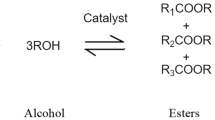
Abstract
This chapter highlights the evolution of eco-friendly lubricants derived from natural oils and fats to green lamellar solid additives to a new class of “greener” functional fluids known as room temperature ionic liquids (RTILs). The attraction to these bio-based lubricants began with vegetable oils due to their low friction and wear properties. These superior tribological characteristics are a result of their chemical composition of triacylglycerol molecules made up of esters derived from glycerol and long chains of polar fatty acids. It is these fatty acids within the natural oils that establish monolayers that enable high lubricity in boundary-lubricated regimes. Despite these accolades, vegetable oils suffer from thermal-oxidative instability, high pour points, and inconsistent chemical compositions. To improve upon the tribological properties, vegetable oils were subjected to additives such as lamellar solid powders to establish more resilient transfer layers to mitigate wear and surface damage. Currently, RTIL lubricants derived from bio-based feedstock represent a promising potential solution to many of the problems associated with previous eco-friendly lubricants. An investigation into RTILs begins with a discussion on the history of ionic liquids and an assessment on their tribological properties. The chapter also includes a case study on the use of RTILs as additives in vegetable oils and as neat lubricants as well as exploring the effects of cation-anion moiety exchange within ionic liquids themselves. Ultimately, the RTILs are compared to more traditional bio-based lubricants for their tribological performance as a new class of eco-friendly lubricants and their potential as a future lubrication technology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Lubrication Fundamentals
A lubricant is a substance introduced between two moving surfaces to reduce friction, minimize wear, distribute heat, remove contaminants, and improve efficiency. The importance of lubricants and sustainable lubrication systems cannot be fully appreciated until understanding the implications of not using an appropriate lubricant or a lubricant at all. In 1979, it was estimated that over $200 billion was spent in North America on machine maintenance [1]. Within the $200 billion, approximately one-third ($66.7 billion) could have been avoided with the use of adequate lubricants. More recently, estimates claim that the amount of energy wasted due to insufficient knowledge applied to the science of friction, lubrication, and wear resulted in roughly 0.4 % of the gross domestic product (GDP) being wasted [2]. In the United States, this means that over $60.36 billion of the $15.08 trillion GDP was wasted due to energy loss [3]. When considering the many macroscale applications that utilize lubricants such as internal combustion engines, turbines, hydraulic systems, compressors, vehicle and industrial gearboxes, and journal and thrust bearings as well as the various micro- and nanoscale applications and metal forming applications, it becomes easy to understand the importance that lubricants play in the compliance, effectiveness, and operation of many of these applications [4–6].
Within the lubrication market there are a vast number of applications which require specifically formulated lubricants that have given rise to the upwards of 10,000 different lubricants that satisfy more than 90 % of all lubricant applications worldwide [2]. Figure 1 analyzes the global lubrication market as of 2004, which consumed roughly 37.4 million tons of lubricant [2]. This figure illustrates how automotive and industrial lubricants are the most prevalent. Industrial lubricants amount to 32 % and were composed of 12 % hydraulic oils, 10 % other industrial oils, 5 % metalworking fluids, 3 % greases, and 2 % industrial gear oils [7, 8]. The 10 % of other industrial oils within the industrial lubricants section consist of a wide range of lubricants such as air and gas compressor oils, bearing and circulating system oils, refrigerator compressor oils, and steam and gas turbine oils. In the automotive lubricants section, the most commonly used liquid lubricants were engine oils (petrol and diesel engine oils), automatic transmission fluids, gearbox fluids, brake fluids, and hydraulic fluids.
2004 Worldwide lubrication consumption [2]
2 History of Eco-friendly Lubricants
Lubricants originally consisted of natural oils and fats derived from plant- and animal-based raw materials which date back to 1400 B.C. The modern lubrication market developed after the first oil well was drilled in 1959 in Titusville, PA. Since then, lubricants have evolved from mineral oils to petrochemically modified synthetic oils arriving in the 1960s, to today’s bio-based eco-friendly lubricants harvested from raw materials derived from the oleochemical industry. Recently, bio-based lubricants have begun to seek prominence for their environmental-friendliness and superior tribological properties. The current trend in the lubrication industry is to develop more bio-based lubricants due to estimates indicating that nearly 50 % of all lubricants sold worldwide pollute the environment, through spillage, evaporation, and total loss applications [9]. An example of lubrication pollution is that of the diesel engine particulate emissions, where approximately one-third of the engine oil vaporizes thus polluting the atmosphere. The large quantity of lubricant loss into the environment is the reason behind the development of eco-friendly lubricants. Although the lubrication market is shifting to become more environmentally responsible by reducing the use of petroleum-based lubricants due to concerns of protecting the environment, depletion of oil reserves, and increases in oil price, mineral oil remains to be the largest constituent and the foundation to most lubricants [9, 10].
The use of biolubricants derived from plant oils and animal fats dates back to antiquity. Scientists have known for centuries that biolubricants provide favorable friction and wear properties. Since the beginning of the twentieth century, investigations into the properties of bio-based oils have received significant attention due to the fact that 50 % of all lubricants worldwide end up in the environment through usage, spill, volatility, or improper disposal [11–13]. Of these lubricants entering the environment, 95 % are derived from petroleum-based oils and are detrimental to many biological ecosystems [14]. More still, within North America alone, over 100 million gallons of toxic lubricants drip, spill, and leak into the environment annually. With the advent of petroleum-based oils in the mid-1800s, the use of bio-based oils as lubricants began to decline dramatically. Recently, there has been a resurgence of eco-friendly lubricants due to increased environmental efforts to reduce the use of petroleum-based lubricants in addition to the depletion of oil reserves, increases in oil price, and rises in lubricant disposal costs [15, 16].
When compared to petroleum-based lubricants, bio-based lubricants have a higher lubricity, lower volatility, higher shear stability, higher viscosity index, higher load carrying capacity, and superior detergency and dispersancy [9, 17, 18], therefore they are excellent alternatives to petroleum-based oils. Traditional eco-friendly lubricants are typically derived from naturally occurring organic substances whose properties and utility vary based on biological factors such as nutrient availability, climate, light, temperature, and water [19–21]. Despite these favorable attributes, the largest drawbacks to many bio-based oils are their poor thermal-oxidative stability, high pour points, and inconsistent chemical composition, which have led to the development of chemically modified synthetic biolubricants, the use of stabilizing additives, and ionic liquids [22, 23].
2.1 Present: Eco-friendly Lubricants
The emphasis placed on bio-based lubricants is a result of the increase in demand for eco-friendly lubricants that are less toxic to the environment, renewable, and provide feasible and economical alternatives to traditional lubricants. Currently, the interest surrounding liquid lubricants derived from various bio-based feedstocks is focused on the use of eco-friendly lubricants derived from plant-based oils. This is the result of the chemical composition consisting of triacylglycerol molecules made up of esters derived from glycerol and long chains of polar fatty acids. It is these fatty acids that are desirable in boundary lubrication for their ability to adhere to metallic surfaces due to their polar carboxyl group, remain closely packed, and create a monolayer film that is effective at reducing friction and wear by minimizing the asperity contact [24, 25]. Much of the work with eco-friendly lubricants has concentrated on understanding the fundamentals of saturated and unsaturated fatty acids with the bulk of the attention focusing on the use of natural oils as neat lubricants, fatty acids as additives in mineral oils, and bio-based feedstock for chemically modified lubricants [12, 17, 26]. Recently, eco-friendly lubricants are finding uses as carrier fluids for lamellar powder additives in sliding contact [9, 12, 27, 28].
Eco-friendly lubricants composed of environmentally benign lamellar powders such as boric acid (H3BO3) and hexagonal boron nitride (hBN) are well-known solid lubricants for their low interlayer friction, ability to form protective boundary layers, and accommodate relative surface velocities [17, 28]. As with many lamellar powders, atoms on the same plane form layers through strong covalent bonds. These layers themselves are held together through the weak van der Waals force, providing the minimal shear resistance, and enabling the low interlayer friction [29]. Lamellar powders are effective in a broad range of environments of extreme pressure and temperature as well as various applications from automotive to aerospace to lower friction and minimize wear [9, 21]. An important property of boron-derived lamellar powders is that they are environmentally benign and inert to most chemicals making them attractive performance enhancing additives to bio-based oils. Experiments have shown that these lamellar particles can be forced out of the contact zone in sliding contact and therefore adding them to natural oils such as canola oil creates a superior eco-friendly lubricant [12, 30]. This new class of eco-friendly lubricant maintains the properties of the powder additives to coalesce and fill in the asperity valleys, thereby establishing a thin, smooth, solid lamellar film between the contacting surfaces, thus decreasing the friction coefficient, wear rate, and surface roughness [9, 31, 32]. In addition, these lubricants maintain boundary lubrication characteristics by establishing the fatty acid adsorption film that thwarts metal-to-metal contact.
2.2 Drawbacks to Eco-friendly Lubricants
The use of lubricants composed of natural plant oils or solid lubricants have their merits; however, they do have their limitations, which have stifled their ability to be widely accepted within the lubrication industry. The drawbacks to these lubricants are summarized below. For natural oils , they suffer from thermal-oxidative instability, high pour points, inconsistent chemical composition, hydrolytic instability, and a severe susceptibility to biological deterioration. For lamellar powders, they suffer from concentration optimization (which affects their price making these lubricants expensive), unwanted abrasive behavior due to particle size and shape, particles can settle out of the colloidal suspension rendering them useless, large particles can block tubes and capillaries within critical engine parts, and they can clog oil filters in circulatory lubrication systems. These shortcomings of traditional eco-friendly lubricants ultimately cause economic issues where the lubricants themselves can become very expensive when modifying their properties for many thousands of potential applications.
3 Future: Eco-friendly Lubricants
3.1 Ionic Lubricants
A new type of eco-friendly lubricant, ionic liquids , is beginning to gain attention. Ionic liquids (ILs) were originally a novel class of solvents typically consisting of an organic cation in combination with any of a wide variety of organic or inorganic anions, exhibit a number of unique and useful characteristics, including high thermal stability, low melting point, a broad liquidus range, and negligible vapor pressure. The last of these properties, particularly minimizing solvent losses due to volatilization (i.e., fugative emissions), have led many to regard ionic liquids as “green solvents,” and over the last decades, they have been evaluated in a wide range of applications, including the fabrication of dye-sensitized solar cells [33], the preparation of electrolytes for electrochemical storage devices [34] and batteries, the electrodeposition of metals [35], the recovery of metal ions from aqueous solutions via liquid–liquid extraction (LLE) [36, 37], and in the development of separation processes for various organic compounds [38]. Many of the same properties that make ILs useful in these applications also make them good candidates as high-performance lubricants [39].
The use of ionic liquids as lubricants was first reported in 1961, when fluoride-containing molten salts (i.e., LiF and BeF2) were subjected to high-temperature (650–815 °C) bearing tests [40]. Nearly four decades later, low melting analogs of classical molten salts, room temperature ionic liquids (RTILs), were first evaluated as synthetic lubricating fluids [41]. Since this time, considerable attention has been devoted to the utilization of ILs as lubricants. Three main applications have been most extensively explored: the use of ILs as base oils, as additives , and as thin films [42]. When employed as base oils, ILs have been reported to exhibit good tribological performance for steel/steel, steel/copper, steel/aluminum, ceramic/ceramic, and steel/ceramic sliding pairs [43–56]. The negligible vapor pressure of ILs makes them good candidates for use under vacuum and in spacecraft applications [42]. ILs are also effective as additives to the main lubricant (e.g., mineral oils), because of their tendency to form strong boundary films, that enhance the tribological performance of the base lubricant [42, 57, 58]. Thin-film lubrication employing ILs has been studied by many researchers with the goal of replacing conventional perfluoropolyether (PFPE) lubricants [54, 55, 59–63].
Although the chemical structure of the cationic and anionic substituents of an IL can vary greatly, the most commonly studied ILs in tribological processes have been those containing a tetrafluoroborate (BF4 −) or hexafluorophosphate (PF6 −) anion [64, 65], the result of the superior tribological properties that boron- and/or phosphorus-containing compounds often exhibit under the high pressures and elevated temperatures that lubricants can encounter [66–69]. The frequent use of boron- and phosphorus-containing ILs as lubricants does not imply that either of them is optimum. Rather, ILs based on these anions are commonly studied because they are readily available and low cost [70]. In fact, BF4 − and PF6 − have been found to cause corrosion of steel under humid conditions. Moreover, other hydrophobic anions, such as bis(trifluoromethanesulfonyl)amide (TFSA) and tris(tetrafluoroethyl)trifluorophosphate (FAP), actually exhibit better tribological properties for steel–steel contact [39, 70, 71]. In general, as the hydrophobicity of the anion increases, both the thermo-oxidative stability and the tribological properties improve [70].
Among the many possible IL cations, the imidazolium ion has probably been studied in the most detail, a result of the high thermal stability of imidazole-based rings [72]. Additionally, the chain length on the imidazolium cation can be readily altered. Increasing the chain length to make the IL more hydrophobic will decrease the friction coefficient in a manner similar to that observed when the anion is made more hydrophobic. In contrast to the improvement in thermo-oxidative stability observed with hydrophobic anions, however, a decrease in stability is observed with more hydrophobic cations [70]. Nonetheless, ILs with longer alkyl chains and lower polarity have been reported to have excellent tribological properties from low to high temperature (−30 to 200 °C) [73]. Other ILs have been studied with the goal of improving their tribological properties include phosphonium [74–76] and ammonium [53, 54, 77–79].
3.2 Room Temperature Ionic Liquid Lubricants
As the industrial marketplace continues to become more ecologically focused with much of the attention centered on novel approaches to achieve efficient energy conservation and sustainability, new classes of green lubricants are being developed that represent the future potential of eco-friendly lubricants. Much of the development aims at creating environmentally friendly lubricants that contain many of the properties of the aforementioned eco-friendly lubricants such as polar molecules (similar to the fatty acids), lamellar crystal structure (like the solid lubricants), derived from bio-based feedstock (natural plant-based oils), high thermal-oxidative stability, physicochemical consistency (which natural oils inherently lack), superior lubricity with minimal wear, and require minimal use of additives [80]. Ionic liquids , particularly those that are fluid at room temperature , represent a promising new class of eco-friendly lubricants that show potential to improve the limitations associated with current petroleum-based oils, bio-based oils, and solid powder additives [42, 43, 81, 82].
Room temperature ionic liquids consist of combinations of a bulky, asymmetric organic cation, and an appropriate organic anion with melting points below 100 °C and a liquid range beyond 300 °C [83, 84]. The atomic structure of an IL is shown in Fig. 2. This structure resembles a lamellar solid crystal structure, except with ILs the anions and the cations form ionic bonds to creating layers and these layers are held together with the weak van der Waals forces [85]. This structure provides ILs with their liquid lamellar crystal structure [86]. Aforementioned, ILs exhibit a number of unique and useful properties that make them well suited as the basis of a new family of lubricants and initial research has already begun investigating the properties of ILs [39, 43, 81, 87–89]. The appeal of ILs as lubricants becomes even more evident when one considers their many potential advantages over other lubricants including: (1) a broad liquid range (low melting and high boiling point); (2) negligible vapor pressure; (3) nonflammability and noncombustibility; (4) superior thermal stability; (5) high viscosity; (6) miscibility and solubility; (7) environmentally benign (nontoxic); (8) lamellar-like liquid crystal structure; (9) long polar anion-cation molecular chains; and (10) economical costs [35, 39, 42, 70, 90, 91].
Additionally, ionic liquids have a consistent and easily tailorable chemical composition that affords them the ability to provide the level of thermal-oxidative stability and lubricity required for a variety of applications in the aerospace, automotive, manufacturing, and magnetic storage industries [35, 44]. The consistent chemical composition allows ILs to have physicochemical properties that are readily reproducible. Furthermore, they can be designed to be eco-friendly by selecting both the cationic and anionic constituents to be nontoxic. In many instances, the ILs can be prepared from nonpetroleum resources. Lastly, their capacity to overcome the variety of environmental, cost, and performance challenges faced by conventional lubricants makes them a potentially attractive alternative eco-friendly lubricant [42, 83].
The possibility of preparing an ionic liquid capable of functioning as an efficient lubricant, while exhibiting a variety of other useful properties is a result of the physicochemical characteristics, inherent tunability, and structural diversity of these novel compounds. Regarding the latter point, it has been estimated that as many as 1018 different combinations of anion and cation moieties are possible [92, 93]. Clearly, this vast assortment of possibilities can pose a significant challenge in ionic liquid design. As the number of desired properties increases, the number of possible candidate ILs declines dramatically. Here, for example, the desire for an environmentally friendly bio-based lubricant means that the use of highly fluorinated anions is unacceptable [94]. Instead, the use of carboxylic anions based on common food additives (e.g., benzoate− and salicylate− are well-known preservatives) or artificial sweeteners (e.g., saccharinate−) are utilized. Similar considerations guide the choice of the cation and suggest that trihexyl(tetradecyl)phosphonium salts (i.e., P +666,14 ), some of which have been found to exhibit antimicrobial and biodegradable properties, can satisfy many of the desired criteria [95]. Along these same lines, the objective of employing renewable feedstocks for the preparation of the ILs suggests the use of certain 1,3-dialkylimidazolium cations, such as can be derived from fructose or other bio-based feedstock [96–100].
4 Case Study: Room Temperature Ionic Liquids
To assess the potential of using ILs as base lubricants, two studies were investigated consisting of imidazolium and phosphonium ILs with carboxylate anions for their ability to address the performance challenges of traditional lubricants [58, 101]. In the first study, a series of experiments were conducted with two conventional ionic liquids, a phosphonium-based (P666,14Tf2N) and an imidazolium-based (C10mimTf2N) ionic liquid that were both mixed with avocado oil in five different proportions as shown in Table 1 to investigate their use as an additive versus a base fluid [101]. In the second study, another set of experiments were conducted by interchanging the cation-anion moieties of the phosphonium-based and imidazolium-based ionic liquids [58]. Multiple IL lubricants having different ion pairs were then compared with various vegetable oils and commercial lubricants. In both studies, pin-on-disk tests were conducted to characterize the tribological performance of ionic liquids as possible lubricants and in particular investigate the performance of bio-based environmentally benign ionic liquid lubricants.
4.1 Study 1: Ionic Liquids as Additives in Natural Oils
Figure 3 shows the variation of the COF for different mixtures of phosphonium-based ILs and avocado oil. In Fig. 3a it can be seen that the lubricant mixtures generally decrease with sliding distance and eventually reach a steady state value at a sliding distance of approximately 1000 m. Figure 3b shows the final COF values at the completion of the tests. It can be seen that as the graph moves from left to right the amount of the IL sequentially decreases from 100 to 0 % in 25 % decrements. This means that on the left, the 100 % IL is a lubricant composed entirely of P666,14Tf2N and on the right, the 0 % IL is a lubricant composed entirely of avocado oil. The lubricant mixtures in the middle are composed of combinations of the two base fluids. Table 1 displays the friction results at the completion of the tests. The correlation between the friction results and the composition of the IL in the base fluid is strong with a negative correlation coefficient (R-value) of −0.982 as shown in Table 1. This indicates that as the percentage of the IL increases in the lubricant mixture the COF decreases.
Having similar results to the phosphonium-based IL and avocado oil lubricant mixtures, Fig. 4 shows the variation of the COF for different mixtures of imidazolium-based IL and avocado oil lubricant mixtures. In Fig. 4a it can be seen that the lubricant mixtures continue to decrease with sliding distance and eventually reach a steady state value at a sliding distance about of 1000 m. Figure 4b shows the final COF values at the completion of the tests. Similarly, it can be seen that as graph moves from left to right the amount of the IL sequentially decreases from 100 to 0 %. On the left the 100 % IL is a lubricant composed entirely of C10mimTf2N and on the right, the 0 % IL is a lubricant composed entirely of avocado oil. The lubricant mixtures in the middle are composed of combinations of the two base fluids. The correlation between the friction results and the composition of the IL in the base fluid is strong with a negative correlation coefficient (R-value) of −0.998, as shown in Table 1. This indicates that as the percentage of the IL increases in the lubricant mixtures the COF decreases.
The friction results indicate that the presence of either the phosphonium-based or imidizolium-based IL as an additive improves the COF. Similar results were witnessed with wear rates. As detailed in Table 1, as the amount of the IL additive increases to the point where it becomes the majority fluid, effectively becoming the base fluid, it continues to impart superior friction properties that outperform the avocado oil. This study demonstrates that ionic liquids can not only be considered as additives in lubricants, but they can also be considered as base fluids in the form of neat lubricants. In the following investigation, various ILs having different anion-cation moieties will be examined for their friction properties. The IL lubricants will be compared to other natural oils and well-known commercial lubricants from both petroleum-based and bio-based feedstocks.
4.2 Study 2: Ionic Liquid Anion-Cation Moiety Manipulation
Figure 5 shows the variation of the coefficient of friction at the completion of the tests for different anion-cation moieties separated into two groups. The first group shown in Fig. 5a, shows a cation study of different ionic liquids all with the same Tf2N anion. Here, an investigation into the influence of cation chain length was performed with the five imidazolium cations having chain lengths of 10, 8, 6, 5, and 3 carbons as well as the phosphonium cation, P666,14 having a chain length of 14 carbon atoms. It can be seen in Fig. 5a and similarly in Table 2 that as the COF decreases, the cation chain length increases, with a strong negative correlation coefficient, R-value of −0.878. It can be seen that having 10 or 14 carbon atoms produces similar friction results with a 10 % difference maintaining values of 0.0526 and 0.0586, respectively. The COF percent difference between 10 and 14 carbon atoms is approximately 10 %, whereas the percent difference between 10 and 8 carbon atoms for two imidazolium-based cations is 47 %. Furthermore, the COF percent difference between 8 and 3 carbon atoms is 50 %. The relative percent differences indicate the importance of larger alkyl chains in the cation help to promote a thicker adsorbed monolayer film that is more effective at minimizing asperity contact. These results are in agreement with previous investigations of ionic liquids [42].
The second group shown in Fig. 5b reveals the anion study of different ionic liquids, all with the same P666,14 cation. This study examines the influence of anion ring size on the phosphonium-based ionic liquids. The anions invested are shown in Table 3 along with their corresponding ring size and final COF values. It can be seen in Fig. 5b and in Table 3, that as the COF decreases, the anion ring size increases, with a strong negative correlation coefficient, of −0.917. It can be seen that the saccharinate with two aromatic rings has the lowest COF value followed by cyclohexane carboxylate. The cyclohexane has a 6-vertexed ring that does not conform to the shape of a perfect hexagon, thus its nonplanar shape is often considered a 3D chair or boat conformation. The benzoate and the salicylate anions demonstrate similar properties as they have very similar aromatic molecular structures consisting of only one ring and a difference of one hydroxyl group as observed in Table 3. The Tf2N and Cl anions have no ring shape and are included for comparative purposes. Interestingly, the ring-shaped anions (saccharinate, cyclohexane, benzoate, and salicylate) maintain a significantly lower COF value than the C10mim cation. The difference between the P666,14Salicylate and the C10mimTf2N reveals a 20 % difference in the friction values and this difference can only increase as the ring size increases. Therefore, the influence of larger ring sized anions tends to improve upon the effect that longer alkyl chain length cations have on lowering friction. Similar results were observed with wear rate as well where studies of the pin worn surfaces reaffirm that aromatic anions and long alkyl chains length cations are important for improved tribological properties. It can be inferred that this is due to density of the monolayer to remain tightly packed on a surface, thus covering more of the surface to prevent unwanted asperity contact and aiding to minimize wear.
4.3 Environmentally Friendly Ionic Liquid Lubricants
The anion-cation investigations thus far have shown that different anionic and cationic constituents influence the performance of ionic liquids . Within the combinations of anion-cation moieties investigated, those ILs with superior tribological properties can serveve as practical lubricants. Further examination will compare the tribological properties of the ILs in particular, the eco-friendly ionic liquids to conventional ILs, bio-based (natural) oils, and petroleum-based oils. Table 4 classifies the lubricants into the four categories based on their source and Table 5 compiles all the friction values for the lubricants tested.
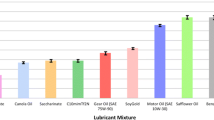
Figure 6 depicts the final COF values at the completion of the tests for all of the lubricants studied in this investigation. The ionic liquid lubricants maintain superior tribological properties when compared to all other lubricants tested. More specifically, the eco-friendly ILs (P666,14Saccharinate, P666,14Salicyate, and P666,14Benzoate) exhibit lower coefficient of friction values better than all conventional ILs (except P666,14Cyclohexane), bio-based oils, and petroleum-based oil. Two trends emerge when analyzing the lubricant chemical composition and the friction values. As denoted previously in Fig. 5, the COF decreases as the alkyl chain length of the cation increases and as the aromatic ring size of the anion increases. These trends remain true with the eco-friendly ILs, as they consist of the largest cation with 14 carbon atoms in the alkyl chain and the largest anions with single or double aromatic rings that enable them to have low friction. Comparing the eco-friendly ILs to the bio-based natural oils, the eco-friendly ILs outperform the bio-based oils in all circumstances. This is speculated to be caused by the resilient monolayers composed of both anion and cation molecules. Here, the ionic liquids are able to maintain lower COF values due to their polar molecules that can adhere to the charged metallic surfaces, establish a monolayer, remain tightly packed, and minimize asperity contact as well as benefit from their lamellar liquid crystal structure that affords the ILs the reduced internal resistance during shearing in the tribo-interface. On the contrary, the bio-based oils maintain high lubricity due to their fatty acid composition consisting of over 70 % of oleic acid (C18:1) and linoleic acid (C18:2) [25]. These fatty acids having 18 carbon atoms and one or two double bonds can form a monolayer that promotes low friction, however, their properties are susceptible to rapid oxidation and chemical inconsistency, which can impede their tribological performance. The synthetic motor oil tested exhibits the highest friction. The friction results are important because they directly reveal the advantages of eco-friendly ionic liquids that make them well suited as a new class of greener lubricants.
5 Conclusions
This chapter explores the history of eco-friendly lubricants from their past as natural impeller pressed plant-based oils, to more complex natural oils with environmentally benign solid particle additives, and now shedding light on a promising new class of environmentally friendly lubricants known as room temperature ionic liquid lubricants . It has been shown that their lamellar-like liquid crystal structure improves lubricity in comparison to traditional eco-friendly lubricants. The dipolar structure allows cations and anions to adsorb on charged worn metal surfaces facilitating self-assembling monolayers that create boundary films that minimize wear, reduce friction, and improve component operation. Eco-friendly ILs were presented and demonstrated to be feasible in design and provide superior tribological properties.
The lubrication industry continues to make new strides toward sustainable eco-friendly lubricants with properties that will lower friction and wear, thereby improving system efficiency and ultimately conserving energy. Continuing this trend, future lubricants formulated from bio-based feedstock should offer the following advantages over petroleum-based oils: a higher lubricity lending to lower friction losses and improved efficiency, affording more power output and better economy. As oil prices rise, environmental awareness grows, and the demand for renewable and sustainable lubricants increases, eco-friendly lubricants will begin to seek prominence. For this reason, fundamental research is an important step to the macroscale development, economical competence, and industrial use of biolubricants for energy conservation and sustainability. Room temperature ionic liquid lubricants represent a new class of novel “greener” lubricants that are nontoxic, obtainable from sustainable (nonpetroleum) resources, and environmentally friendly. They have the potential to satisfy the combination of environmental, health, economic, and performance demands of modern lubricants. Their ability to be tunable establishes them as ‘designer’ lubricants, where the optimization of cation-anion moiety facilitates energy conservation through superior tribological performance.
References
Bannister KE (1996) Lubrication for industry. Industrial Press, New York
Mang T, Dresel W (2006) Lubricants and lubrication. Wiley, Weinheim
United States (1997) Central intelligence A. Central Intelligence Agency, The CIA world fact book. Washington DC
Reeves C, Menezes PL, Lovell M, Jen T-C (2013) Macroscale applications in tribology. In: Menezes PL, Nosonovsky M, Ingole SP, Kailas SV, Lovell MR (eds) Tribology for scientists and engineers. Springer, New York, pp 881–919
Reeves C, Menezes PL, Lovell M, Jen T-C (2013) Microscale applications in tribology. In: Menezes PL, Nosonovsky M, Ingole SP, Kailas SV, Lovell MR (eds) Tribology for scientists and engineers. Springer, New York, pp 921–948
Menezes PL, Reeves C, Kailas S, Lovell M (2013) Tribology in metal forming. In: Menezes PL, Nosonovsky M, Ingole SP, Kailas SV, Lovell MR (eds) Tribology for scientists and engineers. Springer, New York, pp 783–818
Bartz WJ (2006) Automotive and industrial lubrication 15th International Colloquium Tribology. TAE, Ostfildern
Lingg G, Gosalia A (2008) The dynamics of the global lubricants industry: markets, competitors and trends. In: Technische Akademie Esslingen international tribology colloquium proceedings, p 16
Reeves CJ, Menezes PL, Jen T-C, Lovell MR (2012) Evaluating the tribological performance of green liquid lubricants and powder additive based green liquid lubricants. STLE annual meeting and exhibition. STLE, St. Louis
Menezes PL, Reeves C, Lovell M (2013) Fundamentals of Lubrication. In: Menezes PL, Nosonovsky M, Ingole SP, Kailas SV, Lovell MR (eds) Tribology for scientists and engineers. Springer, New York, pp 295–340
Lundgren SM, Persson K, Mueller G, Kronberg B, Clarke J, Chtaib M et al (2007) Unsaturated fatty acids in alkane solution: adsorption to steel surfaces. Langmuir ACS J Surf Colloids 23:10598–10602
Lovell MR, Menezes PL, Kabir MA, Higgs CF III (2010) Influence of boric acid additive size on green lubricant performance. Philos Trans R Soc A Math Phys Eng Sci 368:4851–4868
Lundgren SM, Ruths M, Danerlov K, Persson K (2008) Effects of unsaturation on film structure and friction of fatty acids in a model base oil. J Colloid Interf Sci 326:530–536
Schneider MP (2006) Plant-oil-based lubricants and hydraulic fluids. J Sci Food Agric 86:1769–1780
Deffeyes KS (2009) Hubbert’s peak. Princeton (N.J.). Princeton University Press, Oxford
Goodstein DL (2004) Out of gas: the end of the age of oil, 1st edn. W.W. Norton, New York
Menezes PL, Lovell MR, Kabir MA, Higgs III CF, Rohatgi PK (2012) Green lubricants: role of additive size. In: Nosonovsky M, Bhushan B (eds) Green tribology. Springer, Berlin, pp 265–286
Bennion M, Scheule B (2010) Introductory foods. Prentice Hall, Upper Saddle River
Duzcukoglu H, Sahin O (2011) Investigation of wear performance of canola oil containing boric acid under boundary friction condition. Tribol Trans 54:57–61
Erdemir A (1990) Tribological properties of boric acid and boric acid forming surfaces: part 1, crystal chemistry and self-lubricating mechanism of boric acid. In: Society of tribologists lubrication engineers annual conference. Argonne National Labs, Denver
Lovell M, Higgs CF, Deshmukh P, Mobley A (2006) Increasing formability in sheet metal stamping operations using environmentally friendly lubricants. J Mater Process Technol 177:87
Kumar A, Sharma S (2008) An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.): a review. Ind Crops Prod 28:1–10
Reeves CJ, Menezes PL, Lovell MR, Jen T-C (2015) Science and technology of environmentally friendly lubricants. Environmentally friendly and biobased lubricants. CRC Press, Boca Raton
Grushcow J (2005) High oleic plant oils with hydroxy fatty acids for emission reduction. In: World tribology congress III. American Society of Mechanical Engineers, Washington, DC, pp 485–486
Reeves CJ, Menezes PL, Jen T-C, Lovell MR (2015) The influence of fatty acids on tribological and thermal properties of natural oils as sustainable biolubricants. Tribol Int 90:123–134
Fox NJ, Tyrer B, Stachowiak GW (2004) Boundary lubrication performance of free fatty acids in sunflower oil. Tribol Lett 16:275–281
Grushcow J, Smith MA (2005) Next generation feedstocks from new frontiers in oilseed engineering. ASME Conf Proc 2005:487–488
Reeves CJ, Menezes PL, Lovell MR, Jen T-C (2013) The size effect of boron nitride particles on the tribological performance of biolubricants for energy conservation and sustainability. Tribol Lett 51:437–452
Reeves C, Menezes PL, Lovell M, Jen T-C (2013) Tribology of solid lubricants. In: Menezes PL, Nosonovsky M, Ingole SP, Kailas SV, Lovell MR (eds) Tribology for scientists and engineers. Springer, New York, pp 447–494
Liang H, Jahanmir S (1995) Boric acid as an additive for core-drilling of alumina. J Tribol Journal of Tribology. 1995;117
Reeves CJ, Jen TC, Menezes PL, Lovell MR (2015) The influence of surface roughness and particulate size on the tribological performance of bio-based multi-functional hybrid lubricants. Tribol Int Tribol Int 88:40–55
Reeves CJ, Menezes PL, Jen T-C, Lovell MR (2013) The effect of surface roughness on the tribological performance of environmentally friendly bio-based lubricants with varying particle size. In: STLE annual meeting and exhibition. STLE, Detroit
Li Y, Pang A, Wang C, Wei M (2011) Metal organic frameworks: promising materials for improving the open circuit voltage of dye-sensitized solar cells. J Mater Chem 21:17259–17264
Passerini S, Alessandrini F, Appetecchi GB, Conte M (2006) Ionic liquid based electrolytes for high energy electrochemical storage devices. ECS Trans 1:67–71
Liu W, Ye C, Gong Q, Wang H, Wang P (2002) Tribological performance of room-temperature ionic liquids as lubricant. Tribol Lett 13:81–85
Garvey SL, Hawkins CA, Dietz ML (2012) Effect of aqueous phase anion on the mode of facilitated ion transfer into room-temperature ionic liquids. Talanta 95:25–30
Hawkins CA, Garvey SL, Dietz ML (2012) Structural variations in room-temperature ionic liquids: influence on metal ion partitioning modes and extraction selectivity. SEPPUR Sep Purif Technol 89:31–38
Zaijun L, Jie C, Haixia S, Jiaomai P (2007) Advance of room temperature ionic liquid as solvent for extraction and separation. Rev Anal Chem 26:109–153
Liu X, Zhou F, Liang Y, Liu W (2006) Tribological performance of phosphonium based ionic liquids for an aluminum-on-steel system and opinions on lubrication mechanism. Wear 261:1174–1179
Smith PG (1961) High-temperature molten-salt lubricated hydrodynamic. J Bear ASLE Trans 4:263–274
Ye C, Liu W, Chen Y, Yu L (2001) Room-temperature ionic liquids: a novel versatile lubricant. Chem Commun 2001:2244–2245
Zhou F, Liang Y, Liu W (2009) Ionic liquid lubricants: designed chemistry for engineering applications. Chem Soc Rev 38:2590–2599
Yao M, Liang Y, Xia Y, Zhou F (2009) Bisimidazolium ionic liquids as the high-performance antiwear additives in poly(ethylene glycol) for steel-steel contacts. ACS Appl Mater Interf ACS Appl Mater Interf 1:467–471
Phillips BS, Zabinski JS (2004) Ionic liquid lubrication effects on ceramics in a water environment. Tribol Lett 17:533–541
Wang H, Lu Q, Ye C, Liu W, Cui Z (2004) Friction and wear behaviors of ionic liquid of alkylimidazolium hexafluorophosphates as lubricants for steel/steel contact. Wear 256:44–48
Liu W, Ye C, Chen Y, Ou Z, Sun DC (2002) Tribological behavior of sialon ceramics sliding against steel lubricated by fluorine-containing oils. Tribol Int 35:503–509
Mu Z, Liu W, Zhang S, Zhou F (2004) Functional room-temperature ionic liquids as lubricants for an aluminum-on-steel system. Chem Lett 33:524–525
Lu Q, Wang H, Ye C, Liu W, Xue Q (2004) Room temperature ionic liquid 1-ethyl-3-hexylimidazolium-bis(trifluoromethylsulfonyl)-imide as lubricant for steelsteel contact. Tribol Int 37:547–552
Reich RA, Stewart PA, Bohaychick J, Urbanski JA (2003) Base oil properties of ionic liquids. Lubr Eng 59:16–21
Mu Z, Zhou F, Zhang S, Liang Y, Liu W (2005) Effect of the functional groups in ionic liquid molecules on the friction and wear behavior of aluminum alloy in lubricated aluminum-on-steel contact. Tribol Int 38:725–731
Jimenez AE, Bermudez MD, Iglesias P, Carrion FJ, Martinez-Nicolas G (2006) 1-N-alkyl -3-methylimidazolium ionic liquids as neat lubricants and lubricant additives in steel-aluminium contacts. Wear 260:766–782
Jimenez AE, Bermudez MD, Carrion FJ, Martinez-Nicolas G (2006) Room temperature ionic liquids as lubricant additives in steel-aluminium contacts: Influence of sliding velocity, normal load and temperature. Wear 261:347–359
Omotowa BA, Phillips BS, Zabinski JS, Shreeve JM (2004) Phosphazene-based ionic liquids: synthesis, temperature-dependent viscosity, and effect as additives in water lubrication of silicon nitride ceramics. Inorg Chem 43:5466–5471
Yu G, Zhou F, Liu W, Liang Y, Yan S (2006) Preparation of functional ionic liquids and tribological investigation of their ultra-thin films. Wear 260:1076–1080
Yu B, Zhou F, Mu Z, Liang Y, Liu W (2006) Tribological properties of ultra-thin ionic liquid films on single-crystal silicon wafers with functionalized surfaces. Tribol Int 39:879–887
Xia Y, Wang S, Zhou F, Wang H, Lin Y, Xu T (2006) Tribological properties of plasma nitrided stainless steel against SAE52100 steel under ionic liquid lubrication condition. Tribol Int 39:635–640
Qu J, Bansal DG, Yu B, Howe JY, Luo H, Dai S et al (2012) Antiwear performance and mechanism of an oil-miscible ionic liquid as a lubricant additive. ACS Appl Mater Interf 4:997–1002
Reeves CJ, Menezes PL, Garvey SL, Jen TC, Dietz ML, Lovell MR (2013) The effect of anion-cation moiety manipulation to characterize the tribological performance of environmentally benign room temperature ionic liquid lubricants. In: STLE annual meeting and exhibition (STLE2013). Society of Tribologists and Lubrication Engineers, Detroit
Mo Y, Zhao W, Zhu M, Bai M (2008) Nano/Microtribological properties of ultrathin functionalized imidazolium wear-resistant ionic liquid films on single crystal silicon. Tribol Lett 32:143–151
Zhu M, Yan J, Mo Y, Bai M (2008) Effect of the anion on the tribological properties of ionic liquid nano-films on surface-modified silicon wafers. Tribol Lett 29:177–183
Palacio M, Bhushan B (2008) Ultrathin wear-resistant ionic liquid films for novel MEMS/NEMS applications. Adv Mater 20:1194–1198
Bhushan B, Palacio M, Kinzig B (2008) AFM-based nanotribological and electrical characterization of ultrathin wear-resistant ionic liquid films. J Colloid Interf Sci 317:275–287
Palacio M, Bhushan B (2008) Nanotribological and nanomechanical properties of lubricated PZT thin films for ferroelectric data storage applications. J Vac Sci Technol A Vac Surf Films 26:768–776
Minami I, Inada T, Sasaki R, Nanao H (2010) Tribo-chemistry of phosphonium-derived ionic liquids. Tribol Lett 40:225–235
Zeng Z, Shreeve JM, Phillips BS, Xiao JC (2008) Polyfluoroalkyl, polyethylene glycol, 1,4-bismethylenebenzene, or 1,4-bismethylene-2,3,5,6-tetrafluorobenzene bridged functionalized dicationic ionic liquids: synthesis and properties as high temperature lubricants. Chem Mater 20:2719–2726
Shah F, Glavatskih S, Antzutkin ON (2009) Synthesis, physicochemical, and tribological characterization of S-Di-n-octoxyboron-O, O’-di-n-octyldithiophosphate. ACS Appl Mater Interf 1:2835–2842
Mosey NJ (2005) Molecular mechanisms for the functionality of lubricant additives. Science 307:1612–1615
Mangolini F, Rossi A, Spencer ND (2011) Chemical reactivity of triphenyl phosphorothionate (TPPT) with iron: an ATR/FT-IR and XPS investigation. J Phys Chem C 115:1339–1354
Shah F, Glavatskih S, Höglund E, Lindberg M, Antzutkin ON (2011) Interfacial antiwear and physicochemical properties of alkylborate-dithiophosphates. ACS Appl Mater Interf 3:956–968
Minami I (2009) Ionic liquids in tribology. Molecules (Basel, Switzerland 14:2286–2305
Itoh T, Ishioka A, Hayase S, Kawatsura M, Watanabe N, Inada K et al (2009) Design of alkyl sulfate ionic liquids for lubricants. Chem Lett 38:64–65
Ohtani H, Ishimura S, Kumai M (2008) Thermal decomposition behaviors of imidazolium-type ionic liquids studied by pyrolysis-gas chromatography. Anal Sci Int J Jpn Soc Anal Chem 24:1335–1340
Jimâenez A-E, Bermâudez M-D (2007) Ionic liquids as lubricants for steel-aluminum contacts at low and elevated temperatures. Tribol Lett 26:53–60
Shah FU, Glavatskih S, MacFarlane DR, Somers A, Forsyth M, Antzutkin ON (2011) Novel halogen-free chelated orthoborate-phosphonium ionic liquids: synthesis and tribophysical properties. Chem Phys (Incorporating Faraday Transactions) 13:12865–12873
Sun J, Howlett PC, MacFarlane DR, Lin J, Forsyth M (2008) Synthesis and physical property characterisation of phosphonium ionic liquids based on P(O)2(OR)2 and P(O)2(R)2 anions with potential application for corrosion mitigation of magnesium alloys. Electrochim Acta 54:254–260
Weng L, Liu X, Liang Y, Xue Q (2007) Effect of tetraalkylphosphonium based ionic liquids as lubricants on the tribological performance of a steel-on-steel system. Tribol Lett 26:11–17
Minami I, Kamimura H, Mori S (2007) Thermo-oxidative stability of ionic liquids as lubricating fluids. J Synth Lubr 24:135–147
Kamimura H, Kubo T, Minami I, Mori S (2007) Effect and mechanism of additives for ionic liquids as new lubricants. Tribol Int 40:620–625
Zhao W, Mo Y, Pu J, Bai M (2009) Effect of cation on micro/nano-tribological properties of ultra-thin ionic liquid films. Tribol Int 42:828–835
Reeves CJ, Menezes PL, Lovell MR, Jen TC, Garvey SL, Dietz ML (2013) The tribological performance of bio-based room temperature ionic liquid lubricants: a possible next step in biolubricant technology. World tribology congress—5th. Society of Tribologists and Lubrication Engineers, Torino
Reeves CJ, Garvey SL, Menezes PL, Dietz ML, Jen TC, Lovell MR (2012) Tribological performance of environmentally friendly ionic liquid lubricants. In: ASME/STLE 2012 international joint tribology conference. STLE, Denver
Reeves CJ, Menezes PL, Lovell MR, Jen TC (2014) The effect of particulate additives on the tribological performance of bio-based and ionic liquid-based lubricants for energy conservation and sustainability. In: STLE (ed) STLE annual meeting and exhibition. STLE, Buena Vista
Freemantle M (2010) An Introduction to ionic liquids. RSC Pub, Cambridge
Matlack A (2010) Introduction to green chemistry. Taylor & Francis Group
Manahan SE (1994) Environmental chemistry. Lewis, Boca Raton
Suisse J-M, Bellemin-Laponnaz S, Douce L, Maisse-François AWR (2005) A new liquid crystal compound based on an ionic imidazolium salt. Tetrahedron Lett 46:4303–4305
Yao Y, Wang X, Guo J, Yang X, Xu B (2008) Tribological property of onion-like fullerenes as lubricant additive. Mater Lett 62:2524–2527
Xia Y, Sasaki S, Murakami T, Nakano M, Shi L, Wang H (2007) Ionic liquid lubrication of electrodeposited nickel-Si3N4 composite coatings. Wear 262:765
Battez HA, Alonso DB, Rodriguez RG, Viesca Rodriguez JL, Fernandez-Gonzalez A, Garrido AH (2011) Lubrication of DLC and tin coatings with two ionic liquids used as neat lubricant and oil additives. In: Proceedings of the STLE/ASME international joint tribology conference. American Society of Mechanical Engineers, Los Angeles
Bermúdez MD, Jiménez AE, Sanes J, Carrión FJ (2009) Ionic liquids as advanced lubricant fluids. Molecules 14:2888–2908
Xue H, Tong ZF, Wei FY, Qing SG (2008) Crystal structure of room-temperature ionic liquid 1-butyl-isoquinolinium gallium tetrachloride [(BIQL)GaCl4]. Chem Rec 11:90–94
Canter N (2005) Evaluating ionic liquids as potential lubricants. Tribol Lubr Technol 61:15–17
Sheldon RA, Arends I, Hanefeld U (2007) Green chemistry and catalysis. Wiley, Weinheim
Wang H, Malhotra SV, Francis AJ (2011) Toxicity of various anions associated with methoxyethyl methyl imidazolium-based ionic liquids on Clostridium sp. Chemosphere 82:1597–1603
Atefi F, Garcia MT, Singer RD, Scammells PJ (2009) Phosphonium ionic liquids: design, synthesis and evaluation of biodegradability. Green Chem 11:1595–1604
Handy ST (2003) Greener solvents: room temperature ionic liquids from biorenewable sources. Eur J Chem 9:2938–2944
Gathergood N, Scammells PJ, Garcia TM (2006) Biodegradable ionic liquids part III. The first readily biodegradable ionic liquids. Green Chem 8:156–160
Gathergood N, Garcia TM, Scammells PJ (2004) Biodegradable ionic liquids: part I. Concept, preliminary targets and evaluation. Green Chem 6:166–175
Corma A, Iborra S, Velty A (2007) Chemical routes for the transformation of biomass into chemicals. Chem Inform 38
Zhang ZC (2013) Catalytic transformation of carbohydrates and lignin in ionic liquids. WENE Wiley interdisciplinary reviews: energy and environment
Reeves CJ, Jen T-C, Garvey SL, Dietz ML, Menezes PL, Lovell MR (2014) The effect of phosphonium-and imidazolium-based ionic liquids as additives in natural oil: an investigation of tribological performance
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Reeves, C.J., Menezes, P.L. (2016). Advancements in Eco-friendly Lubricants for Tribological Applications: Past, Present, and Future. In: Davim, J. (eds) Ecotribology. Materials Forming, Machining and Tribology. Springer, Cham. https://doi.org/10.1007/978-3-319-24007-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-24007-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24005-3
Online ISBN: 978-3-319-24007-7
eBook Packages: EngineeringEngineering (R0)