Abstract
The demand for bio-based lubricants has grown considerably in recent years due to increasing environmental awareness among the public, governments, and industries. In this study, soybean-oil-based samples were synthesized via reactions of hydrolysis (FFA, yield > 93% wt.), esterification (BL1, yield > 92% wt.), epoxidation (BL2, yield > 91% wt.), and oxirane ring opening (BLOR, yield > 93% wt.), using a long chain alcohol (2-ethylhexanol). The obtained BL1 and BLOR samples were further subjected to tribological testing in the four-ball configuration. The friction coefficients of the BL1 and BLOR samples were evaluated using speed ramps at different loading forces and temperatures. A hydrotreated mineral oil (HMO) sample was used as a reference to evaluate the lubrication performance of the synthesized bio-based samples. The results indicated that these BL1 and BLOR samples had lower friction coefficients than HMO at all assessed sliding speeds, even with increasing load force and temperature. In the test to evaluate the wear, the samples of BL1 and BLOR presented coefficient of friction smaller than HMO in the order of 31.7% and 46.0%, respectively. Furthermore, an assessment of the wear morphologies indicated that the BL1 and BLOR samples yielded smoother surfaces with shallower grooves than the hydrotreated mineral oil sample. Among the studied bio-based lubricants, BLOR yielded the lowest friction coefficients, wear scar diameter, and surface ripples. The tribology resulting from BL1 and BLOR infers that these biolubricants have potential for applications in mechanical systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bio-based lubricants comprise all lubricants generally obtained from vegetable oils and modified renewable oils (in the form of synthetic ester derivatives). Biolubricant basestock oils are biodegradable and nontoxic to humans and environment [1,2,3]. The demand for these lubricants has grown in recent years due to increasing public environmental awareness, introduction of highly stringent environmental legislations, and industrial approval of biolubricants [4]. However, the commercialization of biolubricants in the lubricant market is still limited by insufficient knowledge of their technical potential [5]. According to recent reported data [6, 7], biolubricants account for approximately 1% of the total lubricant market, and their market share is expected to grow at a compound annual growth rate of 5.2% over the next five years.
Several vegetable oils may be used for biolubricant synthesis because they offer numerous advantages such as excellent lubricity, high viscosity index (VI), and biodegradability [8,9,10]. The raw material for biolubricants depends on the climatic and geographic factors, and hence, it varies with the region. In Europe, rapeseed and sunflower oils are commonly used in biolubricant production, whereas in the USA, the primary source is soybean oil [11]. Although Brazil is the largest world producer of soybean (135.409 million tons in 2021), no solid policy of subsidies, tax incentives, and national and international labeling programs has been implemented thus far in Brazil, unlike Europe and the USA [6, 12]. Considering this scenario, studies proving the conformity of soybean-oil-based biolubricants to the technical specifications of mineral oils would garner much greater interest from industry thus encouraging the Brazilian oleochemical business.
Vegetable oil-based lubricants can be prepared via chemical modifications, such as esterification, transesterification, epoxidation, and epoxy ring opening reactions [13, 14]. These reactions modify the double bonds present in the triglyceride molecules of vegetable oils, which are responsible for the low oxidative stability and poor low-temperature flow properties of the vegetable oils [15]. The physical-property enhancement of the biolubricants due to these chemical modifications should be investigated. Additionally, the ability of these biolubricants to reduce the friction and wear upon application to mechanical systems should be evaluated because the ultimate goal of any lubricant is to achieve adequate performance, through high energy efficiency, long machinery cycles, and long lubricant replacement intervals [16, 17].
On the laboratory scale, biolubricants have been evaluated using tribometers in the four-ball test configuration. This measurement yields friction and wear parameters [18,19,20,21,22,23,24,25], which are determined from the friction coefficient (FC, defined as the ratio between the frictional force and the normal force of the balls in contact [26]) and wear scar diameter (WSD, that results from contact between the balls) respectively. Ribeiro Filho et al. [27] recently reported a study on the lubricity of biolubricants using a tribology accessory mounted on a dynamic shear rheometer (DSR), from which friction coefficients could be determined at varying sliding speeds (Stribeck curves). Consequently, the combined effect of the sliding speed and the normal load on the lubricant properties may be jointly evaluated [27,28,29].
There have been reports on the tribological performance of soybean oil [30] and ester biolubricants produced by transesterification [31] and epoxidation [32] of the soybean oil. However, the tribological behavior of the oxirane ring opening product obtained from soybean oil has not been investigated up to this moment. This type of modification in the molecular structure of the soybean oil triglyceride converts unsaturated bonds into ether-type branching, improving various physicochemical properties such as oxidative stability and low-temperature properties [14]. Identifying whether these changes also improve anti-wear and anti-friction capacity becomes essential to ensure the best performance and durability of biolubricants when applied in machinery and equipment. The present study then aims at synthesizing biolubricants from soybean oil via hydrolysis, esterification, epoxidation, and oxirane ring opening reactions; 2-ethylhexanol was used as the nucleophilic agent to produce ether-type branches. The ability of the biolubricants to reduce friction and wear in a tribological system in the four-ball configuration was also evaluated.
2 Experimental section
2.1 Materials
Refined soybean oil (RSO) was purchased from Soya (Brazil). Hexane (> 98.5% by weight) was purchased from Dinâmica (Brazil). Acetone (> 99.5% by weight), P-toluenesulfonic acid (> 98% by weight), 2-ethylhexanol (> 99% by weight), ethanol (> 99% by weight), hydrochloric acid (37% by weight), potassium hydroxide (> 85% weight), hydrogen peroxide (35% by weight), toluene (> 99% by weight), anhydrous sodium sulfate (> 99% by weight), formic acid (> 85% by weight), Amberlyst 15, potassium bromide, and deuterated chloroform (CDCl3 99.8%) were purchased from Sigma-Aldrich (USA). Commercial nitrogen (> 99.5% purity) was supplied by White Martins Praxair (Brazil). Hydrotreated mineral oil (HMO) sample was kindly provided by Petrobras (Brazil); its main physicochemical properties are listed in Table 1.
2.2 Synthesis procedure
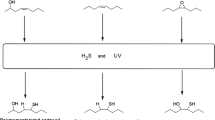
A scheme of the reactions used to synthesize the bio-based lubricants is shown in Fig. 1. A sample of soybean oil was submitted sequentially to hydrolysis, esterification, epoxidation, and oxirane ring opening reaction. The yield was calculated based on the ratio between the amount of product obtained and the sample initially placed in the reactor.
Soybean oil was initially converted to free fatty acids (FFA) by hydrolysis; the procedure was adapted from Pindit et al. [8]. In a three-tube flask in a reflux system (Fig. 2), 100 g of soybean oil was mixed with a 1.75 M potassium hydroxide solution in ethanol at 65 °C for 2 h by stirring. Subsequently, 400 mL distilled water and 500 mL hexane were added to the system, which separated the solution into two phases. A 6 M HCl solution was dripped in until the pH of the solution reached 1. The mixture was then separated into two phases, namely, a hexane- and FFA-rich phase and an aqueous phase. Finally, this mixture was transferred to a separating funnel, wherein the aqueous phase was removed, and the hexane- and FFA-rich phase was washed with distilled water until the pH reached 7. Residual water and catalyst were removed using anhydrous sodium sulfate, whereas the residual hexane was removed using a vacuum rotary evaporator Buchi (Switzerland).
The obtained FFAs (yield > 93% wt.) were then subjected to esterification and epoxidation reactions, following procedures adapted from Rios et al. [33]. For the esterification reaction, 100 g (0.358 mol) of soybean FFA was mixed in a three-tube flask with 140 g (1.08 mol) of 2-ethylhexanol (FFA:alcohol molar ratio = 1:3) and 10 g of p-toluenesulfonic acid (PTSA:FFA mass ratio = 1:10). The reaction occurred at 90 °C under reflux with constant stirring at 900 rpm for 6 h. After this, the mixture was transferred to a separating funnel wherein the organic phase was washed with a solution of NaHCO3 (5% wt.) and distilled water until the pH reached 7. The product was dried with anhydrous Na2SO4 and distilled in a Kugelrohr system Buchi (Switzerland) under vacuum (3 × 10–2 mbar) at 110 °C to remove excess 2-ethylhexanol. This sample was then labeled BL1 (yield > 92% wt.).
The BL1 sample was then subjected to an epoxidation step, during which a solution was prepared in a flat bottom flask containing 70 g (0.183 mol) of BL1, 7.9 mL (0.183 mol) of formic acid, and 50 mL of toluene. Subsequently, 61 mL (0.732 mol) of a hydrogen peroxide solution (BL1:CH2O2:H2O2 molar ratio = 1:1:4) were slowly added. The reaction was allowed to occur at room temperature for 24 h under constant stirring at 900 rpm. After completion of the reaction, the liquid in the flask was transferred to a separating funnel; the upper phase was neutralized with NaHCO3 (5% wt.) and washed with distilled water; and the moisture was removed using anhydrous sodium sulfate. The toluene was then removed using a rotary evaporator under reduced pressure at 70 °C for 40 min. This sample was then labeled BL2 (yield > 91% wt.).
The BL2 sample was then taken to the oxirane ring opening reaction, conducted in a flat bottom flask at 90 °C and 900 rpm for 6 h with 30 g of BL2, 3 g of Amberlyst, and 72 mL of 2-ethylhexanol. The reaction product was neutralized with NaHCO3 (5% wt.). The excess 2-ethylhexanol was distilled using a Kugelrohr system under vacuum (3 × 10–2 mbar) at 110 °C. The sample obtained in this step was labeled BLOR (yield > 93% wt.).
2.3 Compositional and physicochemical characterization
The fatty acid composition of soybean oil (FFA) was determined via ester quantification by gas chromatography [34]. The sample was prepared as follows. In a beaker, 30 mg BL1 ester was weighed, to which 1 mL of 2 mg/mL methyl nonadecanoate solution (internal standard) diluted in chromatographic grade n-hexane was added. The container was capped and shaken manually for a few seconds. The analysis was performed using a VARIAN 450-GC gas chromatography apparatus (USA), which comprised a flame ionization detector (FID) and a capillary column VARIAN CP-WAX with the following dimensions: 60 m length, 0.25 mm diameter, and 0.25 µm film thickness. The operating parameters of the GC are listed in Table 2.
The FFA, BL1, BL2, and BLOR samples were also evaluated by one-dimensional proton nuclear magnetic resonance (1H NMR) spectroscopy. A Bruker Avance DRX-500 spectrometer (USA), operating at 500 MHz, was used with deuterated chloroform as solvent.
Fourier-transform infrared (FTIR) spectroscopy was performed on the BL2 and BLOR samples to confirm the oxirane ring opening, using a potassium bromide (KBr) tablet in a Shimadzu IRTracer-100 (Japan) in the 400–4000 cm−1 range [35]. The tablet was prepared by applying an 8 kN force. Thirty-two scans were acquired at 4 cm−1 resolution.
The ASTM D7042 and ASTM D445 methods were adopted to determine the density at 20 °C and kinematic viscosities at 40 and 100 °C, respectively, using an Anton Paar SVM 3000 apparatus (Austria) [36, 37]. The viscosity index was then calculated using the ASTM D2270 method.
2.4 Tribological evaluation
2.4.1 Friction coefficients in speed sweeps
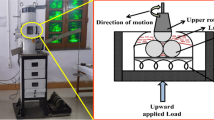
The frictional properties of the synthesized lubricants were assessed using a four-ball tribology accessory associated with a DHR-3 rheometer (TA Instruments, USA). This accessory allows the measurement of the friction coefficient between two solid surfaces under dry or lubricated conditions. Its design secures uniform solid-to-solid contact and axial force distribution, controlling rotational speed and temperature in a wide range of friction measurements. Data are collected through the TRIOS software. The monitored variables are friction coefficient, loading force and friction force. Further details on the tribology accessory may be found elsewhere [38]. During the test, a ball rotated at varying speeds under the action of a loading force against three fixed ball submerged in the lubricant under evaluation, as shown in Fig. 3. The Stribeck curve obtained from this test is used to evaluate the combined effect of sliding speed variation and normal load on the lubricant property.
The balls used in this study were composed of chromium steel alloy (AISI 52100) and 64 HRC and had a diameter of 12.7 mm. For each experiment, 4.5 mL lubricant sample was used. Friction coefficients were determined over sliding speed sweeps between 0–1000 mm/s, under axial forces of 10, 20, and 40 N and at temperatures of 25, 40, and 100 °C. Each experimental condition was repeated three times for each sample. The results will be presented as mean and standard deviation.
2.4.2 Wear characterization
The wear was evaluated under the action of an axial load of 20 N at 40 °C for a constant sliding speed of approximately 228 mm/s for 1 h. The balls were cleaned with acetone and dried under ambient conditions prior to the experiments. The morphology of the worn surface and the WSD were determined using an optical microscope (Zeiss, Germany). A hydrotreated mineral oil (HMO) sample was used as a reference to compare the tribological performance of the biolubricant samples.
3 Results and discussions
3.1 Compositional and physicochemical characterizations
The fatty acid composition of the fresh soybean oil is shown in Table 3. Linoleic (57.28% wt.) and oleic (21.69% wt.) acids are observed in higher concentrations, similar to that previously reported by Parente et al. [39]. The total unsaturated fatty acid content was approximately 85% wt.
The physicochemical properties of the RSO, BL1, BL2 and BLOR samples are presented in Table 4. The values of density at 20 °C decreased slightly for BL1, BL2, and BLOR samples (0.8699, 0.9181, and 0.9118 g/cm3, respectively) relative to that of the original RSO (0.9200 g/cm3). Lubricant densities may range from 0.7 to 0.95 g/cm3 depending on the viscosity, quality, and additive content of the sample [40,41,42]. The American Petroleum Institute (United States) and the Association Technique de L'industrie Européenne des Lubrifiants (Europe) categorize lubricants according to their sulfur content, saturate content, and viscosity index. As per these classifications, samples studied herein belong to group V [40, 43].
Viscosity is a crucial property of lubricants and must be tailored accurately for efficiency in their applications. For instance, by precisely tailoring the viscosity of a lubricant for application in an internal combustion engine, the dissipative losses, which account for 10%-20% energy loss in an automotive engine, can be reduced [44]. The results obtained at 40 ºC and 100 °C indicate that the BL1 sample had a lower viscosity than the RSO sample. This is attributed to the carboxylic functional group present in the RSO molecule, which enables hydrogen bonds formation, thereby leading to stronger intermolecular forces than the interaction forces present in the BL1 ester molecule [45]. Owing to the carbon chain growth, the viscosity increased for BL2 and BLOR [46]. According to Verdier et al. [47], this could be due to enhanced intermolecular van der Waals interactions. BLOR had the highest viscosity among the synthesized products because the oxirane ring opening reaction yields a hydroxyl functional group in the molecule [46, 48].
Viscosity index (VI) is a parameter that evaluates the influence of temperature on viscosity; the higher the VI, the less the temperature-induced changes in viscosity [49]. All synthesized samples (BL1, BL2 and BLOR) had lower VI value than RSO. It has been reported by Durango-Giraldo et al. [49] that, for molecule chains with the same carbon number, an increase in the branching of the carboxylic acid or the alcohol molecules decreases the VI, which explains the results observed in this study.
The 1H NMR spectra of the FFA, BL1, BL2, and BLOR samples are shown in Fig. 4. The spectrum in Fig. 4a corresponded to the free fatty acid sample obtained from soybean oil (FFA), whose peak (a) is associated with the olefin hydrogen attached to carbons 9 and 10 [33, 50], peak (b) corresponds to the terminal chain hydrogen (–CH3), and peaks (c) and (d) are assigned to hydrogen attached to the sp3 carbons of (–CH2–) groups [33, 51]. Peak (e) in Fig. 4b confirmed the occurrence of the esterification reaction because this peak is related to the hydrogen that binds to the carbon near the sp3 oxygen of the ester functional group [52,53,54,55]. The spectrum of the sample yielded by the epoxidation reaction (BL2), shown in Fig. 4c, revealed the disappearance of peak (f), related to unsaturated bonds, and appearance of peak (g), indicating the oxirane ring formation [54, 56]. The spectrum of the BLOR sample, shown in Fig. 4d, confirmed the opening of the oxirane ring; peak (g) was absent, whereas peak (h) was present, which is assigned to the hydrogen bonded to the hydroxyl carbon (–CH(OH)–) [54, 56].
The BL2 and BLOR samples were also evaluated by FTIR spectroscopy (Fig. 5). The comparison of the functional groups in each sample infers that epoxide group bands (825 and 842 cm−1) were present in the BL2 spectrum but not in the BLOR spectrum. Additionally, the BLOR spectrum contained the following vibrational mode bands: C = O stretching (~ 1737 cm−1), C–O of ester and ether (1090, 1172, and 1244 cm−1), and –OH (3470–3448 cm−1), which collectively validate the oxirane ring opening [46, 57,58,59].
3.2 Tribological evaluation
The ramps of friction coefficients (FC) for HMO, BL1, and BLOR samples, subjected to axial forces of 10, 20, and 40 N at 25 °C, are plotted as function of the sliding speed in Fig. 6. The curves show that FC´s decreased with increasing sliding speed, which characterizes the mixed lubrication regime, in which the reduction of friction is influenced by several factors, such as sample viscosity, surface roughness, load, sliding speed, pressure exerted on the contact interface, temperature, among others [60,61,62]. In Fig. 6a, the Stribeck curves of the studied samples show similar qualitative behaviors, although the FC of the BL1 and BLOR samples were low for most sliding speeds that were investigated. As shown in Figs. 6b and c, when the normal force was increased to 20 and 40 N, respectively, the biolubricant samples had significantly lower FC than the HMO sample at all the studied speeds. This behavior is related to the presence of ester functional groups in the biolubricant synthesized samples, which improve the adhesion of the tribofilm to the metal surface of the balls [16, 53,54,55,56,57,58,59,60,61,62,63]. Furthermore, the friction coefficient results shown in Fig. 6 present a decreasing trend with increasing load. This can be explained by the Hertz's contact theory, which states that an increase in the normal load would result in increasing contact stress and friction force at the contact interface [64]. When the increase in contact stress is faster than in the friction force, the friction coefficient is inversely proportional to the application of the normal load [65]. Some previous publications have reported that the friction coefficient decreases with increasing load [30, 66, 67]. According to Sapawe et al. [67], the increase in load can wear out the area of the contact surfaces, accelerating the speed of the ball and thus reducing the friction coefficient.
The speed ramps for 10 N axial force, at 25 °C, 40 °C and 100 °C, are shown in Fig. 7. Even at high temperatures, the BL1 and BLOR samples had lower friction coefficients than the HMO sample. The BLOR sample presented the lowest FC, due to its highest polarity that leads to the lowest frictional torque among all studied samples [68,69,70]. The polar end of the BLOR molecule (an ester group) exhibits a strong affinity to the metal of the balls, facilitating a barrier formation by the nonpolar part of the BLOR molecule (fatty acid carbon chain), thus resulting in surface separation [68,69,70]. Moreover, BLOR has a high degree of branching, which renders a highly stable film upon temperature variations [16].
The FC and WSD results of experimental runs using 20 N axial force and 40 °C are reported in Figs. 8a and b, respectively, to evaluate the wear reduction capability of the samples HMO, BL1 and BLOR, when subjected to the same constant sliding speed (228 mm/s) for 1 h. The HMO sample, with nonpolar molecules, had the least affinity with the surfaces of the balls and thus, the highest FC, but the as-formed film exhibited the most efficient wear reduction. The results obtained for the BL1 and BLOR samples suggest the formation of a monolayer, separating the metallic surfaces of the balls, thereby yielding lower FCs. However, the lubricant film could not sustain the operational conditions of the assay for the entire period of 1 h; hence, they presented higher WSD than the HMO sample [19, 71]. The BLOR sample had a lower WSD than the BL1 sample, due to the formation of more compounds by the oxirane ring opening reaction, which might have better adhesion to the metal surfaces of the balls [72, 73].
In Table 5, the FC and WSD values obtained in the wear test are compared with the results of other studies. As obtained in this study, the polar region of natural or synthesized biolubricants was able to produce a more efficient friction-reducing film than mineral oils, as the polar groups of the biolubricants react with the surface of the balls, reducing the energy of the contact region [74]. However, the chemically modified biolubricant obtained in this research (BLOR) combined excellent anti-friction characteristics (FC = 0.04623) with high anti-wear capacity (WSD = 369.88 µm), due to the hydroxyl group in its molecular structure. A biolubricant with the hydroxyl group in its composition makes it suitable for operation in mixed/EHL and hydrodynamic lubrication regimes, such as a hydraulic fluid, as it creates a viscous lubricant due to intermolecular hydrogen bonds [16, 75, 76]. Additionally, the absence of unsaturations in the BLOR molecule allows for its linear chain alignment, providing greater protection against wear.
The morphologies of the worn surfaces are shown in Fig. 9. The wear caps generated after testing the HMO sample presented deep grooves, undulations, and points indicating removal of the welded material. These aspects are associated with abrasive and adhesive wear, which is characterized by the absence of lubricant in the contact region, causing shearing of the metal asperities of the balls because of friction and removal of the oxide film and metal surface layer [71, 72, 80].
The BL1 and BLOR samples exhibited smooth wear with few undulations, which are characteristics of abrasive wear. Although BL1 and BLOR had similar morphological features, the BLOR sample presented less wear, a lower FC, and fewer surface ripples than the BL1 sample, demonstrating its good potential as bio-based lubricant basestock oil.
3.3 Analysis of the lubrication mechanisms
The lubrication mechanisms of the BL1, BLOR and HMO samples are depicted in Fig. 10. All biolubricant samples have polar groups, which reduce friction because they adsorb onto the metallic structure of the balls [81]. In Fig. 10a, the ester functional group (RCOOR) of the BL1 molecule provided enough adhesiveness for a monolayer formation with extended antifriction capacity, showing low FC’s in all conditions studied. In contrast, the ester functional group (RCOOR) does not have a good anti-wear action due to its moderate adhesiveness and polarities [21, 82]. The carbonic chain of fatty acids (non-polar) from BL1 formed a barrier with little efficiency in protecting the balls, resulting in the highest values of WSD, but the worn surfaces showed shallow and smooth grooves. The higher the polarity of the biolubricant molecule, the higher the possibility of reducing wear; for this reason, BLOR, which presents an association of polar groups in its molecular structure (ester and hydroxyl group), showed the lowest wear among the studied biolubricants samples [16, 81]. In Fig. 10b, the two additional hydroxyl functional groups (-OH), associated with an ester group (RCOOR) are adsorbed at the interface of the balls, producing a lubricating film with higher polarity that results in larger shear strength and better protection against wear. This behavior is due to strong intermolecular hydrogen bonds [16, 83]. Furthermore, the ether-like branches protect the BLOR molecule from physical and chemical interactions due to steric hindrance, which leads to improved tribological characteristics [84,85,86,87]. In Fig. 10c, HMO, a non-polar hydrocarbon molecule, has less affinity with the surfaces of balls and for this reason presented the highest FC, but the lowest WSD, due to its high resistance to shear forces [87].
4 Conclusion
This study evaluated the tribological characteristics of two soybean oil-based biolubricants. BL1 and BLOR were obtained via esterification and oxirane ring opening reactions, respectively. The product yielded by the former presented lower viscosity than that yielded by the latter. Speed ramps evaluations under variable loading forces and temperatures demonstrate that even under high loading and high temperatures, the biolubricant samples effectively reduce the friction coefficients. This behavior is explained by the strong adhesion of the biolubricant molecules to the metallic surfaces of the balls. The results of the friction coefficients under the conditions of temperature of 25 ºC and loads of 20 N and 40 N seem to have a decreasing tendency with increasing load, suggesting that, for these conditions, the friction coefficient is influenced by the increase in the contact stress. Regarding the wear, the biolubricant samples produced smoother wear surfaces with fewer undulations than the HMO sample. The introduction of two hydroxyl functional groups (-OH) through the oxirane ring opening reaction in the BLOR molecule contributed to its polarity increase resulting in a biolubricant with excellent anti-friction and anti-wear properties. The conversion of unsaturations into ether-like branches in the BLOR molecule allowed for its linear chain alignment, providing higher protection against wear. Furthermore, the ether-like branches protect the BLOR molecule from physical and chemical interactions, thus producing a stable film. Therefore, the samples synthesized in this study have immense potential for applications in mechanical systems.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Levizzari A, Voglino M, Volpi P (1999) Environmental and economic impact of re-refined produts: a life cycle analysis. In: Brussels: Proceedings of the 6th International LFE congress
Dorinson A, Ludema KC (1985) Mechanics and chemistry in lubrication (vol. 9). Elsevier
Salimon J, Salih N, Yousif E (2010) Biolubricants: Raw materials, chemical modifications and environmental benefits. Eur J Lipid Sci Technol 112(5):519–530. https://doi.org/10.1002/ejlt.200900205
Market Research Report (2022) Bio Lubricants Market Size, Share e COVID-19 Impact Analysis, By Application (Hydraulic Fluids, Metalworking Fluids, Chainsaw Oils, Mold Release Agents, Two-Cycle Engine Oils, Gear Oils, Greases, and Others), By End-use Industry (Automotive and Other Transportation, Metalworking, Mining, Forestry, Marine, Engines, and Others, and Regional Forecast, 2022–2029. https://www.fortunebusinessinsights.com/bio-lubricants-market-104654. Accessed 11 Jul 2022
Bart J, Gucciardi E, Cavallaro S (2013) Markets for biolubricants. Biolubricants: science and technology, pp 712–754. Elsevier. https://doi.org/10.1533/9780857096326.712
Research and Markets (2022) Biolubricants - Global Market Trajectory & Analytics. https://www.researchandmarkets.com/r/yubum4. Accessed 14 Jun 2022
Portal Lubes (2017) Mercado de biolubrificantes poderá crescer até 5% ao ano. https://portallubes.com.br/2017/11/mercado-de-biolubrificantes/. Accessed 11 Jul 2022
Pindit K, Thanapimmetha A, Saisriyoot M, Srinopakun P (2021) Biolubricant basestocks synthesis using 5-step reaction from jatropha oil, soybean oil, and palm fatty acid distillate. Ind Crop Prod 166:113484. https://doi.org/10.1016/j.indcrop.2021.113484
Hwang HS, Erhan SZ (2001) Modification of epoxidized soybean oil for lubricant formulations with improved oxidative stability and low pour point. J Am Oil Chem Soc 78(12):1179–1184. https://doi.org/10.1007/s11745-001-0410-0
Mobarak HM, Mohamad EN, Masjuki HH, Kalam MA, Al Mahmud KAH, Habibullah M, Ashraful AM (2014) The prospects of biolubricants as alternatives in automotive applications. Renew Sustain Energy Rev 33:34–43. https://doi.org/10.1016/j.rser.2014.01.062
Zainal NA, Zulkifli NWM, Gulzar M, Masjuki HH (2018) A review on the chemistry, production, and technological potential of bio-based lubricants. Renew Sustain Energy Rev 82:80–102. https://doi.org/10.1016/j.rser.2017.09.004
Embrapa (2021) Soja em números (safra 2020/21). https://www.embrapa.br/soja/cultivos/soja1/dados-economicos. Accessed 13 Jul 2022
Syahir AZ, Zulkifli NWM, Masjuki HH, Kalam MA, Alabdulkarem A, Gulzar M, Harith MH (2017) A review on bio-based lubricants and their applications. J Clean Prod 168:997–1016. https://doi.org/10.1016/j.jclepro.2017.09.106
Cecilia JA, Ballesteros Plata D, Alves Saboya RM, Tavares de Luna FM, Cavalcante CL, Rodríguez-Castellón E (2020) An overview of the biolubricant production process: Challenges and future perspectives. Processes 8(3):257. https://doi.org/10.3390/pr8030257
Dehghani Soufi M, Ghobadian B, Atashgaran M, Mousavi SM, Najafi G (2019) Biolubricant production from edible and novel indigenous vegetable oils: mainstream methodology, and prospects and challenges in Iran. Biofuels Bioprod Biorefin 13(3):838–849. https://doi.org/10.1002/bbb.1953
Chan CH, Tang SW, Mohd NK, Lim WH, Yeong SK, Idris Z (2018) Tribological behavior of biolubricant base stocks and additives. Renew Sustain Energy Rev 93:145–157. https://doi.org/10.1016/j.rser.2018.05.024
Sethuramiah A, Kumar R (2016) Chapter 2 - Lubricants and their formulation. In: Modeling of chemical wear, 1st edn. Elsevier, Oxford, pp 25–39. https://doi.org/10.1016/B978-0-12-804533-6.00002-0
Sharma UC, Sachan S (2019) Friction and wear behavior of karanja oil derived biolubricant base oil. SN Appl Sci 1(7):1–11. https://doi.org/10.1007/s42452-019-0706-y
Afifah AN, Syahrullail S, Azlee NIW, Sidik NAC, Yahya WJ, Abd Rahim E (2019) Biolubricant production from palm stearin through enzymatic transesterification method. Biochem Eng J 148:178–184. https://doi.org/10.1016/j.bej.2019.05.009
Shahabuddin M, Mofijur M, Kalam MA, Masjuki HH (2020) Study on the friction and wear characteristics of bio-lubricant synthesized from second generation Jatropha methyl ester. Tribology in Industry 42(1):41–49. https://doi.org/10.24874/ti.2020.42.01.04
Ing TC, Rafiq AKM, Azli Y, Syahrullail S (2012) Tribological behaviour of refined bleached and deodorized palm olein in different loads using a four-ball tribotester. Sci Iran 19(6):1487–1492. https://doi.org/10.1016/j.scient.2012.10.027
García-Zapateiro LA, Franco JM, Valencia C, Delgado MA, Gallegos C (2013) Viscous, thermal and tribological characterization of oleic and ricinoleic acids-derived estolides and their blends with vegetable oils. J Ind Eng Chem 19(4):1289–1298. https://doi.org/10.1016/j.jiec.2012.12.030
Guezmil M, Bensalah W, Mezlini S (2016) Effect of bio-lubrication on the tribological behavior of UHMWPE against M30NW stainless steel. Tribol Int 94:550–559. https://doi.org/10.1016/j.triboint.2015.10.022
Mohd Salleh ZA, Syahrullail S, Norzita N, Nurun Najwa R (2021) Friction study on chemically modified RBD PK oil as a potential renewable resource. J Braz Soc Mech Sci Eng 43(3):1–10. https://doi.org/10.1007/s40430-021-02868-y
Singh N, Agarwal P, Porwal SK (2022) Natural antioxidant extracted waste cooking oil as sustainable biolubricant formulation in tribological and rheological applications. Waste Biomass Valoriz 13(7):3127–3137. https://doi.org/10.1007/s12649-022-01745-6
Maru MM, Tanaka DK (2007) Consideration of stribeck diagram parameters in the investigation on wear and friction behavior in lubricated sliding. J Braz Soc Mech Sci Eng 29(1):55–62. https://doi.org/10.1590/S1678-58782007000100009
Ribeiro Filho PRCF, da Silva SSO, do Nascimento MR, de Aguiar Soares S, de Luna FMT, Cavalcante Jr CL (2022) Tribological properties of bio-based lubricant basestock obtained from pequi oil (Caryocar brasiliensis). J Braz Soc Mech Sci Eng 44(1):1-9https://doi.org/10.1007/s40430-021-03358-x
Stribeck R (1901) Ball bearings for any stress. Z des VDI 45:664
Stribeck R (1902) Die wesentlichenEigenschaften der Gleit-und Rollenlager (The basic properties of plain and roller bearing). Z VerDtschIng 46(36):1342–1348
Ameen NHA, Durak E (2020) Study of the tribological properties the mixture of soybean oil and used (waste) frying oil fatty acid methyl ester under boundary lubrication conditions. Renew Energy 145:1730–1747. https://doi.org/10.1016/j.renene.2019.06.117
Shrivastava S, Prajapati P, Srivastava P, Lodhi AP, Kumar D, Sharma V, Agarwal DD (2023) Chemical transesterification of soybean oil as a feedstock for stable biodiesel and biolubricant production by using Zn Al hydrotalcites as a catalyst and perform tribological assessment. Ind Crop Prod 192:116002. https://doi.org/10.1016/j.indcrop.2022.116002
Abdel-Hameed HS, El-Saeed SM, Ahmed NS, Nassar AM, El-Kafrawy AF, Hashem AI (2022) Chemical transformation of Jojoba oil and Soybean oil and study of their uses as bio-lubricants. Ind Crops Prod 187:115256
Rios IC, Cordeiro JP, Arruda TB, Rodrigues FEA, Uchoa AF, Luna FMT, Ricardo NM (2020) Chemical modification of castor oil fatty acids (Ricinus communis) for biolubricant applications: An alternative for Brazil’s green market. Ind Crop Prod 145:112000. https://doi.org/10.1016/j.indcrop.2019.112000
Saboya RMA (2012) Produção de biodiesel empregando catalisadores nanoestruturados do tipo SBA-15 modificada com lantânio. 2012. 86 f. Dissertation, Universidade Federal do Ceará
dos Santos RCM, Gurgel PC, Pereira NS, Breves RA, de Matos PRR, Silva LP, Lopes RDVV (2020) Ethyl esters obtained from pequi and macaúba oils by transesterification with homogeneous acid catalysis. Fuel 259:116206. https://doi.org/10.1016/j.fuel.2019.116206
ASTM International (2014). ASTM D7042. Standard test method for dynamic viscosity and density of liquids by Stabinger viscometer (and the calculation of kinematic viscosity) ASTM International
ASTM International (2006). ASTM D445. Standard test method for kinematic viscosity of transparent and opaque liquids:(and Calculation of Dynamic Viscosity) ASTM International
TA Instruments (n.d.). Tribo-Rheometry. https://www.tainstruments.com/tribo-rheometry-accessory/. Accessed 26 May 2022
Parente EJ, Marques JPC, Rios IC, Cecilia JA, Rodriguez-Castellon E, Luna FMT, Cavalcante CL (2021) Production of biolubricants from soybean oil: Studies for an integrated process with the current biodiesel industry. Chem Eng Res Des 165:456–466. https://doi.org/10.1016/j.cherd.2020.11.012
API (1509) Appendix E — API base oil interchangeability guidelines for passenger car motor oils and diesel engine oils. https://www.api.org/~/media/files/certification/engine-oil-diesel/publications/annerev043019%20rev043019.pdf. Accessed 13 June 2021
Lin L, Kedzierski MA (2020) Density and viscosity of a polyol ester lubricant: Measurement and molecular dynamics simulation. Int J Refrig 118:188–201. https://doi.org/10.1016/j.ijrefrig.2020.07.004
Ewen JP, Gattinoni C, Thakkar FM, Morgan N, Spikes HA, Dini D (2016) A comparison of classical force-fields for molecular dynamics simulations of lubricants. Materials 9(8):651. https://doi.org/10.3390/ma9080651
Panorama dos óleos básicos no Brasil (2016) Distrito Federal: ANP. http://www.simepetro.com.br/wpcontent/uploads/ANP-RELATORIO-TECNICO-2/2016-SBQ-CPT-DFPANORAMA-DOS-OLEOS-BASICOS-NO-BRASIL.pdf. Accessed 20 Jun 2022
Lee P, Zhmud B (2021) Low Friction Powertrains: Current Advances in Lubricants and Coatings. Lubricants 9(8):74. https://doi.org/10.3390/lubricants9080074
Yunus R, Fakhru’l-Razi A, Ooi TL, Iyuke SE, Perez JM (2004) Lubrication properties of trimethylolpropane esters based on palm oil and palm kernel oils. Eur J Lipid Sci Technol 106(1):52–60. https://doi.org/10.1002/ejlt.200300862
do Valle CP, Rodrigues JS, Fechine LMUD, Cunha AP, Malveira JQ, Luna FMT, Ricardo NMPS (2018) Chemical modification of Tilapia oil for biolubricant applications. J Clean Prod 191:158-166
Verdier S, Coutinho JA, Silva AM, Alkilde OF, Hansen JA (2009) A critical approach to viscosity index. Fuel 88(11):2199–2206. https://doi.org/10.1016/j.fuel.2009.05.016
Yunus R, Luo X (2017) Thermochemical conversion of plant oils and derivatives to lubricants. In Advances in bioenergy (vol. 2, pp. 183–231). Elsevier. https://doi.org/10.1016/bs.aibe.2016.11.001
Durango-Giraldo G, Zapata-Hernandez C, Santa JF, Buitrago-Sierra R (2021) Palm oil as a biolubricant: Literature review of processing parameters and tribological performance. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2021.12.018
Torrentes-Espinoza G, Miranda BC, Vega-Baudrit J, Mata-Segreda JF (2017) Castor oil (Ricinus communis) supercritical methanolysis. Energy 140:426–435. https://doi.org/10.1016/j.energy.2017.08.122
Slivniak R, Domb AJ (2005) Macrolactones and polyesters from ricinoleic acid. Biomacromol 6(3):1679–1688. https://doi.org/10.1021/bm049194r
Salimon J, Salih N, Yousif E (2012) Improvement of pour point and oxidative stability of synthetic ester basestocks for biolubricant applications. Arab J Chem 5(2):193–200. https://doi.org/10.1016/j.arabjc.2010.09.001
Ferreira EN, Arruda TBMG, Rodrigues FEA, Arruda DTD, da Silva Junior JH, Porto DL, Ricardo NMPS (2019) Investigation of the thermal degradation of the biolubricant through TG-FTIR and characterization of the biodiesel–Pequi (Caryocarbrasiliensis) as energetic raw material. Fuel 245:398–405. https://doi.org/10.1016/j.fuel.2019.02.006
Marques JPC, Rios ÍC, ParenteJr EJ, Quintella SA, Luna FMT, CavalcanteJr CL (2020) Synthesis and characterization of potential bio-based lubricant basestocks via epoxidation process. J Am Oil Chem Soc 97(4):437–446. https://doi.org/10.1002/aocs.12317
Rios ÍC, Cordeiro JP, Parente EJ, Quintella SA, Alemán J, Cavalcante CL, Luna FMT (2020) Biodegradable base stock oils obtained from ricinoleic acid using C 8 alcohols and process integration into a biodiesel industry. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00942-4
Kulkarni RD, Deshpande PS, Mahajan SU, Mahulikar PP (2013) Epoxidation of mustard oil and ring opening with 2-ethylhexanol for biolubricants with enhanced thermo-oxidative and cold flow characteristics. Ind Crops Prod 49:586–592. https://doi.org/10.1016/j.indcrop.2013.06.006
Salih N, Salimon J, Yousif E (2011) The physicochemical and tribological properties of oleic acid based triester biolubricants. Ind Crops Prod 34(1):1089–1096. https://doi.org/10.1016/j.indcrop.2011.03.025
Sharma BK, Adhvaryu A, Liu Z, Erhan SZ (2006) Chemical modification of vegetable oils for lubricant applications. J Am Oil Chem Soc 83(2):129–136. https://doi.org/10.1007/s11746-006-1185-z
Ob-eye J, Chaiendoo K, Itthibenchapong V (2021) Catalytic Conversion of Epoxidized Palm Fatty Acids through Oxirane Ring Opening Combined with Esterification and the Properties of Palm Oil-Based Biolubricants. Ind Eng Chem Res 60(44):15989–15998. https://doi.org/10.1021/acs.iecr.1c03974
Stachowiak GW, Batchelor AW (2013) Engineering tribology. Butterworth-heinemann, Oxford
Bhushan B (2013) Introduction to tribology. John Wiley & Sons
Spikes HA (1997) Mixed lubrication—an overview. Lubr Sci 9(3):221–253. https://doi.org/10.1002/ls.3010090302
Bayer RG (1994) Mechanical wear prediction and prevention. Marcel! Dekker, Inc, P. O. Box 5005, Monticello, New York, 12701–5185, USA, 1994. 657
Yu HY, Jiang XY, Zhou ZR (2004) Effects of magnitudes of interference on implant-bone contact stress. Chin. J. Mech. Eng. 40, 89–92. http://qikan.cmes.org/jxgcxb/EN/abstract/abstract25418.shtml. Accessed 14 June 2022
Wang C, Zhang G, Li Z, Zeng X, Xu Y, Zhao S, Ren T (2019) Tribological behavior of Ti-6Al-4V against cortical bone in different biolubricants. J Mech Behav Biomed Mater 90:460–471. https://doi.org/10.1016/j.jmbbm.2018.10.031
Ruggiero A, D’Amato R, Merola M, Valašek P, Müller M (2017) Tribological characterization of vegetal lubricants: Comparative experimental investigation on Jatropha curcas L. oil, Rapeseed Methyl Ester oil, Hydrotreated Rapeseed oil. Tribol Int 109:529–540. https://doi.org/10.1016/j.triboint.2017.01.030
Sapawe N, Hanafi MF, Samion S (2019) The use of palm oil as new alternative biolubricant for improving anti-friction and anti-wear properties. Mater Today Proc 19:1126–1135. https://doi.org/10.1016/j.matpr.2019.11.005
Zulkifli NWM, Kalam MA, Masjuki HH, Al Mahmud KAH, Yunus R (2014) The effect of temperature on tribological properties of chemically modified bio-based lubricant. Tribol Trans 57(3):408–415. https://doi.org/10.1080/10402004.2013.878777
Gellman AJ, Spencer ND (2002) Surface chemistry in tribology. Proc Inst Mech Eng Part J: J Eng Tribol 216(6):443–461. https://doi.org/10.1243/135065002762355352
Canter N (2009) Special report: Boundary lubricity additives. Tribol Lubr Technol 65(9):10
Noorawzi N, Samion S (2016) Tribological effects of vegetable oil as alternative lubricant: a pin-on-disk tribometer and wear study. Tribol Trans 59(5):831–837. https://doi.org/10.1080/10402004.2015.1108477
Prasannakumar P, Edla S, Thampi AD, Arif M, Santhakumari R (2022) A comparative study on the lubricant properties of chemically modified Calophyllum inophyllum oils for bio-lubricant applications. J Clean Prod 339:130733. https://doi.org/10.1016/j.jclepro.2022.130733
Thampi AD, John AR, Arif MM, Rani S (2020) Evaluation of the tribological properties and oxidative stability of epoxidized and ring opened products of pure rice bran oil. Proc Inst Mech Eng Part J: J Eng Tribol 235(6):1093–1100. https://doi.org/10.1177/1350650120950535
Samidin S, Salih N, Salimon J (2021) Synthesis and characterization of trimethylolpropane based esters as green biolubricant basestock. Biointerface Res Appl Chem 11(5):13638–13651. https://doi.org/10.33263/BRIAC115.1363813651
Quinchia LA, Delgado MA, Reddyhoff T, Gallegos C, Spikes HA (2014) Tribological studies of potential vegetable oil-based lubricants containing environmentally friendly viscosity modifiers. Tribol Int 69:110–117. https://doi.org/10.1016/j.triboint.2013.08.016
Quinchia LA, Delgado MA, Valencia C, Franco JM, Gallegos C (2010) Viscosity modification of different vegetable oils with EVA copolymer for lubricant applications. Ind Crops Prod 32(3):607–612. https://doi.org/10.1016/j.indcrop.2010.07.011
Choudhury ND, Saha N, Bhaumik S et al (2023) Production and evaluation of physicochemical, rheological, and tribological properties of Cucurbita pepo L. seed oil. Biomass Conv Bioref. 13:1101–1114. https://doi.org/10.1007/s13399-020-01236-5
Edla S, Thampi AD, Pillai ABK et al (2022) Formulation of rice bran oil-based green cutting fluid with holy basil oil and clove oil as bio-additives. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-02153-x
Campos Flexa Ribeiro Filho PR, Rocha do Nascimento M, Otaviano da Silva SS, Tavares de Luna FM, Rodríguez-Castellón E, Loureiro Cavalcante Jr C (2023) Synthesis and frictional characteristics of bio-based lubricants obtained from fatty acids of castor oil. Lubricants 11(2):57. https://doi.org/10.3390/lubricants11020057
Hsieh PY, Bruno TJ (2015) A perspective on the origin of lubricity in petroleum distillate motor fuels. Fuel Process Technol 129:52–60. https://doi.org/10.1016/j.fuproc.2014.08.012
Kurre SK, Yadav J (2023) A review on bio-based feedstock, synthesis, and chemical modification to enhance tribological properties of biolubricants. Ind Crop Prod 193:116122. https://doi.org/10.1016/j.indcrop.2022.116122
Syahrullail S, Kamitani S, Shakirin AJPE (2013) Performance of vegetable oil as lubricant in extreme pressure condition. Procedia Eng 68:172–177. https://doi.org/10.1016/j.proeng.2013.12.164
Tan CP, Che Man YB (2000) Differential scanning calorimetric analysis of edible oils: comparison of thermal properties and chemical composition. J Am Oil Chem Soc 77(2):143–155. https://doi.org/10.1007/s11746-000-0024-6
Lathi PS, Mattiasson B (2007) Green approach for the preparation of biodegradable lubricant base stock from epoxidized vegetable oil. Appl Catal B 69(3–4):207–212. https://doi.org/10.1016/j.apcatb.2006.06.016
Salimon J, Salih N, Yousif E (2011) Chemically modified biolubricant basestocks from epoxidized oleic acid: Improved low temperature properties and oxidative stability. J Saudi Chem Soc 15(3):195–201. https://doi.org/10.1016/j.jscs.2010.08.004
Madankar CS, Dalai AK, Naik SN (2013) Green synthesis of biolubricant base stock from canola oil. Ind Crops Prod 44:139–144. https://doi.org/10.1016/j.indcrop.2012.11.012
Rudnick LR (ed) (2020) Synthetics, mineral oils, and bio-based lubricants: chemistry and technology. CRC press. London, New York
Funding
Financial support from CNPq (Conselho Nacional de Pesquisa e Desenvolvimento Científico) is gratefully acknowledged. Ribeiro-Filho thanks the FAPEMA (Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão) and Universidade Estadual do Maranhão (UEMA) for financial support.
Author information
Authors and Affiliations
Contributions
Paulo Roberto Campos Flexa Ribeiro Filho performed the experimental measurements, data analysis, writing of original draft; Matheus Rocha do Nascimento assisted the experimental measurements; and Célio Loureiro Cavalcante Jr. and Francisco Murilo Tavares de Luna supervised the work and critically reviewed the results reported in this paper.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ribeiro Filho, P.R.C.F., do Nascimento, M.R., Cavalcante, C.L. et al. Synthesis and tribological properties of bio-based lubricants from soybean oil. Biomass Conv. Bioref. 14, 20509–20521 (2024). https://doi.org/10.1007/s13399-023-04395-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04395-3