of any group of species (Millenium Ecosystem Assessment 2005). Historically, agriculture caused most wetland destruction and degradation, but residential development increasingly overshadows agriculture in its threats (Brady and Flather 1994; Gutzwiller and Flather 2011).
We evaluate how exurbanization alters the biodiversity of wetlands in a rapidly developing region in northeastern U.S.A. (Fig. 1). We predicted that exurbanization would decrease biodiversity by contributing to the loss and degradation of wetland habitats and homogenization of associated communities toward less sensitive species, as suggested by prior work (reviewed in Hamer and McDonnell 2008). We also predicted that at a given housing density, more aggregated housing would support greater species diversity by leaving a greater proportion of undisturbed natural habitat. Thus, we assumed that land-sparing patterns of development during exurbanization would benefit local pond communities more than land sharing (Daniels 1999; Lin and Fuller 2013).
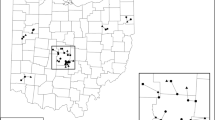
Left, map of study region in the context of the greater northeastern US urban corridor from New York City to Boston. Right, study area detail showing the exurban area centered on Storrs, CT, which is not directly in the suburban limits of Hartford but within commuting distance (i.e., exurban). Circles indicate remnant forest tracts with wetlands in the study. Color version of the figure is available online
Materials and methods
Study site and wetland selection
Southern New England has experienced strong population growth since 1950, and much of this growth occurred outside cities (Brown et al. 2005). Exurban land use increased from 40 to 60% in Northeastern Connecticut during this period (Brown et al. 2005). We studied the region surrounding the campus of the University of Connecticut, which occurs within this region of rapid exurbanization, yet is outside the fringe of development surrounding metropolitan areas that is defined as suburban (Fig. 1). The surrounding landscape is characterized by a mixture of small towns, scattered residential development, agriculture, and small protected areas. Recently, land use has transitioned from agriculture to exurban development, matching more general trends across New England where 3% of forested area was lost from 1990 to 2005, much of it due to residential development (Jeon et al. 2014).
We identified tracts of protected land that occurred within a matrix of residential development. On each tract, we searched for geographically isolated wetlands using satellite imagery followed by intensive ground surveys. When multiple wetlands of sufficient hydroperiods co-occurred on the same tract of land, we selected a wetland at random. We located 38 wetlands that met these criteria. These wetlands occurred in study tracts stratified across residential densities ranging from those characterized as rural (< 0.06 households per ha, Brown et al. 2005) to exurban (0.06–2.5 households per ha) as measured in a 1-km radius. For comparison, we also included three wetlands in higher-density tracts located on and around the campus of the University of Connecticut (4–9 households per ha).
These wetlands ranged from temporary ponds, which dry in late spring 36 days after ice-off, to semi-permanent wetlands, which hold water into the autumn and sometimes year-round, but dry frequently enough to prevent the establishment of permanent fish populations. The median wetland in the study was 349 m2 in total area (range: 36–1970 m2), had a maximum depth of 46 cm (range: 15–104 cm), filled in autumn, and held water until mid-July, which are characteristic of local wetlands (Urban 2004).
Housing density, aggregation, and land use
Using ArcGIS 10.0, we created a map with layers of data from our own fieldwork, satellite imagery, and land use data from Center for Land Use Education and Research at the University of Connecticut. We calculated percent land use at a radius of 300 meters, the mean maximum core terrestrial habitat for amphibians (Semlitsch and Bodie 2003) and a distance that encompasses 90% of flying aquatic insect dispersal events (reviewed in Gratton and Zanden 2009). We also evaluated results at a radius of 1 km that would characterize dispersal distances for some better dispersers (e.g., more mobile dragonflies, Conrad et al. 1999) and obtained similar results. We analyzed the proportion of human land use, including developed land (mostly residential in this region), utility lines, human-maintained turf and grass, barren land (mostly land exposed through mining or agriculture), and agricultural fields (see http://clear.uconn.edu/projects/landscape/project.htm for details of categories). We then used real estate data (www.zillow.com) in combination with ArcGIS’s Bing Satellite map base layer to find every residential unit within a 300 and 1000 m radius of each study wetland.
We estimated housing aggregation at each site. First, we calculated the mean nearest neighbor distance among all houses. We then compared this observed value against the mean nearest neighbor distances of 10,000 iterations of random point locations for the same number of houses. We divided observed mean distances over the random expectation and subtracted it from one to obtain a metric of housing aggregation that ranges from negative (uniform) to zero (random) to one (nonrandomly aggregated).
Physical sampling
We assessed key characteristics known to affect wetland species diversity, including permanence, canopy cover, pH, emergent vegetation, and size (Schneider and Frost 1996; Skelly 2001; Urban 2004). During each sampling visit, we recorded pH, dissolved oxygen, temperature, and depth using a multi-probe meter (DO200, YSI, Ohio, USA) and a pH meter (PCTestr35, Oakton, Illinois, USA) at the surface and at the location of maximum depth. We took the mean of measurements from 3 to 4 sampling visits, with visit number dependent on how long each pond held water. We also quantified the prevalence of aquatic and emergent vegetation by random quadrat sampling. We mapped each wetland on a gridded coordinate system and measured the percentage cover of aquatic vegetation at ten randomly located 1-m radius points. We used a spherical densiometer to quantify percent canopy cover in the center of each wetland. We then estimated the area of each temporary wetland as an ellipse or series of ellipses using measures of the maximum length and width of each wetland or section. We visited wetlands every 3 weeks to record the date they dried. We measured permanence as the number of weeks a wetland held water after spring ice melts the start to the growing season for most wetland species.
Biotic sampling
In 2011, we censused spring breeding amphibian egg masses (wood frogs Rana sylvatica and spotted salamanders Ambystoma maculatum) by conducting visual surveys (Skelly et al. 1999). Beginning 1 week after the egg census and repeated at 3-week intervals, we sampled and cataloged each wetland’s amphibian larvae and macroinvertebrates (operationally defined by net mesh size = 1 mm) by taking 0.8-m long sweeps of every 2 m2 of wetland area with a 22 × 28 cm dip net (Urban 2007; Skelly and Richardson 2009). We repeated sampling until mid-July when few new taxa colonize rapidly drying wetlands.
We sorted morphotaxa in the field and preserved a subset for identification in the lab. We identified invertebrates to genus and amphibians to species using standard keys (Altig 1970; Petranka 1998; Merritt et al. 2008). Online Resource 1 provides species composition and abundances organized by location. We collected, preserved, and identified all rare (< 5 per sampling round) organisms. For easily identifiable and common taxa, we preserved five samples from each pond for up to four sampling periods. For less identifiable and common taxa, including dipteran larvae and small coleopterans, we collected up to 50 individuals for laboratory identification. In the rare cases when more than one genus was identified in the lab from a single morphospecies, we extrapolated identifications proportionally to the total number of morphospecies in that sample. As an additional check to ensure that this sampling protocol did not bias our results, we evaluated family level richness. We found that pond family richness explained 97% of the variation in richness estimated at the lowest possible resolution. Moreover, both richness measures were similarly correlated with permanence (r = 0.64 and 0.65 for fine and family resolution, respectively), which was an important predictor of richness in the study.
To estimate species richness, we calculated an abundance-based coverage estimator (ACE) of asymptotic richness in EstimateS (Colwell 2013) based on randomized species accumulation curves. ACE estimates total richness with limited bias due to differences in sampling effort and abundances (Colwell and Coddington 1994; Chazdon et al. 1998). We also calculated alternative richness indices, including Chao’s diversity and Michaelis–Menten saturation diversity curves, and found them to be highly correlated with ACE (ρ > 0.90). ACE also correlated well with Simpson’s diversity, which accounts for both richness and evenness (ρ = 0.57). However, owing to the weaker correlation, we performed all analyses for both ACE richness and Simpson’s diversity (hereafter referred to as richness and diversity, respectively). Overall, the choice of richness or diversity metric had minor effects on results.
Statistics
We used standard generalized linear models available in the ‘lm’ function in R (version 3.2.2, R Core Team 2015) to analyze relationships between residential density, percent human land use, and diversity. To understand how local wetland characteristics were associated with biodiversity, we applied a model-averaging approach based on the Akaike information criterion with a correction for finite sample sizes (AICc) (Burnham and Anderson 2002). We averaged across models incorporating all possible combinations of the five characteristics expected to explain diversity based on prior literature and calculated confidence intervals in ‘MuMIn’ package (Barton 2016) in R using the subset of models corresponding to the 95% confidence subset of AICc weights (Burnham and Anderson 2002).
We evaluated alternative hypotheses for the relationships among factors and their association with diversity in wetlands using structural equation modeling in the ‘lavaan’ package in R (Rosseel 2012). Our original model was that human household density would affect diversity directly and indirectly through land use changes, including road proximity, and through the three factors mediating wetland habitat characteristics identified in the previous analysis. After evaluating the full model, we iteratively removed non-significant links and chose the model with minimum AIC.
We performed a multivariate regression of community composition for the three wetland characteristics that proved significant in initial analyses and human land development using redundancy analysis (RDA) in the ‘vegan’ package in R (Oksanen et al. 2017). We used 10,000 permutations to test for significant contributions of land development.
We next looked for indicator species associated with human land use and wetland permanence. The indicator value index describes sets of species that characterize pre-defined habitats (Dufrêne and Legendre 1997). We excluded rare species, defined as < 10 individuals across all sampling periods to prevent them from disproportionately affecting results (Dufrêne and Legendre 1997). We calculated the indicator value index and its significance using 10,000 permutations in the ‘multiplatt’ function in R package ‘indicspecies’ (Cáceres and Legendre 2009). We used Holm’s procedure to correct for multiple tests to identify taxa significantly associated with developed wetlands and permanent wetlands. We split wetlands equally into four groups for percentage human land use: 0–10, 10–20, 20–36, and > 36%. This same procedure yielded four groups of varying permanence: < 53, 74, 97, > 119 days of water.
Finally, we evaluated community nestedness along a gradient in permanence to examine if more permanent wetlands provided habitat for the same species as temporary wetlands. We used a nestedness metric based on overlap and decreasing fill named NODF (Almeida-Neto et al. 2008). We performed 10,000 simulations using the quasi-swap fixed row and fixed column algorithm, which has been shown to have correct type-I error rates (Ulrich and Gotelli 2007), and determined the probability that the observed nested patterns overlapped with simulated patterns in ‘oecosimu’ function in the package ‘vegan’ in R (Oksanen et al. 2017).
Results
We first evaluated which local wetland characteristics correlated with wetland species richness. The best (minimum AICc) model included canopy cover, wetland permanence, and percent aquatic vegetation and explained 57% of the variation in wetland richness, and the next most competitive models (with delta AICc < 2; Table S1) also included these variables, together with pH or area. These same three variables appeared in the best model for Simpson’s diversity, which incorporates evenness. Wetland richness decreased with higher tree canopy cover and increased in semi-permanent wetlands and wetlands with dense aquatic vegetation (model-averaged coefficients in Fig. 2). Wetland area and pH did not significantly explain richness on their own.
Model-averaged standardized regression coefficients of correlates of animal richness organized in order of decreasing effect size (N = 38). The magnitude of the coefficient indicates its effect on wetland richness relative to other factors. Error bars represent the 95% confidence interval of the model-averaged coefficients of all models weighted by their AICc values. Overlap between error bars and zero indicates non-significance
We next evaluated regional factors at a 300-m radius around wetlands, which reflects the typical movement of wetland taxa. Residential housing density was significantly and positively related with proportional human land use (normal GLM, β = 0.59 ± 0.24 SE, F 1,36 = 5.9; P = 0.020) at this scale, explaining 27% of the variance. However, this relationship was highly variable at low housing densities where human land use varied from 0 to 30% (Fig. 3a).
Housing density was significantly associated with greater percent human land use a, but not greater species richness b, within a 300-m buffer radius. Housing aggregation ranged from randomly dispersed (0) to highly clumped (0.75), but was not significantly correlated with percent human land use c or species richness d. Only significant (P < 0.05; N = 38) relationships are indicated with regression lines. Note ln (x + 1) x-axis scale for household density
In contrast to predictions, housing density, housing aggregation, and their interaction did not directly explain wetland richness or diversity (Fig. 3b, d; P > 0.3). Moreover, housing aggregation did not significantly affect human land use individually or interact with housing density, as originally assumed in the land-sparing hypothesis (Fig. 3c; P > 0.7). Instead, household density correlated positively with human land use, which in turn was significantly associated with wetland biodiversity (Fig. 4a). Surprisingly, increasing human land use was associated with significantly more, not less, species richness and diversity in focal wetlands (normal GLM, β = 29 ± 12 SE, F 1,36 = 6.2, P = 0.018; β = 4.7 ± 1.5 SE, F 1,36 = 9.8, P = 0.003, respectively).
Percent human land use is correlated with higher species richness a. This effect is mediated by higher proximity to roads in more developed landscapes b. Wetlands near roads tend to be more permanent c. More permanent wetlands, in turn, support greater species richness d. Wetlands are more permanent in more developed landscapes e. All relationships are significant (P < 0.05; N = 38) and retained in the best structural equation model in Fig. 5
To understand this unexpected pattern, we combined local habitat and regional landscape factors into a series of alternative structural equation models. In the best (minimum AIC) model for both species richness and diversity, a combination of direct effects of wetland characteristics, human land use, and its indirect effects on wetland permanence via changes in wetland proximity to roads explained 66% of the variation in local wetland richness (Fig. 5; Table S2). Specifically, roads often occurred near wetlands in more developed landscapes (Fig. 4b; β = − 3057 ± 850 SE), and these roadside wetlands often were more permanent (Fig. 4c; β = − 0.021 ± 0.006 SE). Wetland permanence determined wetland richness and diversity directly (Fig. 4d; β rich = 0.36 ± 0.08 SE, β div = 0.039 ± 0.012 SE) and indirectly by decreasing canopy cover, which increased aquatic vegetation density and pond richness (Fig. 5). We also found support for a similar structural equation model using a 1000-m buffer, except that human land use was no longer associated with road proximity at this coarser scale and did not directly affect species richness.
The full and reduced (minimum AIC) structural equation model explaining wetland richness and diversity in exurban landscapes. Gray dotted arrows indicate relationships that were not retained in the minimum AIC model. All remaining links were significant (P < 0.05; N = 38). The standardized regression estimates are indicated next to arrows. R 2 values indicate the variance explained for the associated factor by all factors connected with black arrows. For display purposes, we omitted arrows from housing aggregation, which were not retained in the minimum AIC model and were also not significant. A higher χ 2 probability and smaller root mean square error of approximation (RMSEA) indicates a better model fit
We next examined the composition of wetland communities across exurban gradients using redundancy analysis. The best (minimum AIC) model included permanence and aquatic vegetation, but other models within 2 ΔAIC included different combinations of the other factors (Table S3). We included percent land development at 300 m with these variables in a new redundancy analysis to understand how these variables relate to exurban land use. The first axis explained 75% of the constrained variation and was positively associated with permanence, aquatic vegetation, and human land use (Fig. 6). Aquatic isopods (Caecidotea spp.), fingernail clams (Musculium spp.), phantom midge larvae (Chaoborus spp.), and ram’s horn snails (Planorbella trivolvis) were associated with permanent wetlands in developed landscapes, whereas wood frogs (Rana sylvatica) and mosquitoes (Aedes spp.) were associated with temporary wetlands in more natural landscapes (Fig. 6).
Redundancy analysis bi-plot of wetland community composition versus environmental attributes of wetland permanence and percent aquatic vegetation and percent human land use (N = 38). Most taxa occur close to the center. Outlying taxa include mosquitoes (Aedes = Aed), wood frogs (Rana sylvatica = RaSy), spotted salamanders (Ambystoma maculatum = AmMa), phantom midge larvae (Chaoborus = Cha), isopods (Caecidotea = Cae), damselfly larvae (Lestes = Les), ram’s horn snails (Planorbella trivolvis = PlTr), gray drake mayflies (Siphlonurus = Sip), and fingernail clams (Musculium = Mus)
We also conducted an indicator species analysis, which identifies the most characteristic species of wetlands that differed in human land use and permanence (Dufrêne and Legendre 1997). The ram’s horn snail (Planorbella trivolvis) was the only species significantly indicating wetlands in developed landscapes (> 35% development; indicator value: 0.68, P = 0.005). We looked at indicator species for wetland permanence to see if the two groups of indicator species overlapped. We detected five significant indicator species for semi-permanent wetlands after correcting for multiple tests. Ram’s horn snails also indicated semi-permanent wetlands, providing the highest indicator value for wetland permanence (= 0.89, P = 0.0001). Other semi-permanent wetland indicator species included the diving beetle Matus, phantom midge larva Chaoborus, water beetle Enochrus, and water boatman Trichocorixa. Taxa indicating temporary wetlands included Helophorus beetles and Aedes mosquitoes.
Finally, we assessed if losing temporary wetlands in exurban landscapes reduced overall species richness, which would be the case if not all species inhabited more permanent wetlands. If most species that occur in more permanent wetlands also occur in temporary wetlands, then species occurrence patterns would be significantly nested along a gradient of permanence. When wetlands were ordered by permanence and secondarily by percent human land use, we found no evidence for significant nestedness (Fig. 7; observed NODF = 50.8, simulated NODF = 51.3, lower and upper 95% confidence limits = 49.8, 52.7, respectively). This outcome suggests that some species occur only in temporary wetlands.
Nestedness plot of species occurrence patterns for 38 wetlands. Columns are wetlands ordered by decreasing permanence and then by decreasing human land use. Rows indicate species ordered by declining occurrence from top to bottom. Presences indicated by shading and absences by white. Absences in the upper left corner and presences toward the lower right contribute to non-nestedness
Discussion
We expected a decrease in freshwater animal diversity with increasing exurban development consistent with the findings of similar studies (e.g., Houlahan and Findlay 2003; Pillsbury and Miller 2008). In particular, several reviews demonstrate an overall negative effect of urbanization on wetland amphibian diversity by degrading and destroying habitat and homogenizing communities (Hamer and McDonnell 2008; Scheffers and Paszkowski 2011). Yet, we found no effect of household density along the exurbanization gradient we studied, and a positive rather than a negative effect of human land use on wetland animal diversity.
This result seems counterintuitive given the common finding of decreasing species diversity in wetlands with increasing urbanization (McKinney 2002; Hansen et al. 2005; Rubbo and Kiesecker 2005; Urban et al. 2006; Hamer and McDonnell 2008). However, studies from other taxonomic groups that focus on exurban or suburban areas rather than the entire urban–rural gradient demonstrate heterogeneous diversity responses to residential development, and some studies even demonstrate a peak in diversity in exurban regions (McKinney 2002; Hansen et al. 2005). Exurban peaks are commonly attributed to increased contributions from non-native species, but we only found native species in our study, which is typical for temporary wetlands in this region. We did, however, observe a shift in the characteristics of exurban ponds.
More permanent communities
Structural equation modeling suggested that the higher diversity of wetland species in exurban wetlands occurs because wetlands generally became more permanent with increasing land development (Fig. 4e), which was positively correlated with household density (r = + 0.55). Other studies also found a shift toward more permanent wetlands in exurban regions, supporting a more general pattern (Kentula et al. 2004; Rubbo and Kiesecker 2005; Johnson et al. 2013).
Wetland permanence supports greater freshwater diversity both directly and indirectly. More permanent wetlands directly promote higher species diversity by providing a longer development time, which accommodates both rapidly and slowly developing species (Schneider and Frost 1996; Wellborn et al. 1996; Urban 2004; Hamer and McDonnell 2008). Wetland permanence also was associated with less tree canopy cover, which in turn, correlated positively with denser aquatic vegetation (Fig. 5). Trees often perform poorly in more permanent water bodies, limiting overhead canopy cover (Relyea 2002). As trees colonize wetlands, they also can reduce hydroperiods by increasing evapotranspiration (Werner and Glennemeier 1999). Wetlands without extensive tree canopy cover are warmer and sunnier and therefore support warm-adapted species and dense aquatic vegetation (Skelly et al. 1999, 2002). This aquatic vegetation can create habitat complexity and provide food to a more diverse assemblage of species (Werner and Glennemeier 1999; Relyea 2002; Skelly et al. 2002; Urban 2004).
The road to permanence
Permanent wetlands can dominate exurban landscapes because humans create new permanent wetlands, make temporary wetlands more permanent, or eliminate temporary ponds completely. We found that few permanent wetlands occurred in rural areas, and few temporary wetlands occurred in more developed landscapes (Fig. 4e). This pattern, together with the region’s history, suggests a combination of wetland conversion and loss.
Conversion or creation of permanent wetlands appears likely in this region. Only road proximity significantly explained wetland permanence in exurban areas in the best structural equation model. Exurban wetlands often occurred adjacent to roads, and the most temporary wetlands (< 2-month inundation) only occurred more than 1.0 km from roads (Fig. 4b). Roads can enhance permanence by damming natural water courses, concentrating road runoff, or providing access for permanence-enhancing alterations (Forman et al. 2003). In our study, 13 exurban wetlands showed signs of enhanced permanence: nine from roads, three from agricultural dams, and one from a historic stone wall. Similarly, 60% of wetlands around Portland, Oregon showed hydrological manipulations, and after 8 years of rapid exurban development, wetlands had become deeper and more permanent (Kentula et al. 2004). Roads normally are associated with loss of diversity in wetlands (Johnson et al. 2013), but in our case, the positive effects of enhanced permanence might have outweighed its negative effects.
Besides conversion to permanent wetlands, temporary wetlands might become lost entirely. We lack historic records to substantiate historic temporary wetland loss in our study tracts. However, filling temporary wetlands was a common practice in this region. Historical records indicate that 74% of wetlands were lost from 1780 to 1980 in Connecticut (Dahl 1990). Wetland draining was taught at the Storrs Agricultural School (now University of Connecticut), located in the center of the study region (Metzler and Tiner 1992). Moreover, forested wetlands continue to disappear in this region because they are filled during residential development or converted to permanent wetlands (Tiner et al. 2013). Overall, the loss of temporary wetlands and creation of more permanent wetlands fits well with our understanding of the contemporary picture of an exurban landscape of open-canopy grasslands dotted with permanent—not temporary—ponds (Gobster 1994; McKinney 2006; Suarez-Rubio et al. 2011).
Land sparing versus land sharing
We expected greater diversity with land sparing via clustered, high-impact development that leaves wild areas intact rather than land sharing characterized by more expansive, but low-impact development (Lin and Fuller 2013). Although housing aggregation ranged from random (0.0) to highly aggregated (0.75), it did not significantly affect diversity or other wetland characteristics. However, more diffuse development could affect species that perceive the landscape over greater spatial scales such as birds and large mammals with large habitat requirements (Laurance et al. 2002). In our system, we found no significant link between housing aggregation and proportional human land use after statistically accounting for differences in housing density. Hence, we rejected the underlying assumption that more concentrated housing disturbs less land. In theory, clustered housing developments could be designed that reduce landscape disturbance and enhance the diversity of local wildlife, but existing development patterns did not produce this effect in our study region.
This finding of a relatively intact natural landscape despite moderate exurban development likely holds the key to understanding why we found little negative effect and even positive impacts of exurbanization on wetland biodiversity. In general, residential development often occurred without significant loss of forest cover. For instance, even at moderate housing densities of one household per hectare, usually more than half the landscape remained undeveloped because landowners maintained substantial woodland on their property and an active local land trust protected intervening parcels. Other research also suggests that wetland permanence can dominate other factors in wooded landscapes that remain relatively intact despite exurban development (Baldwin et al. 2006).
We sampled wetlands from remnant forest tracts on private and public lands that varied in surrounding land development. We chose this sampling design because we were interested in the degree to which small parcels of protected land within an exurban landscape could protect local freshwater diversity. We likely would have encountered more negative effects of exurbanization if we had also included wetlands directly abutting developments. However, the strength of our study is that it shows how exurbanization affects aquatic diversity even within the relatively undisturbed, but small, forest tracts that commonly dot exurban landscapes.
Negative aspects of permanence
Even though exurban landscapes support an overall greater diversity of freshwater organisms, they support a different composition. We failed to detect significant nestedness in species across a gradient in permanence and human land use (Fig. 7). The more permanent wetlands found in exurban landscapes excluded certain temporary wetland specialists. Temporary wetland specialists are adapted to grow rapidly in ephemerally inundated habitats, but often cannot compete with or avoid predation from permanent wetland species (Werner and Anholt 1993; Wellborn et al. 1996; Snodgrass et al. 2000). Therefore, conserving the full assemblage of freshwater species in a region requires protecting less diverse temporary wetlands and the specialist species that they harbor.
By promoting more permanent wetlands, exurbanization also can facilitate the spread of disease. The ram’s horn snail (Planorbella trivolvis) was the only indicator species of exurban wetlands. Planorbella snails are the only intermediate host of the Ribeiroia ondatrae parasite, which can cause amphibian limb deformities (Johnson et al. 1999). This increase in limb deformities is posited to occur because anthropogenic land use increases nutrients in wetlands, supporting greater algal food resources for snail vectors (Johnson et al. 2007). Although we did not measure nutrients, we found that P. trivolvis was strongly associated with both exurbanization and wetland permanence. Independent of nutrient load, exurbanization might create the permanent, warm, sunlit wetlands that facilitate the growth and spread of this wildlife disease vector.
The processes that create longer hydroperiods in exurban wetlands could produce fully permanent ponds that never dry seasonally. Permanent ponds support very different species, including fish, which strongly alter freshwater diversity and composition (Wellborn et al. 1996; Snodgrass et al. 2000). We do not know when hydroperiods changed in our study, but other surveys suggest that wetland permanence can change quickly, even within a decade (Kentula et al. 2004). Hence, exurbanization could more strongly affect diversity in the future if ponds transition into fully permanent ponds.
Although we find increasing diversity with urbanization, our study does not address potential differences in the performance of species inhabiting more developed ponds or ponds located near roads. For instance, other studies suggest lower population sizes and survival for amphibians exposed to road salt (Karraker et al. 2008; Collins and Russell 2009). Population-level performance and its link to community patterns along this gradient remain topics for future study.
Conclusions
Much of our understanding about how land conversion affects biological diversity has focused on the stark transition from rural to urban areas and not surprisingly found a negative effect on aquatic diversity. However, these analyses also suggest a puzzling range of responses at the moderate development levels that characterize exurban areas. We demonstrate that moderate exurbanization can maintain or even enhance diversity in wetlands by creating more permanent wetlands that support more species. Exurban development that protects sufficient natural habitat in the landscape could conserve the upland habitat and movement corridors that more typical land development removes yet are critical for maintaining regional aquatic diversity (Gibbs 1998; Semlitsch and Bodie 2003; Urban et al. 2006). A promising message from our research is that protecting sufficient forest cover in a region could moderate exurban development’s effect on local freshwater diversity. Intact landscapes together with wetland permanence likely support the exurban peak in diversity in the region.
However, this development decreases temporary wetland specialists and could promote wildlife disease. To maintain the full diversity of freshwater species in a region, land managers should consider strategies that protect, maintain, and even create temporary wetlands that dry by mid-summer. These habitats maintain abundant populations of certain frogs, salamanders, dragonflies, and other insects that are susceptible to predation in more permanent wetlands. These species provide substantial ecosystem services to the surrounding countryside, e.g., by controlling pest species (Zedler and Kercher 2005), and thus represent important conservation targets for both natural and human interests.
References
Almeida-Neto M, Guimarães P, Guimarães PR, Loyola RD, Ulrich W (2008) A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos 117:1227–1239. https://doi.org/10.1111/j.0030-1299.2008.16644.x
Altig R (1970) A key to the tadpoles of the continental United States and Canada. Herpetologica 26:180–207
Baldwin R, Calhoun A, DeMaynadier P (2006) The significance of hydroperiod and stand maturity for pool-breeding amphibians in forested landscapes. Can J Zool 84:1604–1615
Barton K (2016) MuMIn: Multi-Model Inference
Berube A, Singer A, Watson J, Frey W (2006) Finding exurbia: America’s Fast-Growing Communities at the Metropolitan Fringe Washington. The Brookings Institution, Living Cities Census Series, DC
Brady SJ, Flather CH (1994) Changes in wetlands on nonfederal rural land of the conterminous United States from 1982 to 1987. Environ Manage 18:693–705. https://doi.org/10.1007/BF02394634
Brown DG, Johnson KM, Loveland TR, Theobald DM (2005) Rural land-use trends in the conterminous United States, 1950–2000. Ecol Appl 15:1851–1863
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Cáceres MD, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574
Chazdon RL, Colwell RK, Denslow JS, Guariguata MR (1998) Statistical methods for estimating species richness of woody regeneration in primary and secondary rain forests of NE Costa Rica. In: Dallmeier F, Comiskey JA (eds) Forest biodiversity research, monitoring and modeling: Conceptual background and Old World case studies. Parthenon Publishing, Paris, pp 285–309
Collins SJ, Russell RW (2009) Toxicity of road salt to Nova Scotia amphibians. Environ Pollut 157:320–324
Colwell RK (2013) EstimateS: Statistical estimation of species richness and shared species from samples. Version 9, User’s Guide and application published at: http://purl.oclc.org/estimates. Accessed 1 Feb 2014
Colwell RK, Coddington JA (1994) Estimating terrestrial biodiversity through extrapolation. Philosophical Transactions of the Royal Society of London. Series B Biol Sci 345:101–118
Conrad K, Willson K, Harvey I, Thomas C, Sherratt T (1999) Dispersal characteristics of seven odonate species in an agricultural landscape. Ecography 22:524–531
Core Team R (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Costanza R et al (1997) The value of the world’s ecosystem services and natural capital. Nature 387:253–260
Dahl TE (1990) Wetlands losses in the United States, 1780’s to 1980’s. Report to the Congress. U.S. Department of the Interior, Fish and Wildlife Service, Washington
Dahl TE (2000) Status and trends of wetlands in the conterminous United States 1986–1997. Department of the Interior, Fish and Wildlife Service, Washington
Daniels T (1999) When city and country collide: managing growth in the metropolitan fringe. Island Press, Washington
Distribución de la Reproducción de Anfibios en un Paisaje Urbanizado. Conserv. Biol. 19:504-511. https://doi.org/10.1111/j.1523-1739.2005.000101.x
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366 10.1890/0012-9615(1997) 067[0345:SAAIST]2.0.CO;2
Forman RTT et al (2003) Road ecology: science and solutions. Island Press, Washington
Gibbs JP (1998) Distribution of woodland amphibians along a forest fragmentation gradient. Landsc Ecol 13:263–268. https://doi.org/10.1023/a:1008056424692
Gobster PH (1994) The Urban Savanna Reuniting Ecological Preference and Function. Ecol Restor 12:64–71
Gratton C, Zanden M (2009) Flux of aquatic insect productivity to land: comparison of lentic and lotic ecosystems. Ecology 90:2689–2699
Grimm NB et al (2008) Global change and the ecology of cities. Science 319:756–760. https://doi.org/10.1126/science.1150195
Gutzwiller KJ, Flather CH (2011) Wetland features and landscape context predict the risk of wetland habitat loss. Ecol Appl 21:968–982. https://doi.org/10.1890/10-0202.1
Hamer AJ, McDonnell MJ (2008) Amphibian ecology and conservation in the urbanising world: a review. Biodivers Conserv 141:2432–2449
Hansen AJ et al (2002) Ecological causes and consequences of demographic change in the New West. Bioscience 52:151–162
Hansen AJ et al (2005) Effects of exurban development on biodiversity: patterns, mechanisms, and research needs. Ecol Appl 15:1893–1905
Houlahan JE, Findlay CS (2003) The effects of adjacent land use on wetland amphibian species richness and community composition. Can J Fish Aquat Sci 60:1078–1094
Irwin EG, Bockstael NE (2007) The evolution of urban sprawl: evidence of spatial heterogeneity and increasing land fragmentation. Proc Natl Acad Sci USA 104:20672–20677
Jeon SB, Olofsson P, Woodcock CE (2014) Land use change in New England: a reversal of the forest transition. J Land Use Sci 9:105–130
Johnson PT, Lunde KB, Ritchie EG, Launer AE (1999) The effect of trematode infection on amphibian limb development and survivorship. Science 284:802–804
Johnson PTJ et al (2007) Aquatic eutrophication promotes pathogenic infection in amphibians. Proc Natl Acad Sci USA 104:15781–15786. https://doi.org/10.1073/pnas.0707763104
Johnson PT, Hoverman JT, McKenzie VJ, Blaustein AR, Richgels KL (2013) Urbanization and wetland communities: applying metacommunity theory to understand the local and landscape effects. J Appl Ecol 50:34–42
Karraker NE, Gibbs JP, Vonesh JR (2008) Impacts of road deicing salt on the demography of vernal pool-breeding amphibians. Ecol Appl 18:724–734
Kentula ME, Gwin SE, Pierson SM (2004) Tracking changes in wetlands with urbanization: sixteen years of experience in Portland, Oregon, USA. Wetlands 24:734–743
Laurance WF et al (2002) Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conserv Biol 16:605–618
Lin BB, Fuller RA (2013) FORUM: sharing or sparing? How should we grow the world’s cities? J Appl Ecol 50:1161–1168
McCauley LA, Jenkins DG (2005) GIS-based estimates of former and current depressional wetlands in an agricultural landscape. Ecol Appl 15:1199–1208
McKinney ML (2002) Urbanization, biodiversity, and conservation. Bioscience 52:883–890
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biodivers Conserv 127:247–260
Merritt RW, Cummins KW, Berg MB (2008) An introduction to the aquatic insects of North America, 4th edn. 3rd edn. Kendall Hunt Publishing, Dubuque, Iowa
Metzler KJ, Tiner RW (1992) Wetlands of Connecticut. State Geological and Natural History Survey of Connecticut, Department of Environmental Protection, Hartford
Millenium Ecosystem Assessment (2005) Ecosystems and human well-being: current state and trends. Island Press, Washington
Oksanen J et al (2017) Vegan: Community ecology package
Pejchar L, Reed SE, Bixler P, Ex L, Mockrin MH (2015) Consequences of residential development for biodiversity and human well-being. Front Ecol Environ 13:146–153
Petranka JW (1998) Salamanders of the US and Canada. Smithsonian Institution, Washington
Pillsbury FC, Miller JR (2008) Habitat and landscape characteristics underlying anuran community structure along an urban-rural gradient. Ecol Appl 18:1107–1118
Radeloff VC, Hammer RB, Stewart SI, Fried JS, Holcomb SS, McKeefry JF (2005) The wildland-urban interface in the United States. Ecol Appl 15:799–805
Relyea RA (2002) Local population differences in phenotypic plasticity: predator-induced changes in wood frog tadpoles. Ecol Monogr 72:77–93
Rosseel Y (2012) lavaan: an R Package for Structural Equation Modeling. J Stat Softw 48:1–36
Rubbo MJ, Kiesecker JM (2005) Amphibian Breeding Distribution in an Urbanized Landscape
Scheffers BR, Paszkowski CA (2011) The effects of urbanization on North American amphibian species: identifying new directions for urban conservation. Urban Ecosyst 15:133–147. https://doi.org/10.1007/s11252-011-0199-y
Schneider DW, Frost TM (1996) Habitat duration and community structure in temporary ponds. JNABS 15:64–86
Semlitsch RD, Bodie JR (2003) Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Conserv Biol 17:1219–1228
Seto KC, Güneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci USA 109:16083–16088. https://doi.org/10.1073/pnas.1211658109
Skelly DK (2001) Distributions of pond-breeding anurans: an overview of mechanisms. Isr J Zool 47:313–332
Skelly DK, Richardson JL (2009) Larval sampling. In: Dodd CK (ed) Amphibian ecology and conservation: a handbook of techniques. Oxford University Press, New York
Skelly DK, Werner EE, Cortwright SA (1999) Long-term distributional dynamics of a Michigan amphibian assemblage. Ecology 80:2326–2337
Skelly DK, Freidenburg LK, Kiesecker JM (2002) Forest canopy and the performance of larval amphibians. Ecology 83:983–992
Snodgrass JW, Komoroski MJ, Bryan AL, Burger J (2000) Relationships among isolated wetland size, hydroperiod, and amphibian species richness: implications for wetland regulations. Conserv Biol 14:414–419. https://doi.org/10.1046/j.1523-1739.2000.99161.x
Suarez-Rubio M, Leimgruber P, Renner SC (2011) Influence of exurban development on bird species richness and diversity. J Ornithol 152:461–471
Theobald DM (2004) Placing exurban land-use change in a human modification framework. Front Ecol Environ 2:139–144
Tiner RW, McGuckin K, Herman J (2013) Changes in Connecticut Wetlands: 1990 to 2010. In: Service UFaW (ed), Hadley, MA
Ulrich W, Gotelli NJ (2007) Null model analysis of species nestedness patterns. Ecology 88:1824–1831. https://doi.org/10.1890/06-1208.1
Urban MC (2004) Disturbance heterogeneity determines freshwater metacommunity structure. Ecology 85:2971–2978
Urban MC (2007) Predator size and phenology shape prey survival in temporary ponds. Oecol 154:571–580
Urban MC, Burchsted D, Price W, Lowry S (2006) Stream communities across a rural–urban landscape gradient. Divers Distrib 12:337–350
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst 27:337–363
Werner EE, Anholt BR (1993) Ecological consequences of the trade-off between growth and mortality rates mediated by foraging activity. Am Nat 142:242–272
Werner EE, Glennemeier KS (1999) Influence of forest canopy cover on the breeding pond distributions of several amphibian species. Copeia 1999:1–12
Whitney GG (1996) From coastal wilderness to fruited plain: a history of environmental change in temperate North America from 1500 to the present. Cambridge University Press, Cambridge
Zedler JB (2003) Wetlands at your service: reducing impacts of agriculture at the watershed scale. Front Ecol Environ 1:65–72. https://doi.org/10.1890/1540-9295(2003)001[0065:WAYSRI]2.0.CO;2
Zedler JB, Kercher S (2005) Wetland resources: status, trends, ecosystem services, and restorability. Annu Rev Environ Resour 30:39–74
Acknowledgements
Dana Drake provided insightful comments, and Chelsey Gribbin helped sample ponds. We thank Joshua’s Land Trust and the many private landowners that provided access to their land. MCU was supported by National Science Foundation award DEB-1555876 and UConn’s Center of Biological Risk.
Author information
Authors and Affiliations
Contributions
RR conceived of the project, conducted field sampling, and identified specimens. RR and MCU conducted statistical analyses and wrote the manuscript.
Corresponding author
Ethics declarations
Statement of animal rights
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Leon A. Barmuta.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Urban, M.C., Roehm, R. The road to higher permanence and biodiversity in exurban wetlands. Oecologia 186, 291–302 (2018). https://doi.org/10.1007/s00442-017-3989-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3989-y