Abstract
Manganese(III), cobalt(II), nickel(II) and copper(II) complexes of a salen-type ligand, namely 6,6′-((1E,1′E)-(ethane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(5-isopropyl-2-methylphenol) (H2L), have been synthesized and characterized by physicochemical and spectroscopic methods. In addition, single-crystal X-ray analysis confirmed the formulae of the manganese and nickel complexes as [Mn(OAc)(L)] and [Ni(L)], respectively. The free Schiff base and its complexes have been screened for in vitro antibacterial activity by colony count methods, and the antioxidant activity was assayed by DPPH radical scavenging. The ability of free H2L and its complexes to mediate DNA cleavage was studied by agarose gel electrophoresis.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DNA remains a long-term target for the diagnosis and treatment of human diseases [1]. DNA plays an essential role in the life process since it encodes all the genetic information required for the cellular functions [2]. Metal complexes of Schiff bases derived from salicyaldehyde have been found to be effective in DNA cleavage and can also possess anticancer and antibacterial activities [3,4,5,6]. DNA cleavage may lead to various pathological changes in living organisms and is also relevant to the synthetic restriction enzymes, chemotherapeutic drugs and DNA footprinting agents [7]. Recently, the ability of transition metal complexes to mediate DNA cleavage in the presence of oxidants such as O2, H2O2 or peracids has been extensively studied. Efforts have been made to design transition metal complexes as chemical nucleases suitable for nicking or direct DNA strand scission [8, 9]. Cobalt complexes with different oxidation states have been found to possess DNA cleavage activity [10]. In this context, we report here the synthesis of an N2O2 donor tetradentate Schiff base ligand H2L-6,6′-((1E,1′E)-(ethane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(5-isopropyl-2-methylphenol), prepared by the condensation of ethane-1,2-diamine with 2-hydroxy-6-isopropyl-3-methyl benzaldehyde, together with its manganese(III), cobalt(II), nickel(II) and copper(II) complexes. All the compounds were characterized by physicochemical and spectroscopic methods and further screened for antibacterial, antioxidant and DNA cleavage activities.
Experimental
Materials and methods
Carvacrol, ethane-1,2-diamine, ethidium bromide, tris-boric acid-EDTA buffer (10X TBE buffer), loading dye mix, agarose gel, l-histidine, DABCO, d-mannitol and H2O2 30% were purchased from Sigma-Aldrich. Sodium hydroxide, triethylamine, chloroform, ethanol, methanol, hexane, ethyl acetate, acetonitrile, DMF, DMSO, EDTA, manganese acetate tetrahydrate, cobalt acetate tetrahydrate, nickel chloride hexahydrate and copper(II) acetate monohydrate were Loba Chemie products. All the chemicals and solvents were used without further purification. The DNA pBR322 and DNA loading dye were purchased from Bangalore Genei. The electronic spectra were recorded as DMSO solutions on a Shimadzu UV 2450 series spectrophotometer in the range 200–800 nm. FTIR spectra were recorded as KBr pellets on a Shimadzu FTIR-8400 spectrometer in the range 4000–400 cm−1. NMR spectra were measured on a Bruker Avance III (400 MHz) spectrometer, and proton chemical shifts were recorded in ppm relative to tetramethyl silane as an internal standard using CDCl3 as solvent. LCMS spectra were recorded on a Waters Micromass Q-Tof Micro instrument. The elemental analyses were obtained with a ThermoFinnigan elemental analyzer. The X-band ESR spectrum of the copper complex was recorded on a JES-FA200 ESR spectrometer at liquid nitrogen temperature (77 K) in DMF solution, using DPPH as a standard. Magnetic susceptibilities were measured at room temperature on a Gouy balance using Hg[Co(NCS)4] as reference. Molar conductivities were measured on a Systronic conductivity bridge with a dip-type cell using 10−3 M solutions in DMF.
Preparation of the Schiff base (H2L)
The Schiff base (H2L) was prepared according to our previously reported method [11], in which a solution 2-hydroxy-6-isopropyl-3-methyl benzaldehyde (0.356 g, 2 mmol) plus ethane-1,2-diamine (0.060 g, 1 mmol) in methanol (30 ml) was boiled under reflux for 2 h (Scheme 1).
Yellow solid; Yield: 73%; Anal. calcd for C24H32N2O2 (%): C 75.7, H 8.4, N 7.3, Found: C 75.5, H 9.1, N 7.5; UV–Vis (DMF) λ max (nm): 273, 334; FTIR (KBr pellet, cm−1) ν max: 3498 (OH), 1609 (C=N), 1462 (C=C), 1259 (C–O); 1H-NMR (CDCl3, 400 MHz) (δ, ppm): 14.60 (brs, 2H, OH), 8.72 (d, 2H, –HC=N), 7.09 (d, 2H, Ar–CH, J = 8 Hz), 6.61 (d, 2H, Ar–CH, J = 8 Hz), 3.95 (s, 4H, –CH2), 3.28–3.21 (m, 2H, –CH of isopropyl group), 2.18 (s, 6H, 2-CH3), 1.11 (d, 12H, J = 4 Hz, 4-CH3); 13C-NMR (CDCl3, 400 MHz) (δ, ppm): 164.59, 160.74, 147.26, 133.74, 123.52, 114.30, 114.10, 59.63, 27.78, 23.90, 15.52; LCMS (m/z): calcd 381.52, obs 381.30.
Preparation of the manganese(III) complex
A solution of Mn(OAc)2·4H2O (0.322 g, 1.31 mmol) in methanol (25 ml) was added to a stirred solution of H2L (0.5 g, 1.31 mmol) in methanol (25 ml). The mixture was refluxed for 6–8 h; then the precipitate was filtered off, washed with methanol and dried under vacuum. Yield 0.5 g (78%).
Reddish brown Solid; Anal. calcd for C26H33MnN2O4 (%): C 63.4, H 6.7, N 5.6, Found: C 63.6, H 6.7, N 5.7; UV–Vis (DMF) λ max (nm): 275, 333, 417; FTIR (KBr, pellet cm−1) ν max: 1585 (C=N), 1276 (C–O), 1379 (C=C), 518 (M–O), 452 (M–N); LCMS (m/z): calcd 492.49, obs 433.1 [M-OAc]; μ eff: 4.82 B.M.; Conductance (Λ M, Ω−1 cm2 mol−1) in DMF: 19.60.
Preparation of the cobalt(II) complex
A solution of Co(OAc)2·4H2O (0.327 g, 1.31 mmol) in methanol (25 ml) was added to a stirred solution of H2L (0.5 g, 1.31 mmol) in methanol (25 ml). The mixture was refluxed for 6–8 h; then the precipitate was filtered off, washed with methanol and dried under vacuum. Yield 0.41 g (71%).
Green solid; Anal. calcd for C24H30CoN2O2 (%): C 65.9 H 6.9, N 6.4, Found: C 65.0, H 7.3, N 6.9; UV–Vis (DMF) λ max (nm): 269, 410, 632; FTIR (KBr pellet, cm−1) ν max: 1564 (C=N), 1234 (C–O), 1456 (C=C), 520 (M–O), 412 (M–N); LCMS (m/z): calcd 437.44, obs 437.2 μ eff: 4.23 B.M. Conductance (Λ M, Ω−1 cm2 mol−1) in DMF: 14.35.
Preparation of the nickel(II) complex
A solution of NiCl2·6H2O (0.312 g, 1.31 mmol) in methanol (25 ml) was added to a stirred solution of H2L (0.5 g, 1.31 mmol) in methanol (25 ml). The mixture was refluxed for 3 h; then the precipitate was filtered off, washed with methanol and dried under vacuum. Yield 0.45 g (79%).
Orange solid; Anal. calcd for C24H30N2NiO2 (%): C 65.9, H 6.9, N 6.4 Found: C 65.6, H 6.7, N 6.5; UV–Vis (DMF) λ max (nm): 268, 349, 352, 442, 552; FTIR (KBr pellet, cm−1) ν max: 1560 (C=N), 1234 (C–O), 1452 (C=C), 474 (M–O), 432 (M–N); LCMS (m/z): calcd 437.20, obs 437; Conductance (Λ M, Ω−1 cm2 mol−1) in DMF: 15.64.
Preparation of the copper(II) complex
A solution of Cu(OAc)·H2O (0.312 g, 1.31 mmol) in methanol (25 ml) was added to a stirred solution of H2L (0.5 g, 1.31 mmol) in methanol (25 ml). The mixture was stirred for 3–4 h; then the precipitate was filtered off, washed with methanol and dried under vacuum. Yield 0.42 g (72%).
Dark brown solid; Anal. calcd for C25H32CuN2O2(%): C 65.2, H 6.8, N 6.3, Found: C 65.6, H 6.9, N 6.6; UV–Vis: (DMF) λ max (nm): 284, 379, 560, 572; FTIR (KBr, cm−1) ν max: 1560 (C=N), 1240 (C–O), 1408 (C=C), 520 (M–O), 410 (M–N); LCMS (m/z): calcd 442.05, obs 442.05. μ eff: 1.69; Conductance (Λ M Ω−1 cm2 mol−1): 12.67.
Single-crystal X-ray diffraction
Crystals of the manganese and nickel complexes were each mounted on an APEX 2 (Bruker 2004) diffractometer, respectively. The cell refinement and data reduction were performed using APEX2/SAINT (Bruker 2004) and SAINT/XPREP (Bruker 2004), respectively [12]. The structures were solved by using SIR92 [13], and the structures were refined by SHELXL-2014/7 [14]. The computing molecular graphics were prepared with ORTEP 3.0 [15] and Mercury [16] software. Figure 1 shows ORTEP representations of [Mn(OAc)(L)] and [Ni(L)], with the common atomic numbering scheme. The crystallographic data and structure refinement for both complexes are summarized in Table 1. Selected bond lengths and angles for [Mn(OAc)(L)] and [Ni(L)] are given in Tables 2 and 3, respectively.
Biological studies
Antibacterial activity
The antibacterial activities of the compounds were assayed by the colony count method, using the selected test organisms Bacillus subtilis, Staphylococcus aureus and Pseudomonas aeruginosa. In this procedure, cells of the test organisms were grown in nutrient broth until mid-log phase and used as inoculums for performing antibacterial tests. Approximately 1 × 106 cells/ml of the test organisms were each inoculated with 0–500 μg/ml concentrations of the test compounds and incubated for 16–18 h at 37 °C. After incubation, the number of viable cells was assayed by spreading an aliquot from the broth on Muller–Hilton agar and counting the number of colony forming units per milliliter (CFU/ml). DMSO was used as negative control. Minimum inhibitory concentration (MIC) was determined. Ciprofloxacin, ampicillin and streptomycin were used as positive controls for the comparison of antibacterial activity [17].
DPPH radical scavenging activity
Radical scavenging activity was assayed by the bleaching of a purple-colored methanol solution of the stable radical 1,1-diphenyl-1-picrylhydrazyl (DPPH) by the test compound [18]. Aliquots (1 ml) of the required test compound concentrations (5, 10, 25, 50 and 100 μg/ml) in methanol were added to 4 ml of a 0.004% (w/v) methanol solution of DPPH. After 30 min of incubation at room temperature, the absorbance was measured against blank at 517 nm. The percent inhibition (I %) of free radical production from DPPH was calculated by the following equation:
where ‘A control’ is the absorbance of the control reaction (containing all reagents except the test compound) and ‘A sample’ is the absorbance of the test compound. Tests were carried out in triplicate.
DNA cleavage experiments
The DNA cleavage potential of the free Schiff base and its complexes was studied by gel electrophoresis on a total sample volume of 10 μl in 0.5 ml transparent Eppendorf microcentrifuge tubes containing pBR322 DNA (200 ng). For the gel electrophoresis experiments, supercoiled pBR322 DNA was treated with the test compounds concentrations (10, 20, 50, 100, 150 and 200 μM) and the mixtures were incubated in the dark for 30 min at 37 °C. The reaction was quenched by adding 2 μl DNA loading dye (two dye mixture containing bromophenol blue and xylene cyanol), and the samples were loaded in wells prepared for 1% agarose gel electrophoresis (tris-boric acid-EDTA (TBE) buffer, pH 8.2) for 3 h at 40 V. The pBR322 DNA bands were stained using ethidium bromide, and the level of cleavage of pBR 322 DNA was determined by measuring the intensities of the bands using a UVITECH gel documentation system. For mechanistic investigations, initial experiments were carried out in the presence of H2O2 at different sample concentrations [19].
Results and discussion
The manganese(III), cobalt(II), nickel(II) and copper(II) complexes were synthesized by reactions of the corresponding metal salts with 6,6′-((1E,1′E)-(ethane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(5-isopropyl-2-methylphenol) Schiff base (H2L) (Scheme 1). Coordination of the ligand to each metal through its oxygen and nitrogen donor sites was confirmed by elemental analysis, electronic spectra, FTIR spectra, ESR and LCMS spectroscopy, magnetic susceptibility and molar conductance measurements. Furthermore, the structures of the manganese(III) and nickel(II) complexes were confirmed by single-crystal X-ray crystallography.
The electronic spectrum of the free Schiff base shows two intense bands at 273 and 332 nm which can be assigned to the π → π* transitions of the aromatic rings and the imines group, respectively [20]. These bands were also observed at similar positions in the UV–Vis spectra of the complexes. A new band at 417 nm and weak absorbance at higher wavelengths in the spectrum of the manganese complex are assigned to metal-to-ligand charge transfer and d–d transitions, respectively, in accordance with previously reported observations closely for related manganese(III) complexes [21]. A band at 632 nm in the spectrum of the cobalt(II) complex is indicative of tetrahedral geometry. The electronic spectrum of the nickel(II) complex shows an absorption band below 600 nm, and the lack of any transition at longer wavelength indicates a large crystal-field splitting, consistent with a square-planar geometry for nickel(II). The spectrum of the copper(II) complex shows a shoulder at 560 nm which is assigned to d–d transitions in a square-planar geometry [22].
The FTIR spectrum of the free Schiff base shows a υ(OH) vibration at 3438 cm−1 which is attributed to the phenolic OH groups. This band is absent from the spectra of the metal complexes. An absorption in the region of 1609–1560 cm−1 in the spectrum of H2L is assigned to the azomethine group (–C=N), and the position of this band is found to be shifted upon complexation. The characteristic absorption bands for the C=C and C–O groups of H2L were observed at 1462 and 1259 cm−1, respectively. There was a significant change in the positions of both of these bands upon complexation. An absorption in the ranges of 410–452 and 510–525 cm−1 in the spectra of the metal complexes can be assigned to the (M–N) and (M–O) stretching vibrations [23, 24].
In the 1H NMR spectrum of H2L, peaks in the region 6.61–7.09 δ are attributable to the aromatic ring protons. A sharp singlet at 8.72 ppm assigned to the azomethine proton (–CH=N–) confirms formation of the Schiff base linkage. Another singlet at 2.18 ppm is observed for the methyl group protons, while a broad peak at 14.60 ppm is assigned to the phenolic OH group. A doublet and multiplet at 1.13 and 3.2 ppm are assigned to isopropyl group CH3 and CH protons, respectively. A singlet at 3.95 δ is assigned to the ethylene diamine protons.
The LCMS spectrum of the free Schiff base showed the molecular ion at m/z = 381.3, i.e., M+ [Calcd. 380.52] confirming the empirical formula. The LCMS spectra of the manganese(III), cobalt(II), nickel(II) and copper(II) complexes each showed the molecular ion peaks exactly equivalent with the empirical formulas (see “Experimental” section).
The molar conductivities of 10−3 M solutions of the complexes in DMF were measured at room temperature. The observed values were in the range of 12.67–19.60 mol−1 cm−2, showing that all of these complexes are nonelectrolytic [25].
The magnetic moments of the manganese(III), cobalt(II) and copper(II) complexes were 4.82, 4.23 and 1.69 B.M, respectively. These values are consistent with square pyramidal, tetrahedral and square-planar geometries, respectively [26, 27]. The nickel(II) complex was found to be diamagnetic, as expected for the square-planar geometry confirmed through the single-crystal structure.
The EPR spectrum of the copper complex at 77 k was recorded in DMF solvent. The g-tensor values of copper complexes can be used to derive the ground state [28]; hence, from the ESR spectrum of this complex the values of g|| = 2.43, g⊥ = 2.077, G = 5.76, g avg = 2.195, f = 136.01 were derived. These values show that g|| > g⊥ > ge with f in the range of 105–135 cm−1; this pattern has been reported for the square-planar complexes. The data indicate that the unpaired electron lies in the d x2–y2 orbital, giving 2B1g as the ground state [29]. Furthermore, g|| at 2.34 is within the range of 2.3–2.4 indicating the presence of mixed copper–nitrogen and copper–oxygen bonds in this complex [30]. The geometric parameter, G = (g|| − 2/g⊥ − 2), for axial spectra measures the exchange interaction between copper(II) centers in the polycrystalline state; if G > 4.0, the exchange interaction is negligible, whereas if it is less than 4.0 considerable exchange interaction is indicated. In the present complex, G is 5.76 indicating negligible exchange [31].
Crystal structure studies
X-ray studies of the manganese and nickel complexes reveal that each central metal is coordinated by a single ligand through one five-membered and two six-membered chelate rings. The manganese(III) complex has an irregular square pyramidal five-coordinate environment, with the basal plane formed by N2O2 atoms of the Schiff base ligand from its two deprotonated phenol oxygens and two imino nitrogens. An acetate ligand occupies the apical position. The observed Mn–N, Mn–O and Mn–OAc bond lengths are in the ranges of previously reported manganese complexes [32]. Similarly, in the nickel complex the Ni–O and Ni–N distances are within the ranges of 1.83–1.87 and 1.83–1.89 Å, respectively, as described for N2O2 square-planar [Ni(L)] complexes [33]. In [Ni(L)], the Ni–O and Ni–N bond lengths are in the ranges of 1.831–1.832 and 1.829–1.825 Å, respectively. The bond angles around the metal center N2–Ni–O2, O1–Ni–O2, N2–Ni–N1 and O1–Ni–N1 take values of 93.95(15), 87.47(12), 85.7(3) and 92.8(3)°, respectively. The N(2)–Ni(1)–O(1) and N(1)–Ni(1)–O(2) bond angles are 178.99(18) and 178.78(18)°, respectively. These values indicate that the complex has a distorted square-planar geometry. Both structures are monoclinic with a beta angle close to 90°. They have been refined as pseudomerohedral twins.
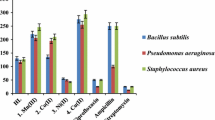
Antibacterial activity
Both the free Schiff base ligand and its complexes were tested for antibacterial activity against P. aeruginosa as a Gram-negative bacteria and B. subtilis and S. aureus as Gram-positive bacteria, with the results presented as MIC values (Table 4).
The results indicated that the Schiff base possesses activity against B. subtilis and S. aureus which is comparable to the standard ampicillin, while the manganese(III) complex shows excellent activity with MIC values of 85, 87 and 70 µg/ml against B. subtilis, S. aureus and P. aeruginosa, respectively. However, all of these complexes displayed poor activities as compared to ciprofloxacin and streptomycin [34].
Antioxidant activities
The antioxidant activities of the complexes were assayed by quenching of the DPPH radical, which absorbs at 517 nm. Results are reported as the IC50 values (Table 5).
The antioxidant activities of free H2L and its complexes were compared with those of butylated hydroxyl toluene (BHT), ascorbic acid and α-tocopherol as standards. Free H2L was found to have poor antioxidant activity; however, several of its complexes showed much better activities. In particular, the nickel(II) and copper(II) complexes showed the activities better than BHT, and comparable to ascorbic acid and α-tocopherol. In contrast, the manganese(III) complex exhibited poor activity.
DNA cleavage activity
We have utilized pBR322 DNA in order to study agarose gel electrophoresis. DNA cleavage is controlled by relaxation of supercoiled circular form DNA into nicked circular and linear forms. When circular DNA is analyzed by electrophoresis, the fastest migration will be observed for the supercoiled form (Form I). If one strand is cleaved, the supercoils will convert to a slower-moving open-circular form (Form II), and if both the strands are cleaved, a linear form (Form III) will be generated; this migrates in between Forms I and II. We found that the free Schiff base ligand and its manganese(III), nickel(II) and copper(II) complexes do not have any effect on DNA in the presence or absence of hydrogen peroxide, suggesting that these compounds do not mediate the oxidative cleavage of DNA. However, the cobalt(II) complex possesses DNA cleavage activity at higher concentration, 200 µM in the presence of oxidant H2O2, as evident (Lane 7) from Fig. 2, where the conversion of supercoiled DNA to nicked DNA occurs (Fig. 3).
Changes in the agarose gel electrophoretic pattern of pBR 322 DNA induced by H2O2 and the cobalt(II) complex. Lane 1, DNA + H2O2; lane 2, DNA + 10 µM sample + H2O2; lane 3, DNA + 20 µM sample + H2O2; lane 4, DNA + 50 µM sample + H2O2; lane 5, DNA + 100 µM sample + H2O2; lane 6, DNA + 150 µM sample + H2O2; lane 7, DNA + 200 µM sample + H2O2
Thereafter, we carried out experiments with the cobalt(II) complex at a constant concentration (200 μM) in the presence of different radical scavengers, namely d-mannitol, DMSO DABCO, H2O2 and l-histidine. In the presence of H2O2, the Form I DNA was to Form II, while partial conversion occurred in the presence of DABCO and there was no effect of d-Mannitol, DMSO and l-histidine on Form I DNA. This suggests that oxidative DNA cleavage is mediated by hydroxyl radicals in the presence of the complex. The mechanism of DNA cleavage in similar systems has been described in the literature, and mechanistic studies are in progress [35].
Conclusion
Manganese(III), cobalt(II), nickel(II) and copper(II) complexes of a salen-type Schiff base ligand were synthesized and characterized. Square pyramidal and distorted square-planar geometries were confirmed through single-crystal X-ray crystallographic techniques. The manganese(III) and copper(II) complexes were found to possess antibacterial activities against the B. subtilis, S. aureus and P. aeruginosa as compared to standard ampicillin. The nickel(II), copper(II) and cobalt(II) complexes exhibited good antioxidant activities. The cobalt(II) complex was found to cleave pBR322 DNA in the presence of H2O2.
Supplementary materials
‘CCDC numbers 1554257 and 1544860 contain the supplementary crystallographic data for this paper. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.’
References
Zhang HZ, He SC, Peng YJ, Zhang HJ, Gopala L, Tangadanchu VK, Gan LL, Zhou CH (2017) Eur J Med Chem 136:165–183
Rizzotto M (2012) Complexes as antimicrobial agents. In: A search for antibacterial agents. InTech. http://www.intechopen.com/books/a-search-for-antibacterialagents
Peng YL, Liu XL, Wang XH, Zhao ZG (2014) Chem Pap 68:401–408
Alekshun MN, Levy SB (2007) Molecular mechanisms of antibacterial multidrug resistance. Cell 128:1037–1050
Lakshmi SS, Geetha K, Gayathri M, Ganesh S (2016) J Chem Sci 128:1095–1102
Shrivastava HY, Devaraj SN, Nair BU (2004) J Inorg Biochem 98:387–392
Peng Y, Zhong H, Chen ZF, Liu YC, Zhang GH, Qin QP, Liang H (2014) Chem Pharm Bull 62:221–228
Chakravarty AR, Anreddy PA, Santra BK, Thomas AM (2002) Copper complexes as chemical nucleases. J Chem Sci 114:391–401
Pogozelski WK, Tullius TD (1998) Oxidative strand scission of nucleic acids: routes initiated by hydrogen abstraction from the sugar moiety. Chem Rev 98:1089–1108
Vaidyanathan VG, Nair BU (2003) J Inorg Biochem 94:121–126
Tadavi SK, Rajput JD, Bagul SD, Hosamani AA, Sangshetti JN, Bendre RS (2017) Res Chem Intermed 43:4863–4879
Version I. SAINT (version 7.03), and SADABS (version 2.11). Bruker advanced X-ray solutions
Altomare A, Cascarano G, Giacovazzo C, Guagliardi A (1993) J Appl Crystallogr 26:343–350
Sheldrick GM (2014) SHELXL-2014/7: program for the solution of crystal structures. University of Göttingen, Göttingen
Farrugia LJ (2002) ORTEP-3 for windows-a version of ORTEP-III with a Graphical User Interface (GUI). J Appl Crystallogr 30:565
Bruno IJ, Cole JC, Edgington PR, Kessler M, Macrae CF, McCabe P, Pearson J, Taylor R (2002) Acta Crystallogr Sect B Struct Sci 58:389–397
Samina KT, Jamatsingh DR, Suresh DR, Jaiprakash NS, Amar AH (2017) Mod Chem Appl. https://doi.org/10.4172/2329-6798.1000211
Burits M, Bucar F (2000) Antioxidant activity of Nigella sativa essential oil. Phytother Res 14:323–328
Bhat SS, Kumbhar AA, Heptullah H, Khan AA, Gobre VV, Gejji SP, Puranik VG (2010) Inorg Chem 50:545–558
Ghaffari A, Behzad M, Dutkiewicz G, Kubicki M, Salehi M (2012) J Coord Chem 65:840–855
Damercheli M, Dayyani D, Behzad M, Mehravi B, Shafiee Ardestani M (2015) J Coord Chem 68:1500–1513
Chattopadhyay S, Ray MS, Chaudhuri S, Mukhopadhyay G, Bocelli G, Cantoni A, Ghosh A (2006) Inorg Chim Acta 359:1367–1375
Ikiz M, Ispir E, Aytar E, Ulusoy M, Karabuğa Ş, Aslantaş M, Çelik O (2015) New J Chem 39:7786–7796
Alan I, Kriza A, Badea M, Stanica N, Olar R (2013) J Therm Anal Calorim 111:483–490
Abdallah SM, Mohamed GG, Zayed MA, El-Ela MS (2009) Spectrochim Acta Part A Mol Biomol Spectrosc 73:833–840
Temel H, Şekerci M (2001) Synth React Inorg Met Org Chem 31:849–857
Satpathy KC, Mishra HP, Mishra R (1981) J Inorg Nucl Chem 43:2765–2769
Srinivasan S, Athappan P, Rajagopal G (2001) Transit Met Chem 26:588–593
Raj BB, Kurup MP, Suresh E (2008) Spectrochim Acta Part A Mol Biomol Spectrosc 71:1253–1260
Raman N, Raja YP, Kulandaisamy A (2001) Proc Indian Acad Sci Chem Sci 113:183–190
Hathaway B, Billing DE (1970) Coord Chem Rev 5:143–207
Hulme C, Pritchard R, McAuliffe C, Bermejo M (1997) J Chem Soc Dalton Trans 11:1805–1814
Ghosh M, Fleck M, Mahanti B, Ghosh A, Pilet G, Bandyopadhyay D (2012) J Coord Chem 65:3884–3894
Wissner A, Berger DM, Boschelli DH, Floyd MB, Greenberger LM, Gruber BC, Johnson BD, Mamuya N, Nilakantan R, Reich MF, Shen R (2000) J Med Chem 43:3244–3256
Tadavi SK, Yadav AA, Bendre RS (2018) J Mol Struct 1152:223–231
Acknowledgements
Authors gratefully thank UGC, New Delhi for sanctioning Major Research Project File No. 42 374/2013(SR) and for Rajiv Gandhi National Fellowship for ST Candidates (Grant No. F1 17.1/2013-14/RGNF-2013-14-ST-MAH-45781/(SA-III/WEBSITE)). We are also grateful to IIT Madras for extending help in solving crystal structure of manganese(III) and nickel(II) complexes.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bendre, R.S., Tadavi, S.K. & Patil, M.M. Synthesis, crystal structures and biological activities of transition metal complexes of a salen-type ligand. Transit Met Chem 43, 83–89 (2018). https://doi.org/10.1007/s11243-017-0196-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-017-0196-y