Abstract
Hydroxy fatty acids, such as those derived from castor and lesquerella seed oils, make ideal substrates for the synthesis of bio-lubricants, cosmetics, coatings, plastics, and lubricant additives. However, feedstocks of such fatty acids suffer from major drawbacks, such as a lack of a cropping system to produce those seeds or toxic by-products in generating the seed oil, all of which limit availability and thus add to costs. In this study, we explore lubrication properties of microbially derived hydroxy fatty acids and demonstrate that such microbial ω − 1 hydroxy fatty acids, and their derivatives, exhibit lubrication traits (e.g., anti-friction and anti-wear properties) comparable to those of seed-derived hydroxy fatty acids. These ω − 1 hydroxy fatty acids can be recovered from sophorolipids produced by the yeast Candida bombicola ATCC 22214, or by bioengineering bacterial systems to produce them from sugar (Garg et al. in Metab Eng 35:9–20, 2016). Optimization of this latter system can pave the way for a less costly and sustainable alternative to plant-derived bio-lubricants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As the performance capabilities of newly manufactured machines, automobiles, and engines continue to improve, suppliers of high-performance lubricants are being challenged to provide new and innovative molecular solutions which improve lubricant function, decrease energy consumption and increase mechanical efficiency. Often, petroleum-based lubricants are insufficient to meet these demands; their maximum performance capabilities have plateaued after significant research and development efforts [2]. In addition to this developmental challenge, increasing awareness of changes in global climate, as well as efforts to reduce the dependence of USA on imported crude oil, have placed an increasing focus on developing bio-based, sustainable, next-generation lubricants [3].
Previous work investigating the tribological properties of the hydroxy fatty acids, such as ricinoleic acid and lesquerolic acid, has shown that these compounds demonstrate favorable cold-temperature properties [4], oxidative stability [5], anti-friction [6], and anti-wear [7] properties. Significant safety and production concerns [8,9,10,11] limit the widespread use of these hydroxy fatty acids in industrial settings. Hydroxy fatty acids also occur naturally in glycolipids, namely sophorolipids [12, 13], that are synthesized by fermentation of long-chain fatty acids and other long-chain compounds by certain yeasts, such as Candida bombicola [14], Torulopsis magnoliae [12] and Torulopsis gropengiesseri [15]. Recently, synthesis of such hydroxy branched fatty acids has been demonstrated in metabolically engineered E. coli hosts [1]. A potential high-yielding, readily accessible, and safe biosynthesis pathway of structurally distinct (ω − 1) hydroxy branched-chain fatty acid-containing lipids (ω − 1 HBFAs), occurs in Candida bombicola ATCC 22214, and offers novel chemistries and routes to new lubricants. This organism produces large quantities of ω − 1 HBFA-containing sophorolipids, which can occur in lactone and free acid forms (Fig. 1a, b, respectively). Extremely high titers of these sophorolipids (up to 120 g/L) with a 0.60 carbon source yield can be obtained when this organism is grown in the presence of glucose and a lipid feedstock [16].
Although sophorolipids have been widely studied for their potential applications as surfactants, emulsifiers, and therapeutic agents [17,18,19,20], accessing and chemically derivatizing the ω − 1 HBFAs for other uses, such as lubricants, have not been investigated. Given these observations and the potential applications of both sophorolipids and ω − 1 HBFAs as bio-lubricants, this work seeks to evaluate the anti-friction, anti-wear, and viscosity properties of these molecules.
2 Methods
2.1 Growth of Candida bombicola
The yeast strain, Candida bombicola ATCC 22214, was cultured according to described methods [16]. The organism was grown overnight on YM media and stationary phase cells were harvested, washed with 0.9% sodium chloride, and used to inoculate production media. The production media consisted of 100 g/L olive oil (Hy-Vee, West Des Moines, IA), 100 g/L glucose (Fisher, Waltham, MA), and 5 g/L yeast extract (BD Difco, Franklin Lakes, NJ). These production cultures were grown in shake flasks maintained in an incubator shaker (Thermo Scientific, Waltham, MA) at 30 °C and 250 RPM for 7 days.
2.2 Isolation and Analysis of Sophorolipids
Sophorolipids were extracted from spent media in a manner consistent with previous reports [16, 21], with the following modifications: after 1 week of growth, cultures were placed in an appropriately sized separatory funnel, and the sophorolipid content was allowed to settle out of suspension; the residual oil substrate, as well as the aqueous phases, were removed from the top of the separatory funnel and extracted twice with two volumes n-hexane followed by extraction twice with one volume ethyl acetate. The sophorolipid fraction was dissolved in ethyl acetate, combined with the ethyl acetate extraction of the aqueous and residual oil phase, and dried with anhydrous sodium sulfate. Ethyl acetate was removed by evaporation, and the resulting sophorolipids were washed twice with n-hexane to remove any residual oil feedstock. Production and purity of sophorolipids were confirmed using thin layer chromatography (TLC) analysis. The mobile phase consisted of chloroform/methanol/water in a 65/35/5 ratio. Visualization was achieved by spraying the plate with a 40% sulfuric acid solution using an all-glass atomizer and then heating the plate at 100 °C for 10 min [22].
2.3 Production and Characterization of ω − 1 HBFAMEs and their Polymers
ω − 1 HBFAMEs were produced from isolated sophorolipids by reflux for 2 h at 85 °C in acidic methanol (2 M HCl). Following acid reflux, the resulting fatty acid methyl esters (FAMEs) were extracted using 1 volume of diethyl ether [23]. Ether was removed from the sample by evaporation under nitrogen.
Analysis of the resulting ω − 1 HBFAMEs was carried out using gas chromatography-mass spectroscopy (GC–MS). Prior to analysis, 200 µL aliquots of sample dissolved in ethyl acetate were silylated by addition of 70 microliters of N,O-bis (trimethylsilyl)trifluoroacetamide (BSFTA) (Sigma-Aldrich, St. Louis, MO) followed by heating at 65 °C for 30 min. Reactions were cooled to room temperature, evaporated under nitrogen, and re-dissolved in 200 microliters of chloroform prior to injection.
Samples were injected with an injection volume of 1 µL into an Agilent Technologies (Agilent Technologies, Santa Clara, CA) model 6890 gas chromatograph coupled to a model 5973 mass-selective detector set to scan from m/z 40 to m/z 550 at a scan rate of 20 Hz. The column used was an Agilent Technologies HP-5MS (30 m × 0.25 mm). The oven temperature was programmed with a variable ramp rate of 25 °C/min from 120 to 213 °C, 6 °C/min from 213 to 260 °C, and 20 °C/min from 260 to 325 °C. The temperature of the injector port was 250 °C and the mass specific detector (MSD) transfer line temperature was 280 °C. The carrier gas was helium at a flow rate of 1 mL/min with a split ratio of 100:1.
Polyester linkages of the ω − 1 HBFAMEs were formed by addition of 5% (w/w) of Amberlyst-15 catalyst (Sigma-Aldrich, St. Louis, MO) to a tube containing 300 milligrams of ω − 1 HBFAME with no additional solvent. The tube was then sealed with a Teflon cap, kept under nitrogen atmosphere, and stirred at 90 °C for 24 h. After the reaction was complete, the polymerized transesterification product was extracted with ethyl acetate and transferred to a vial. The solvent was then removed under reduced pressure and the sample placed under high vacuum for 16 h. Polymers were analyzed using direct infusion to a Bruker (Bruker, Billerica, MA) FT-ICR set in the positive ion mode with a mass acquisition range of m/z 300 to m/z 2000.
2.4 Tribological Properties
Several lubricant additives were tested and compared: Olive oil (O.O.), fatty acid methyl esters (FAMEs), sophorolipids (SLs), ω − 1 HBFAMEs, and the polymerized ω − 1 HBFAMEs. The FAMEs were derivatives of the O.O, while the SLs and the ω − 1 HBFAMEs were products of the O.O. and yeast culture. Each was blended in commercially available isoparaffinic API Group III base oil (NEXBASE 3043) at concentrations of 1 and 5% by weight. Technical data from the manufacturer for the base oil has been provided in Table 1. In addition, neat base oil was studied as a positive control, while the olive oil served as a negative control. Olive oil was chosen because it was a key component of the production media. Friction and wear testing was conducted on these samples at room temperature using an in-house ball-on-flat microtribometer as seen in Fig. 2. This apparatus consists of two linear stages mounted perpendicular to one another which provide both lateral movement of the sample and vertical displacement to induce the load. The friction and normal forces are measured using a double cantilever system where each beam is fitted with semiconductor strain gauges in a half-bridge configuration. The cantilevers hold the probe which is brought into contact with the substrate and lubricant of interest. The resulting output is collected with an A/D converter and LabView software.
Friction testing was performed at additive concentrations of 1 and 5% by weight using a ramped load test from 10 to 70 mN at a sliding speed of 10 mm/s over a stroke length of 35 mm on two interfaces: one a 5 mm diameter 440c stainless steel probe on AISI 8620 steel and the other a 4 mm diameter SiC probe on 6061 aluminum. Four replicates were performed for each lubricant. A steel–steel interface was chosen because it is ubiquitous in machinery, while the SiC–Al interface was chosen because the two constituent materials have a relatively large difference in hardness and therefore would potentially accelerate wear testing. For each interface, the estimated maximum Hertzian contact pressure was approximately 0.4 GPa and expected to be within the elastic regime for the bulk materials. The substrate surface roughness was characterized using a Zygo optical profilometer over a scan area of 1.41 mm × 1.88 mm. The steel and aluminum substrates exhibited an average roughness (Ra) of 116 ± 20 and 251 ± 14 nm, respectively. Six measurements on each sample were made.
Anti-wear properties were measured using a cyclic wear test conducted on the SiC–Al interface at additive concentrations of 5% by weight. The test was limited to 50 cycles over a stroke length of 8 mm at a load of 0.61 N with an estimated maximum Hertzian contact pressure of 0.5 GPa and constant sliding speed of 10 mm/s. The surface along the wear scar (four scan areas) was then measured with the optical profilometer, and the average depth was calculated in MATLAB. Prior to all testing and surface analysis, the substrates were cleaned in an ultrasonic bath of acetone for 10 min. This test was replicated one other time. After completion of the testing, SEM and EDS analysis was performed.
The kinematic viscosity of these lubricants was characterized at 5% by weight at ambient temperature using a microVISC viscometer (RheoSense, San Ramon, CA) at shear rates of 50, 100, and 500 1/s. Due to limited sample volumes of the additives, this device was chosen because it required small sample sizes (less than a mL). However, it lacked a temperature controller and thus, during the course of the measurements, the internal temperature of the test chamber fluctuated with the environment by approximately 4 °C. Accordingly, the average temperature per sample is reported.
3 Results
3.1 Isolation and Characterization of ω − 1 HBFA-Containing Lipids
Prior optimization studies [16, 21] have shown that sophorolipids can be obtained in high titer from Candida bombicola ATCC 22214 through manipulations of media composition and growth conditions. Here, we use those optimized conditions to obtain sophorolipids in sufficient quantity to analyze the tribological properties of sophorolipids, as well as their methyl-ester and polyester derivatives. Figure 3a demonstrates the production and partial purification of sophorolipids derived from a culture provided with an olive oil feedstock. TLC analysis of the extracted products shows the near-complete conversion of the olive oil triacylglycerol (TAG) (lane 1) to a mixture of sophorolipid products (lane 2). The sophorolipid identification was based on the observation of a dark purple color of the spots in lane 2 upon staining and heating the TLC plate with 40% sulfuric acid, which is indicative of the presence of glycolipids [22]. Furthermore, the occurrence of multiple purple TLC spots in lane 2, with a range of Rf values indicates a mixture of sophorolipid molecules. This chemical diversity is consistent with reports of mixed lactone and acid structures [24, 25], as well as with a variety of acyl chain lengths with different degrees of unsaturation in the sophorolipid, determined by the presence of such different acyl chains in the olive oil feedstock [26].
Analysis of sophorolipid and ω − 1 HBFAME production. a Results of TLC analysis of olive oil feedstock (lane 1) and the resulting mixture of sophorolipid s (lane 2) produced by Candida bombicola after 1 week of growth. The dark purple color observed in the spots in lane 2 indicates the presence of glycolipids. The range of R f values observed in lane 2 is consistent with a mixture of forms of sophorolipid as well as a mixture of chain length and unsaturation from the initial feedstock. b Representative GC profile of the ω − 1 HBFAMEs produced upon acid methanolysis of isolated sophorolipids. As expected a mixture of chain lengths and degrees of unsaturation in both the straight chain FAMEs resulting from initial feedstock as well as in the corresponding ω − 1 HBFAMEs is observed
Direct demonstration of the acyl chain diversity was demonstrated by the GC–MS analysis of ω − 1 HBFAMEs produced by acidic methanolysis of the isolated sophorolipids (Fig. 3b). This experiment identifies a mixture of ω − 1 HBFAMEs and non-hydroxylated FAMES consisting of 16:0, 16:1, 18:0 and 18:1 acyl chains, with the hydroxylated forms accounting for ~30%. The relative abundance of these acyl chains is similar to that found in the olive oil feedstock used in culturing C. bombicola [26].
3.1.1 Chemical Synthesis of ω − 1 HBFA-Containing Lipids
Acid catalysis using the Amberlyst 15 catalyst was used to trans-esterify the isolated ω − 1 HBFAMEs and non-hydroxylated FAME mixture to produce polyester polymers. Figure 4 shows the characterization of the products of this reaction using direct infusion Fourier transform–ion cyclotron–resonance mass spectrometry (FT-ICR-MS). The results of this characterization demonstrate that the major product of this reaction is a dimer, with minor amounts of trimers and tetramers. These oligomers are a mixture of non-hydroxylated-FAs and ω − 1 HBFAs monomers.
Characterization of polyesters formed by ω − 1 HBFAMEs FT-ICR profile of poly ω − 1 HBFAME. m/z ratios indicate a major product corresponding to two ω − 1 HBFAMEs of either 16 or 18 carbon chain length with the major fraction of this product composed of two 18 carbon chains. The minor products of this reaction correspond to polymers of 3 and 4 ω − 1 HBFAMEs
3.1.2 Testing Tribological Properties of ω − 1 HBFA-Containing Lipids
The tribological properties of a variety of ω − 1 HBFA-containing lipids were determined and compared with neat base oil, olive oil and the FAMEs derived from olive oil. These properties were evaluated by measuring the friction coefficient (Fig. 5), wear depth (Figs. 6, 7), and kinematic viscosity (Fig. 8) of each additive. The friction coefficient was determined for each additive at 1% (wt/wt) and 5% (wt/wt) concentrations in base oil. These measurements were made using the SiC–Al interface (Fig. 5a) and a steel–steel interface (Fig. 5b). For both interfaces, the additives reduced the coefficient of friction with the higher concentration on average outperforming the lower concentration. The reductions were far more significant for the SiC–Al interface with decreases as low as 36, 38, and 42% on average for the ω − 1 HBFAMEs (COF 0.21), sophorolipids (COF 0.20) and FAMEs (COF 0.19), respectively, when compared to the base oil (COF 0.33). Note that the overall friction coefficients for the steel–steel interface were significantly lower than the values for the SiC–Al interface and as such, provided less room for improvement. As with the other interface, the ω − 1 HBFAMEs, sophorolipids, and FAMEs were among the top performing additives with reductions in friction of over 20% on average.
The wear testing was conducted using the SiC–Al interface, and the results are summarized in Fig. 6. Olive oil, ω − 1 HBFAMEs, and poly (ω − 1 HBFAMEs) additives each demonstrated an average wear depth roughly half the magnitude of the scar generated with the base oil. The sophorolipid and olive oil-FAME samples also showed improved wear resistance, but to a lower extent. No wear was observed on the SiC probes after the tests. Examination of the wear scar width indicated no measureable differences among the tested additives.
Upon completion of the wear tests, the substrates and probes were examined by SEM (Fig. 7) and EDS analysis (data not shown). Figure 7 shows a series of secondary electron images taken of the wear scars for each lubricant sample. Note that the scar has been outlined for clarity. A burnishing of the surface asperities can be observed indicating plastic deformation at the asperities and mild abrasion. The EDS analyses showed no discernable difference in the chemical composition inside and outside of the wear scar. Similarly, no transfer material could be found on the probes. The wear observed in the presence of additive is limited primarily to the asperity level, whereas for the base oil, the scar is significantly deeper.
Figure 8 shows the kinematic viscosity of the base oil in the presence of each additive. The viscosity was measured at three shear rates. As shown in Fig. 8, the sophorolipid, ω − 1 HBFAMEs, and olive oil (O.O.) have nearly the same viscosity over the given shear rate range, while the base oil and olive oil FAMEs have viscosities that are approximately 10 cSt greater.
4 Discussion
The suitability of bio-based lubricants derived from oils that contain such hydroxy fatty acids as ricinoleic and lesquerolic acids (extracted from castor and lesquerella seeds, respectively) has previously been investigated [4, 5, 7, 27,28,29] as a means to address the growing need for novel high-performance lubricants [2]. These prior studies have indicated the improved lubricant characteristics of such hydroxy fatty acids in several key properties (e.g., friction coefficients and wear resistance) relative to other sources [5,6,7,8]. Sources of these hydroxy fatty acids have significant limitations that impact their industrial viability [8,9,10,11]. In this work, we evaluated an alternative source of a hydroxy fatty acid (i.e., Candida bombicola ATCC 22214) that offers a different chemistry; namely the hydroxyl group is situated near the end of the alkyl chain of the fatty acids, whereas in ricinoleic and lesquerolic acids the hydroxyl group is situated mid-chain. There are several additional advantages of C. bombicola-sourced hydroxy fatty acid-containing lipids. These include: (a) the production platform for this lipid has already undergone significant optimization because of the widespread potential applications of sophorolipids [17,18,19,20]; (b) the relative ease and safety of producing hydroxy fatty acids; (c) the ease of chemically converting sophorolipid derived hydroxy fatty acids to esters suitable for lubrication applications [23]. Further, recent work has demonstrated the ability of C. bombicola to utilize treated cellulosic hydrolysates in isolation or with soybean oil supplementation to produce sophorolipid at titers which exceed standard glucose media [30]. The characterizations presented herein demonstrate that the ω − 1 HBFA-containing lipids, produced by C. bombicola, can serve as an efficient bio-based lubricant, likely acting through an adsorbed tribofilm.
A significant feature of ω − 1 HBFA-containing lipids is the availability of the hydroxyl functional group at the ω − 1 carbon. The presence of this functional group allows for many potentially significant chemical modifications, including the ability to form polyol-esters, which may improve tribological properties of such molecules. Previous work has demonstrated improved characteristics of polyesters in several key tribological properties [27]. The polymers synthesized in this work consist primarily of dimers and trimers of differing acyl chain lengths. The advantage of ω − 1 HBFA over previously produced diester and triester molecules is that the latter are dependent on the need for a polyol molecule substrate [27,28,29, 31], whereas ω − 1 HBFA can be used to directly produce dimer and trimer esters, without an exogenous source of a polyol substrate.
Anti-friction and anti-wear testing results show that the friction coefficient and the degree of wear protection afforded by the compounds tested in this work are of the same order as those described previously for ester derivatives of ricinoleic acid [6]. The evaluations conducted in this study were interpreted by assuming boundary lubrication. That is, under the given temperature and loading conditions, the calculated lambda ratios for the tests fell significantly below one in accordance to EHL theory. As previously mentioned, all tests were conducted at room temperature—a range which is relevant to many applications. While both temperature extremes are worth further investigation, it is pertinent to mention that the addition of the additives at their relatively low concentrations did not appear to adversely affect the pourability of the bulk lubricant. As for higher temperatures, the additives were not expected to form beneficial films as a result of chemical reactions. Therefore, these results provide significant insights into the mechanism by which ω − 1 HBFA-containing esters function as efficient lubricants.
The protection supplied by ω − 1 HBFA-containing esters appear to act via an adsorbed tribofilm, which allows the mating surfaces to slide past one another more efficiently. This can be described in greater detail by analyzing the additives’ molecular structure. Generally, surface active additives such as friction modifiers and anti-wear agents like those presented in this work consist of a polar functional group which adsorbs onto the substrate and an oleophilic hydrocarbon group that allows solubility. Oxygen-containing functional groups such as esters, which occur in the studied additives, are among the most common and strongest adsorbing due to oxygen’s electronegativity. The strength of this polar group combined with the length of the hydrocarbon chain determines the additive’s behavior. The nonpolar chains extend outward from the surface and associate with one another to form the film. Short nonpolar chains have lower solubility but lead to greater surface affinity and vice versa. In addition, short and branched ligands tend to have higher coefficients of friction than long and linear chains [32].
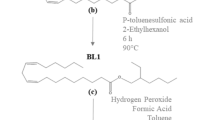
What differs between the presented additives is the ligand structure. The difference between the FAMEs and ω − 1 HBFAMEs (as seen in Fig. 1c) is clearly the hydroxyl group which presents a second polar site. Despite this addition, these two reduced friction by nearly the same amount with the FAMEs slightly outperforming on average though the difference was insignificant. In fact, the SLs (as seen in Fig. 1a, b) reduced friction equally well even with the presence of the sugar molecule. However, the branching is believed to have caused some minor solubility issues.
An additive’s ability to mitigate wear is primarily dependent on its film-forming capability. Under shear, the film is sacrificed as opposed to the interacting asperities beneath. Thus, anti-wear agents require high surface affinity so that the molecules can readily adsorb onto the surface and therefore, tend to have shorter nonpolar chains [32]. This mechanism of wear protection is indicated by a burnishing of the surface asperities that can be observed, indicating localized plastic deformation and mild abrasion. However, while the wear observed in the presence of additives is limited primarily to the asperity level, the scar resulting from the sole use of the base oil is significantly deeper, indicating that the base oil does not provide equal protection on its own, in contrast to the tangible level of wear protection provided by ω − 1 HBFA-containing esters. The additional polar site on the ω − 1 HBFAMEs may have led to better adsorption and thus provided superior wear protection over the FAMEs. However, the EDS analyses showed no discernable difference in the chemical composition inside and outside of the wear scar. This suggests that any film formed of the order of a micron thick is not robust and is not chemisorbed onto the substrate surface under the testing conditions utilized in this study. Similarly, no transfer material could be found on the probes. Thinner adsorbed films not detectable by EDS cannot be ruled out, however.
5 Conclusion
This study provides evidence that sophorolipid-based production, purification, and isolation of hydroxy fatty acid represents a novel source of effective bio-lubricants. The friction and anti-wear behavior at the boundary lubrication regime of these novel hydroxy fatty acid based bio-lubricants were positively compared to olive oil and its derivatives. The studies show that each of the additives succeeded in reducing the friction coefficient when tested in the boundary lubrication regime. Some of the greatest reductions were observed with the ω − 1 HBFAMEs and FAMEs as compared to the neat base oil for the SiC–Al interface. In addition, the ω − 1 HBFAMEs provided superior anti-wear protection by mitigating the wear depth. Therefore, ω − 1 HBFAMEs demonstrate a combination of improved friction reduction and anti-wear properties relative to base oil and non-hydroxy fatty acid additives. The mechanism behind these enhanced tribological behaviors was rationalized to stem from their ability to generate weak, yet effective, films.
References
Garg, S., Rizhsky, L., Jin, H., Yu, X., Jing, F., Yandeau-Nelson, M.D., et al.: Microbial production of bi-functional molecules by diversification of the fatty acid pathway. Metab. Eng. 35, 9–20 (2016)
Lubricants market by type (mineral oil lubricants, synthetic lubricants, bio-based lubricants and greases), by application (transportation, and industrial machinery and equipment)–global trends and forecasts to 2019. Inc., M.a.M. http://www.marketsandmarkets.com/market-reports/lubricants-market-182046896.html (2014). Accessed 28 Sept 2016
Kodali, D.R.: High performance ester lubricants from natural oils. Ind Lubr Tribol 54, 165–170 (2002)
Cermak, S.C., Brandon, K.B., Isbell, T.A.: Synthesis and physical properties of estolides from lesquerella and castor fatty acid esters. Ind. Crop. Prod. 23, 54–64 (2006)
Isbell, T.A., Lowery, B.A., DeKeyser, S.S., Winchell, M.L., Cermak, S.C.: Physical properties of triglyceride estolides from lesquerella and castor oils. Ind. Crop. Prod. 23, 256–263 (2006)
Yao, L.X., Hammond, E.G., Wang, T., Bhuyan, S., Sundararajan, S.: Synthesis and physical properties of potential biolubricants based on ricinoleic acid. J. Am. Oil Chem. Soc. 87, 937–945 (2010)
Adhvaryu, A., Erhan, S.Z., Perez, J.M.: Tribological studies of thermally and chemically modified vegetable oils for use as environmentally friendly lubricants. Wear 257, 359–367 (2004)
Chen GQ, van Erp H, Martin-Moreno J, Johnson K, Morales E, Browse J, et al (2016) Expression of castor LPAT2 enhances ricinoleic acid content at the sn-2 position of Triacylglycerols in Lesquerella Seed. Int J Mol Sci 17
Schieltz, D.M., McWilliams, L.G., Kuklenyik, Z., Prezioso, S.M., Carter, A.J., Williamson, Y.M., et al.: Quantification of ricin, RCA and comparison of enzymatic activity in 18 Ricinus communis cultivars by isotope dilution mass spectrometry. Toxicon 95, 72–83 (2015)
Thorpe, S.C., Kemeny, D.M., Panzani, R., Lessof, M.H.: Allergy to castor bean. 1. Its relationship to sensitization to common inhalant allergens (Atopy). J. Allergy Clin. Immunol. 82, 62–66 (1988)
Thorpe, S.C., Kemeny, D.M., Panzani, R.C., Mcgurl, B., Lord, M.: Allergy to castor bean. 2. Identification of the major allergens in castor bean-seeds. J Allergy Clin Immun 82, 67–72 (1988)
Gorin, P.A.J., Spencer, J.F.T., Tulloch, A.P.: Hydroxy fatty acid glycosides of sophorose from Torulopsis magnoliae. Can. J. Chem. 39, 846–855 (1961)
Asmer, H.-J., Lang, S., Wagner, F., Wray, V.: Microbial production, structure elucidation and bioconversion of sophorose lipids. J. Am. Oil Chem. Soc. 65, 1460–1466 (1988)
Daniel, H.-J., Reuss, M., Syldatk, C.: Production of sophorolipids in high concentration from deproteinized whey and rapeseed oil in a two stage fed batch process using Candida bombicola ATCC 22214 and Cryptococcus curvatus ATCC 20509. Biotech. Lett. 20, 1153–1156 (1998)
Jones, D.F., Howe, R.: Microbiological oxidation of long-chain aliphatic compounds. I. Alkanes and alk-1-enes. J. Chem. Soc. Perkin 1 22, 2801–2808 (1968)
Casas, J.A., Garcia-Ochoa, F.: Sophorolipid production by Candida bombicola: medium composition and culture methods. J. Biosci. Bioeng. 88, 488–494 (1999)
Nguyen, T.T., Sabatini, D.A.: Characterization and emulsification properties of rhamnolipid and sophorolipid biosurfactants and their applications. Int. J. Mol. Sci. 12, 1232–1244 (2011)
Otto, R.T., Daniel, H.J., Pekin, G., Muller-Decker, K., Furstenberger, G., Reuss, M., et al.: Production of sophorolipids from whey II. Product composition, surface active properties, cytotoxicity and stability against hydrolases by enzymatic treatment. Appl. Microbiol. Biot. 52, 495–501 (1999)
Shah, V., Doncel, G.F., Seyoum, T., Eaton, K.M., Zalenskaya, I., Hagver, R., et al.: Sophorolipids, microbial glycolipids with anti-human immunodeficiency virus and sperm-immobilizing activities. Antimicrob. Agents Chemother. 49, 4093–4100 (2005)
Van Bogaert, I.N.A., Saerens, K., De Muynck, C., Develter, D., Soetaert, W., Vandamme, E.J.: Microbial production and application of sophorolipids. Appl. Microbiol. Biot. 76, 23–34 (2007)
Ribeiro, I.A., Bronze, M.R., Castro, M.F., Ribeiro, M.H.L.: Sophorolipids: improvement of the selective production by Starmerella bombicola through the design of nutritional requirements. Appl. Microbiol. Biot. 97, 1875–1887 (2013)
Kates, M.: Separation of lipid mixtures. In: Burdon, R.H., van Knippenberg, P.H. (eds.) Techniques of lipidology: isolation, analysis, and identification of lipids, pp. 239–240. Elsevier, Place Elsevier (1986)
Nunez, A., Ashby, R., Foglia, T.A., Solaiman, D.K.Y.: Analysis and characterization of sophorolipids by liquid chromatography with atmospheric pressure chemical ionization. Chromatographia 53, 673–677 (2001)
Davila, A.M., Marchal, R., Vandecasteele, J.P.: Sophorose lipid production from lipidic precursors–predictive evaluation of industrial substrates. J. Ind. Microbiol. 13, 249–257 (1994)
Hu, Y.M., Ju, L.K.: Sophorolipid production from different lipid precursors observed with LC-MS. Enzyme Microb. Technol. 29, 593–601 (2001)
Beltran, G., Del Rio, C., Sanchez, S.N., Martinez, L.: Influence of harvest date and crop yield on the fatty acid composition of virgin olive oils from cv. Picual. J. Agric. Food Chem. 52, 3434–3440 (2004)
Sharma, B.K., Adhvaryu, A., Liu, Z.S., Erhan, S.Z.: Chemical modification of vegetable oils for lubricant applications. J. Am. Oil Chem. Soc. 83, 129–136 (2006)
Linko, Y.Y., Tervakangas, T., Lamsa, M., Linko, P.: Production of trimethylolpropane esters of rapeseed oil fatty acids by immobilized lipase. Biotechnol. Technol. 11, 889–892 (1997)
Uosukainen, E., Linko, Y.Y., Lamsa, M., Tervakangas, T., Linko, P.: Transesterification of trimethylolpropane and rapeseed oil methyl ester to environmentally acceptable lubricants. J. Am. Oil Chem. Soc. 75, 1557–1563 (1998)
Samad, A., Zhang, J., Chen, D., Liang, Y.: Sophorolipid production from biomass hydrolysates. Appl. Biochem. Biotechnol. 175, 2246–2257 (2015)
Yunus, R., Fakhru’l-Razi, A., Ooi, T.L., Iyuke, S.E., Idris, A.: Development of optimum synthesis method for transestrification of palm oil methyl esters and trimethylolpropane to environmentally acceptable palm oil-based lubricant. J. Oil Palm. Res. 15, 35–41 (2003)
Rizvi, S.: A comprehensive review of lubricant chemistry, technology, selection, and design. ASTM International, Baltimore (2009)
Technical Data Sheet: NEXBASE 3043. NESTE. https://www.neste.com/sites/default/files/attachments/tds_nexbase_3043.pdf. Accessed 22 Jan 2015
Acknowledgements
This work was supported by Iowa Energy Center’s opportunity grant (Grant Number: OG-16-010). The author Derek White wishes to acknowledge Professor Jonathan Claussen (Iowa State University) and graduate student, Allison Cargill, for facilitating the viscosity measurements. Additionally, thanks and acknowledgment go to the W. M. Keck Metabolomics Facility at Iowa State University.
Author information
Authors and Affiliations
Contributions
SG and BJN conceived the scientific idea of the study, designed experiments, contributed to the manuscript for intellectual content, and participated in writing and revision of the manuscript. RS executed experiments, collected data, and led the effort to write the manuscript. DW, KLV, TH collected data, and contributed to writing the manuscript. SS oversaw design and execution of tribological analysis experiments, data interpretation and analysis, provided important intellectual inputs to the study, and participated in revision of the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Sturms, R., White, D., Vickerman, K.L. et al. Lubricant Properties of ω − 1 Hydroxy Branched Fatty Acid-Containing Natural and Synthetic Lipids. Tribol Lett 65, 99 (2017). https://doi.org/10.1007/s11249-017-0883-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11249-017-0883-z