Abstract
Diamond-like carbon (DLC) coatings are designed to work under severe lubrication conditions. Depending on working environment, lubrication condition, and counterpart material, DLC coatings can exhibit crucial variations in their tribological behaviors. In the search of a counterpart material to improve tribological performances of DLC coatings in desired environment, we found out in previous work that carbon diffusion is an important parameter that influences the tribological behaviors of amorphous hydrogenated (a-C:H) coating in boundary lubrication. a-C:H coating exhibits high wear against steel counterpart material that has a high carbon affinity/solubility, while its exhibits lower wear against chromium counterpart material that has lower carbon affinity/solubility than steel. These recent findings imply that consideration of the carbon affinity/solubility when choosing a counterpart material for a-C:H coating in boundary lubrication might be of significant importance to improve tribological performances of a-C:H coating. This research investigates tribological behaviors of a-C:H coating against germanium counterpart material that has extremely low carbon affinity/solubility. Results show that there is no atomic interaction between a-C:H coating and germanium during friction and wear test, followed with a very low wear of a-C:H coating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Diamond-like carbon (DLC) hard coatings have gained popularity in recent years for a very wide range of applications to control friction and wear for environmental and fuel efficiency. They are some of the hardest materials known, with several outstanding properties such as high mechanical strength, chemical inertness, and very attractive friction and wear properties. Robertson, Erdemir, and Donnet give a detail classification of DLC coatings, different deposition methods, and relationship between deposition method, coating structure, and coating properties [1,2,3]. Tribological behaviors of various types of DLC coatings have been extensively investigated to enlarge our understanding of their tribological properties, in order to increase efficiency in their applications. Much research have been conducted to meet the needs of clarification of DLC coatings properties in different environments and working conditions, such as vacuum environment, gas environment (dry nitrogen, oxygen or hydrogen), or lubricated environment (water, oil or oil plus additive). A general review of friction and wear properties of various DLC coatings is presented by Grill [4, 5]. Anderson et al. investigated the influence of different environments of tribological behaviors of DLC coatings [6]. Amorphous hydrogenated (a-C:H) coating was reported to give ultralow friction coefficient in vacuum condition. On the other hand, hydrogen-free DLC (ta-C) coating shows high friction coefficient in vacuum condition, and addition of water molecules to the testing environment leads to a drastic decrease of the friction coefficient. Mahmud et al. investigated tribological behaviors of hydrogenated DLC (a-C:H) coating and hydrogen-free DLC (ta-C) coating in commercial lubricating oil [7]. ta-C coating was reported to sustain temperature as high as 150 °C in opposition to a-C:H coating. Tasdemir et al. compared friction properties of hydrogen-free DLC (ta-C) coating in pure PAO oil to its friction properties in glycerol mono-oleate (GMO) friction modifier additive containing PAO oil, Zinc dialkyldithiophosphate (ZnDTP) anti-wear additive containing PAO oil, and GMO + ZnDTP containing PAO oil [8]. ta-C was reported to give ultralow friction in pure PAO oil followed by high wear of the coating, while GMO enhanced durability of the coating. ZnDTP was reported to induce different tribological behaviors of ta-C coating depending on the presence or absence of ferrous surface, while combination of ZnDTP and GMO additives does not show any synergetic correlation. de Barros et al. investigated differences between tribological behaviors of hydrogen containing DLC (a-C:H), titanium-containing DLC (Ti-C:H), and hydrogen-free DLC (a-C) coatings when lubricated in pure PAO oil to their tribological behaviors in ZnDTP anti-wear additive containing PAO oil and ZnDTP + MoDTC (molybdenum dithiocarbamate) friction modifier additive containing PAO oil [9]. Podgornik et al. compared friction and wear compatibility of hydrogenated DLC (a-C:H) coating and tungsten-doped hydrogenated DLC coating with different types of oil lubricants [10]. Carbon diffusion from DLC coating into the counterpart material has been found lately to be among key parameters that influence wear and friction of hydrogenated amorphous carbon (a-C:H) DLC coating during friction and wear test in boundary lubrication as we published in a recent work [11]. Diffusion of carbon atoms from one material to another is influenced by several parameters, among which is the carbon affinity/solubility of the reception material. It was found that there is high diffusion ratio of carbon from a-C:H coating into steel due to high solubility of carbon in iron, while chromium plating exhibits less carbon diffusion due to chromium’s ability to decrease carbon diffusion. Proportionally, a-C:H coating exhibits higher wear against steel than against chromium plating [12]. Therefore, our hypothesis is that a counterpart material with less carbon affinity/solubility than chromium would improve the wear resistance of a-C:H coating in boundary lubrication.
Germanium is a hard metalloid with a diamond-like crystalline structure and is largely utilized in the semiconductor industry. Germanium has been reported to have extremely low carbon solubility [13,14,15]. The purpose of this research is therefore to investigate the tribological behaviors of a-C:H coating when tested against germanium counterpart material in boundary lubricated condition and verify our hypothesis about tribological behaviors of a-C:H coating against a low carbon solubility material.
2 Experimental Details
2.1 Material Characterization and Lubricants
In this study, a-C:H coatings were tested against germanium disks. The coating was deposited on cylindrical JIS SUJ2 (equivalent to AISI 52100 or DIN 100Cr6) bearing steel substrate, with a thickness of 0.8 µm. Cylindrical steel pins were 5 mm in diameter and 5 mm in length. A thin metal interlayer was used to increase the adhesion between the coating and steel. The supplier of the coating was KURITA SEISAKUSHO, Japan. a-C:H coating depositions were conducted using carbon 13 rich methane gas.
Tested germanium disks were optical grade germanium, with 99.99% purity. Germanium disks were 22.5 mm in diameter and 4 mm in thickness, with 0.01 μm average roughness (Ra), and were supplied by TEST MATERIALS, Japan.
Tribological behaviors of a-C:H coating when tested against germanium are compared in this work to its behaviors when tested against chromium plating and steel. Friction and wear tests were conducted using chromium-plated circular disks and S55C steel circular disks supplied by TEST MATERIALS, Japan. Chromium-plated disks and S55C steel disks were 22.5 mm in diameter and 4 mm in thickness. Important characteristic properties of tested materials are shown in Table 1.
The base oil used in this study was a synthetic poly-alpha-olefin (PAO4) having a 19 mm2/s viscosity and a 17.08 GPa−1 pressure–viscosity coefficient at 40 °C.
Hardness and Young’s modulus of tested materials were measured by nano-indentation with a Berkovich indenter (Elionix, ENT-1100a). Surface roughness was evaluated by ZYGO white-light interference microscopy. Hydrogen content in a-C:H coatings was measured using elastic recoil detection analysis (ERDA).
2.2 Tribological Experiments and Surface Analysis
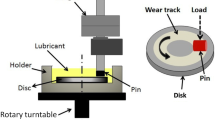
Boundary lubrication tests were carried out using a standard pin-on-disk type unidirectional tribotester illustrated in Fig. 1. The mating disk was mounted on a steel holder fixed to a rotary turntable, while the a-C:H-coated cylindrical pin was rubbed against the disk, under a normal load of 5 N (Fig. 1). Boundary lubrication friction and wear tests were performed at 80 °C, 100 °C, and 120 °C with 0.1 m/s average linear speed, and 1-h duration.
Surface of tested samples were analyzed using optical microscope and X-ray Photoelectron Spectroscopy (XPS). XPS is a technique which analyzes the elements constituting the sample surface, its composition, and chemical bonding state. Further details are described elsewhere [16].
High-resolution spectra of Ge3d, C1s, O1s, and Ar2p regions were acquired from the wear track on germanium samples after experiments. Collected spectra were analyzed using the CasaXPS software. Spectrum calibration was done using the Ar2p peak with a fixed value of 241.9 eV. A Shirley backgrounds was used to analyze the Ge3d spectra.
3 Results
3.1 Friction Results
Friction experiments of a-C:H-coated pin rubbed against germanium disk were performed in pure PAO oil, at different temperatures. 3 experimental runs were performed for each temperature setting. Figure 2 presents friction curves of lowest friction coefficient obtained for each temperature. On the right-hand side of the figure are presented the average fiction coefficients for the last 2000 cycles for each experiment. Similarly to our previous work [11, 16], we observed that as the temperature increases, the friction coefficient decreases, and reaches the lowest value of 0.0545 at 120 °C. Figure 3 shows a comparison of friction behaviors of a-C:H coating versus chromium plating and steel, as function of temperature. Values of the friction coefficient reported in Fig. 3 are average values of the friction coefficient for the last 2000 cycles of three experiments with standard deviation error bars. All tested tribopairs show a decreasing friction coefficient with increasing temperature, and the lowest friction coefficient is obtained with a-C:H coating/Cr plating tribopair, followed by a-C:H coating/S55C steel tribopair, and the highest friction coefficient is obtained with a-C:H coating/germanium tribopair.
3.2 Wear Results
Figure 4a–e shows wear scars on a-C:H-coated pins after friction and wear tests for a-C:H coating/germanium contact, a-C:H coating/Cr plating contact, and a-C:H coating/S55C steel contact. On a-C:H-coated pins after friction tests at 80 °C and 100 °C against germanium, we can distinguish a contact area but there is almost no wear scar to be measured. On a-C:H-coated pin after friction tests at 120 °C against germanium, we can distinguish a wear scar, visible enough to perform measurement and calculate the specific wear rate, but it is still very small in comparison to the wear scar obtained when a-C:H-coated pin is rubbed against Cr plating (Fig. 4d) and S55C steel (Fig. 4e). This clearly indicates a high wear resistance capability of a-C:H coating when rubbed against germanium counterpart material. Figure 5 shows a comparison of specific wear rate of a-C:H coating versus germanium, chromium plating, and steel, where we can appreciate the high wear resistance of a-C:H coating against germanium.
Specific wear rate of a-C:H coating as a function of temperature against S55C steel and Cr plating after friction and wear test in boundary base oil lubrication. Specific wear rate of a-C:H coating against S55C steel and Cr plating are reported from our previous work [11]
To observe how far a-C:H coating can resist to wear when rubbed against germanium, friction, and wear tests were performed for a longer sliding time (2 h, 3 h), under higher normal load (20 N, 30 N) at 120 °C. Figure 6a, b shows wear scars on a-C:H-coated pins after friction and wear tests for a-C:H coating/germanium contact for 2 h and 3 h under 20 N and 30 N loads, respectively. We can appreciate that in comparison to the wear scar obtained on a-C:H-coated pin after friction and wear test under 5 N load at 120 °C, wear scars obtained under 20 N and 30 N loads are wider.
Figure 7 shows a comparison of wear behaviors of a-C:H coating in boundary lubrication against S55C steel, Cr plating, and germanium at 120 °C. Specific wear rates of a-C:H coating have been evaluated in our previous work [11, 16]. We can observe that in comparison to its wear behaviors against other tested counterpart materials, a-C:H coating exhibits very low wear rate when rubbed against germanium, and even with an increase of the sliding time up to 3 h and an increase of the normal load up to 30 N, the specific wear rate of a-C:H coating against germanium at 120 °C is very low comparatively to the specific wear rate against S55C steel and Cr plating after 1 h at 120 °C.
Specific wear rate of a-C:H coating against S55C steel, Cr plating, and germanium after friction and wear test in boundary base oil lubrication. All experiments were conducted at 120 °C. Friction and wear tests in boundary base oil lubrication were conducted for a-C:H coating/germanium tribopair for 1 h under 5 N load, 2 h under 20 N load, and 3 h under 30 N load. Friction and wear tests in boundary base oil lubrication were conducted for a-C:H coating/S55C steel and a-C:H coating/Cr plating tribopairs for 1 h under 5 N load. Specific wear rate for a-C:H coating/S55C steel and a-C:H coating/Cr plating tribopairs are reported from our previous work [11]
Figure 8 presents a summary of friction coefficient of a-C:H coating when rubbed against germanium counterpart material in PAO oil at 120 °C under different normal load after 1 h of sliding. We can appreciate that in addition to showing a high resistance to wear, when rubbed against germanium counterpart material, a-C:H coating exhibits a friction coefficient relatively stable between 0.04 and 0.06 regardless of the increase of the normal load.
In order to understand the above described friction and wear behaviors of a-C:H coating, surface analysis of germanium specimens tested at 120 °C were performed using XPS, to observe atomic interaction between a-C:H coating and germanium disks.
3.3 XPS Results
3.3.1 Ge3d Region Investigation Results
Figure 9a–i shows spectra of Ge3d region acquired from wear track of tested germanium specimens, while Fig. 9j shows a typical Ge3d spectrum for pure germanium obtained from a sample as received.
Ge3d XPS spectrum acquired from the wear track on germanium disk after friction and wear test in boundary base oil lubrication at 120 °C. a, c, e, g show depth profiling of Ge3d region for germanium samples tested for 1 h/5 N, 1 h/10 N, 2 h/20 N, and 3 h/30 N, respectively. For samples tested under 5 N, 10 N, and 20 N load (a, c, e), from 6 nm to 36 nm under the surface, the Ge signal remains relatively constant, in shape and intensity. For the sample tested under 30 N load (g), the Ge signal remains relatively constant, in shape and intensity from 12 nm to 36 nm under the surface. Peak deconvolution of the signal at 6 nm for that sample i reveals that there is about 5% oxidation at 6 nm depth. b, d, f, h show peak deconvolution of the Ge signal at the surface of all investigated Ge samples which reveal that there is only pure Ge and Ge oxides (GexOy) present at the surface, and no carbon- and germanium-involved compound (GexCy) observed on Ge disks after experiment. j shows peak deconvolution of typical Ge3d spectrum for pure germanium obtained from a sample as received
Figure 9a, c, e, g shows depth profiling of Ge3d region for samples tested at 120 °C for 1 h/5 N, 1 h/10 N, 2 h/20 N, and 3 h/30 N, respectively. For each sample, spectra obtained at the top surface and successively at 6 nm, 12 nm, and 36 nm depth are displayed. All spectra are obtained from the same point. We can observe 2 broad peaks at the surface suggesting that Ge bonded with at least one other element at the surface of all investigated Ge samples. In Fig. 9a, c, e, depth profiling shows a relatively good superposition of the Ge3d signal from 6 to 36 nm under the surface for germanium samples tested for 1 h/5 N, 1 h/10 N, and 2 h/20 N, suggesting that for those samples there is only pure germanium under the surface. However, Fig. 9g shows that for the germanium sample tested for 3 h/30 N, there is relatively good superposition of the Ge3d signal from 12 to 36 nm under the surface but the signal at 6 nm shows a little difference in the shape comparatively to Ge3d signals obtained from deeper under the surface, highlighting a difference in the chemical composition of that sample at 6 nm depth. Peak deconvolution of the Ge3d signal at 6 nm depth for the germanium sample tested for 3 h/30 N shown in Fig. 9i reveals that in addition to pure germanium, there is some germanium oxide compound at 6 nm under the surface. We can then understand that for germanium samples tested for, 1 h/5 N, 1 h/10 N, 2 h/20 N, there is only pure germanium under the surface but the sample tested for 3 h/30 N was oxidize up to 6 nm under the surface.
Figure 9b, d, f, h shows peak deconvolution of the Ge3d signal at the surface for samples tested at 120 °C for 1 h/5 N, 1 h/10 N, 2 h/20 N, and 3 h/30 N, respectively. Peak deconvolution reveals that there is only pure germanium and germanium oxides (GexOy) presents at the surface, and no carbon- and germanium-involved compound (GexCy) observed on germanium disks after experiment. This suggests that during the experiment, there were no atomic interactions between germanium disks and the a-C:H coating is mainly composed of carbon atoms. Investigations on the C1 s region are needed to observe if GexCy compound is present or not at the C1s region. Results of investigations on the C1s region are presented and discussed in the next section.
3.3.2 C1s Region Investigation Results
Figure 10a–h shows spectra of C1s region acquired from the wear track of tested germanium specimens.
C1s XPS spectrum acquired from the wear track on germanium disk after friction and wear test in boundary base oil lubrication at 120 °C. a, c, e, g show depth profiling of C1s region for germanium samples tested for 1 h/5 N, 1 h/10 N, 2 h/20 N, and 3 h/30 N, respectively. No carbon signal is detected under the surface of all tested samples. b, d, f, h show peak deconvolution of the C1s signal at the surface of germanium samples tested for 1 h/5 N, 1 h/10 N, 2 h/20 N, and 3 h/30 N, respectively. Only carbon, carbon oxide, and carbon hydroxide are detected at the surface of all tested samples
Figure 10a, c, e, g shows depth profiling of C1s region for samples tested at 120 °C for 1 h/5 N, 1 h/10 N, 2 h/20 N, and 3 h/30 N, respectively. For each sample, all spectra obtained from the same point, at the top surface and successively at 6 nm, 12 nm, and 36 nm depth, are displayed. We can observe 1 broad peak at the surface suggesting the presence of carbon-involved compound at the surface. However, the depth profiling shows that no signal was detected under the surface, suggesting that there is no carbon diffusion into the germanium disk.
Figure 10b, d, f, h shows peak deconvolution of the C1s signal at the surface of all investigated germanium samples, which reveals that there are only carbon, carbon oxide, and hydroxide present at the surface, and no GexCy is observed on germanium disks after experiment. This confirms that during the experiment, there were no atomic interactions between germanium disks and the DLC coating is mainly composed of carbon atoms.
3.3.3 O1s Region Investigation Results
Figure 11a–d shows spectra of O1s region acquired from the wear track germanium samples tested at 120 °C for 1 h/5 N, 1 h/10 N, 2 h/20 N, and 3 h/30 N, respectively. We can observe one broad peak at the surface for all investigated samples, suggesting the presence of oxygen-involved compound. However, the depth profiling shows that no signal was detected under the surface, suggesting that under the surface there is only pure Ge, with the exception of the sample tested under 30 N load for which germanium oxide was observed at 6 nm under the surface at Ge3d region.
Depth profile of O1s XPS spectrum acquired from the wear track on germanium disk after friction and wear test in boundary base oil lubrication at 120 °C, for 1 h/5 N, 1 h/10 N, 2 h/20 N, and 3 h/30 N, respectively. Oxygen signal is observed at the surface of all tested samples but not under the surface
4 Discussion: Tribological Behaviors of a-C:H Coating Against Germanium Material
Friction and wear mechanisms of DLC coatings have been investigated in a wide range, to determine which materials are the most suitable to control friction and wear of DLC coatings in desired environments and working conditions. Following our previous works on a-C:H coating/S55C steel tribopair [16] and a-C:H coating/Cr plating tribopair [11], the objective of the present study is to investigate the effect of carbon diffusion on tribological behaviors of a-C:H coating against germanium counterpart in boundary base oil lubrication. Our hypothesis was that a-C:H hard coating, mainly composed of carbon atoms, will show lower wear against low carbon diffusion tolerance counterpart material.
Friction results obtained in this study show that when tested against germanium counterpart, the friction coefficient of a-C:H coating decreases with the temperature, and a-C:H coating shows higher friction coefficient when rubbed against germanium counterpart, in comparison to steel and Cr plating counterpart (Fig. 3). In addition, the friction coefficient of a-C:H coating was found to keep relatively stable values between 0.04 and 0.06 with increasing load, when a-C:H coating is rubbed against germanium counterpart (Fig. 7).
Wear results obtained in this study show that a-C:H coating exhibits high wear resistance when rubbed against germanium counterpart, in comparison to steel and Cr plating counterpart (Fig. 7)
Carbon diffusion from a-C:H coating was found to be a key parameter in the accelerated wear of a-C:H coating when tested against steel counterpart. Carbon atoms easily diffuse into high carbon-soluble metals such as iron and steel [17]. Chromium is known to decrease carbon diffusion [18], and carbon diffusion rate in Cr plating is lower in comparison to carbon diffusion in steel [11]. Germanium is known to have extremely low carbon solubility. Scace et al. reported that below 2780 °C the amount of carbon dissolved in liquid germanium was too small to measure accurately [14]. No investigation report on the tribological behavior of DLC coatings against germanium was found prior to this work, suggesting that DLC coatings have never been tested against germanium in the past. In our previous works, we evidenced
-
carbon diffusion from a-C:H coating into steel, with iron carbide (Fe3C) formation within the first 12 nm under the surface of the steel disk and high wear of a-C:H coating when tested against steel counterpart;
-
carbon diffusion from a-C:H coating into Cr plating, with chromium carbide (Cr7C3) formation at the surface of the Cr-plated disk and lower wear of a-C:H coating when rubbed against Cr plating counterpart, in comparison to steel counterpart.
In this work, we observed no carbon diffusion from a-C:H coating into germanium counterpart, no germanium- and carbon-involved compound observed at a-C:H coating/germanium contact, and very much lower wear of a-C:H coating when rubbed against germanium counterpart, in comparison to steel and Cr plating counterparts. We can understand that there were no atomic interaction between the a-C:H coating and the germanium disk during the friction and wear test, which is believed to be a key parameter of the high wear resistance of a-C:H coating when rubbed against germanium counterpart. Those results show very strong agreement with our starting hypothesis, and we can deduce that the wear of a-C:H coating decreases with the carbon diffusion affinity of its counterpart material. We can propose germanium or a germanium-based surface coating as counterpart material for a-C:H coating in tribosystems where high wear resistance of a-C:H coating is needed.
5 Conclusion
This research investigated the effects of carbon diffusion on friction and wear properties of a-C:H hard coating when rubbed against germanium counterpart. The results showed that
-
a-C:H coating exhibits very high wear resistance when rubbed against germanium counterpart in boundary lubricated condition;
-
a-C:H coating exhibits relatively stable friction coefficient value between 0.04 and 0.06 against germanium counterpart in boundary lubricated condition at 120 °C, even when the normal load increases up to 30 N;
-
there is no carbon diffusion from a-C:H coating into germanium surface and no germanium–carbon compound observed after friction and wear test. Therefore, there were no atomic interactions between a-C:H coating and germanium counterpart.
This research reveals to the scientific and industrial world that germanium and the series of germanium–carbon alloys are a whole new series of materials that can be used as counterpart material for DLC hard coatings to control friction and wear in boundary lubrication. It therefore paves the way for new researches on tribological behavior of DLC hard coatings against different germanium–carbon alloys.
References
Robertson, J.: Diamond-like amorphous carbon. Mater. Sci. Eng. R 37, 129–281 (2002). https://doi.org/10.1016/S0927-796X(02)00005-0
Bhushan, B.: Modern Tribology Handbook. CRC Press, Boca Raton (2001)
Donnet, C., Erdemir, A.: Tribology of Diamond-Like Carbon Films: Fundamentals and Applications. Springer, Boston (2008)
Grill, A.: Review of the tribology of diamond-like carbon. Wear 168, 143–153 (1993). https://doi.org/10.1016/0043-1648(93)90210-D
Grill, A.: Tribology of diamondlike carbon and related materials: an updated review. Surf. Coat. Technol. 94–95, 507–513 (1997). https://doi.org/10.1016/S0257-8972(97)00458-1
Andersson, J., Erck, R.A., Erdemir, A.: Friction of diamond-like carbon films in different atmospheres. Wear 254, 1070–1075 (2003). https://doi.org/10.1016/S0043-1648(03)00336-3
Al Mahmud, K.A.H., Varman, M., Kalam, M.A., Masjuki, H.H., Mobarak, H.M., Zulkifli, N.W.M.: Tribological characteristics of amorphous hydrogenated (a-C: H) and tetrahedral (ta-C) diamond-like carbon coating at different test temperatures in the presence of commercial lubricating oil. Surf. Coat. Technol. 245, 133–147 (2014). https://doi.org/10.1016/j.surfcoat.2014.02.052
Abdullah Tasdemir, H., Wakayama, M., Tokoroyama, T., Kousaka, H., Umehara, N., Mabuchi, Y., Higuchi, T.: Ultra-low friction of tetrahedral amorphous diamond-like carbon (ta-C DLC) under boundary lubrication in poly alpha-olefin (PAO) with additives. In: Tribology International, pp. 286–294. Elsevier (2013)
De Barros’Bouchet, M.I., Martin, J.M., Le-Mogne, T., Vacher, B.: Boundary lubrication mechanisms of carbon coatings by MoDTC and ZDDP additives. Tribol. Int. 38, 257–264 (2005). https://doi.org/10.1016/j.triboint.2004.08.009
Podgornik, B., Sedlaček, M., Vižintin, J.: Compatibility of DLC coatings with formulated oils. Tribol. Int. 41, 564–570 (2008). https://doi.org/10.1016/j.triboint.2007.12.004
Aboua, K.A.M., Umehara, N., Kousaka, H., Deng, X., Tasdemir, H.A., Mabuchi, Y., Higuchi, T., Kawaguchi, M.: Effect of carbon diffusion on friction and wear properties of diamond-like carbon in boundary base oil lubrication. Tribol. Int. 113, 389–398 (2017). https://doi.org/10.1016/j.triboint.2016.10.047
Aboua, K.A.M., Umehara, N., Kousaka, H., Tokoroyama, T., Murashima, M., Mabuchi, Y., Higuchi, T., Kawaguchi, M.: Effect of carbon diffusion on friction and wear behaviors of diamond-like carbon coating against Cr-plating in boundary base oil lubrication. Tribol. Online 13, 290–300 (2018). https://doi.org/10.2474/trol.13.290
Olesinski, R.W., Abbaschian, G.J.: The C-Ge (Carbon-Germanium) System. Bull. Alloy Phase Diagr. 5, 484–486 (1984). https://doi.org/10.1007/BF02872901
Scace, R.I., Slack, G.A.: Solubility of carbon in silicon and germanium. J. Chem. Phys. 30, 1551–1555 (1959). https://doi.org/10.1063/1.1730236
Lee, J.H., Lee, E.K., Joo, W.J., Jang, Y., Kim, B.S., Lim, J.Y., Choi, S.H., Ahn, S.J., Ahn, J.R., Park, M.H., Yang, C.W., Choi, B.L., Hwang, S.W., Whang, D.: Wafer-scale growth of single-crystal monolayer graphene on reusable hydrogen-terminated germanium. Science 344, 286–289 (2014). https://doi.org/10.1126/science.1252268
Aboua, K.A.M., Umehara, N., Kousaka, H., Deng, X., Tasdemir, H.A., Mabuchi, Y., Higuchi, T., Kawaguchi, M.: Effect of carbon diffusion on friction and wear properties of diamond-like carbon in boundary base oil lubrication. Tribol. Int. 113, 389–398 (2017). https://doi.org/10.1016/j.triboint.2016.10.047
Chen, Y., Zhang, L.: Polishing of Diamond Materials. Springer, London (2013)
Krishtal, M.A.: Diffusion processes in iron alloys. Isr. Progr. Sci. Transl. Jerusalem., pp. 135–137 (1970)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aboua, K.A.M., Umehara, N., Kousaka, H. et al. Effect of Carbon Diffusion on Friction and Wear Behaviors of Diamond-Like Carbon Coating Against Germanium in Boundary Base Oil Lubrication. Tribol Lett 67, 65 (2019). https://doi.org/10.1007/s11249-019-1179-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11249-019-1179-2