Abstract
A new series of transition metal complexes with (1) Mn(III), (2) Co(II), (3) Ni(II) and (4) Cu(II) have been synthesized by the reaction of [6,6′-((1E,1′E)-(propane-1,3-diylbis(azanylylidene))bis(methanylylidene))bis(5-isopropyl-2-methylphenol)] with suitable metal salts. The synthesized complexes have been characterized by elemental analysis and spectroscopic techniques. The results of single crystal structures show that the metal is bonded to the ligand through the phenolic oxygens and imino nitrogens. Synthesized complexes have been evaluated for antibacterial activity and antioxidant activity, which shows considerable results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past few decades, considerable attention has been paid to the chemistry of metal complexes of Schiff bases containing nitrogen and oxygen as donor atoms, which have been found to occupy an important role in the development of the chemistry of chelate systems [1, 2]. Schiff bases have been reported to possess antimicrobial, antiviral, anticancer and anti-inflammatory activity [3]. The salen-type schiff base ligands feature two covalent and two coordinate covalent sites situated in a planar array. This makes the ligands ideal for the equatorial coordination of transition metals, leaving the two axial sites open for ancillary ligands [4]. The salen-type ligands thus closely resemble metallo-proteins [5]. A good quality of work has been reported on the synthesis and structural investigation of different types of Schiff bases and their complexes [6]. These N2O2 donor Schiff bases coordinate to d-block transition metals in a tetradentate fashion to afford stable mononuclear complexes [7]. The field of salen complexes is fast growing because of the wide variety of possible structures for the ligands, depending on the type of aldehyde and amine used. Transition metal salen complexes have been studied as catalysts in olefin epoxidations [8], reversible oxygen transport, potent antiviral and antitumor agents, in enantioselective and asymmetric catalysis and as models for vitamin B12 [9], for interaction with DNA [10–12], several metal complexes have been used as chemosensors [13–15] and metalloproteins involved in important biological electron transfer reactions, as well as in redox processing of molecular oxygen [16].
To contribute to research on the synthesis of symmetrical Schiff base complexes, we report the synthesis, characterization, crystal structures of mononuclear (3) Ni(II) and 4 Cu(II) complexes, and have studied their electrochemical properties as well as antibacterial and antioxidant activities derived from a 6,6′-((1E,1′E)-(propane-1,3-diylbis(azanylylidene))bis(methanylylidene))bis(5-isopropyl-2 methylphenol).
Experimental
All the solvents and chemicals were of commercial reagent grade and were used as received from Aldrich. The electronic spectra of ligand and complexes are recorded as DMF solutions in the range 200–800 nm on a UV 2400 Series spectrophotometer. FT-IR spectra were recorded as KBr pellets on a SHIMADZU FT-IR-8400 spectrometer in the range 4000–400 cm−1. 1H and 13C-NMR spectra were measured with a BRUKER AVANCE III (400 MHz) spectrometer and proton chemical shifts have been recorded in ppm relative to tetramethyl silane as an internal standard using CDCl3 as solvent, while the LC–MS spectra of complexes have been recorded on a Waters Micromass Q-Tof Micro instrument. The elemental analyses of ligand and complexes were carried out with a ThermoFinnigan elemental analyzer. The X-band ESR spectra of copper complex have been recorded at liquid nitrogen temperature (77 K) as a DMF solution on a JES-FA200 ESR spectrometer using DPPH as standard. Magnetic susceptibilities are measured at room temparature on a Guoy balance using Hg[Co(NCS)4] as reference. Thermogravimetric analysis was carried out on Shimadzu TGA 50, Japan and SEM–EDS analysis was performed on a scanning electron microscope (SEM; JEOL JSM 6360 and JEOL JSM 5400; Japan. Molar conductivities of metal complexes were measured on a Systronic conductivity bridge with dip-type cell using a 10−3-M solution in DMF. Electrochemical properties of the complexes were studied by cyclic voltammetry. Cyclic voltammetry study was carried out by an electrochemical analyser (model 608 D, CH instrument) employing a three-electrode system of glassy carbon, Pt wire and Ag/AgCl as the working, counter and reference electrodes, respectively. All cyclic voltammograms were recorded at 100 mVs−1 scan rate for the potential range from 1.6 to −1.6 V in DMF containing 0.1 M TBAP as electrolyte.
Procedure for the synthesis of ligand (HL): HL = 6,6′-((1E,1′E)-(propane-1,3-diylbis(azanylylidene)) bis(methanylylidene)) bis(5-isopropyl-2-methylphenol)
The ligand was prepared by dropwise addition of a solution of propane-1,3-diamine (1 mmol) in ethanol to a constantly stirred solution of 2-hydroxy-6-isopropyl-3-methyl benzaldehyde (2 mmol) in ethanol. The reflux condition was maintained until the formation of a solid product. The product was filtered, washed with cold ethanol and recrystallized from ethanol and dried over sodium sulphate. Spectral data for ligand HL: Color: yellow solid; Yield: 67%; M. P. 87 °C; UV–Vis (DMF) λ max(nm): 273, 330; FT-IR (KBr pellet, cm−1) ν max: 3221 (OH), 1610 (C=N), 1463 (C=C), 1259 (C–O); 1H-NMR (CDCl3, 400 MHz) (δ, ppm): 14.87 (brs, 2H, –OH), 8.85 (s, 2H, HC=N), 7.14 (d, 2H, Ar–H, J = 8 Hz), 6.68 (d, 2H, Ar–H, J = 8 Hz), 3.77–3.74 (m, 4H, CH2), 3.39–3.32 (m, 1H, CH), 2.23–2.20 (m, 6H, 2(CH3)), 2.17–2.10 (m, 2H, CH2), 1.27–1.63 (m, 12H, 4(CH3)); 13C-NMR (CDCl3,400 MHz) (δ, ppm): 163.06, 160.95, 147.02, 133.68, 123.70, 114.26, 56.81, 31.81, 27.84, 24.10, 15.59; MS (m/z): calcd 394.55, obs 395.3; Anal. calcd for C25H34N2O2(%): C, 76.10, H, 8.69, N, 7.10, Found: C, 77.77, H, 9.07, N, 6.85.
Procedure for the synthesis of symmetrical mononuclear metal complexes
Complex 1
Mn(III) was prepared by dropwise addition of a 1-mmol ethanolic solution of manganese acetate tetrahydrate to a stirred 1-mmol solution of ligand in ethanol in basic condition under nitrogen atmosphere. The color of the solution changed instantly. The reaction mixture was refluxed after completion of the reaction, and the solution obtained was evaporated and the product collected, and thenwashed with cold ethanol followed by diethyl ether. Spectral data for (1) Mn(III) complex: Color: reddish-brown solid; Yield: 65%; UV–Vis (DMF) λ max (nm): 266, 327; FT-IR (KBr, cm−1) ν max: 1560 (C=N), 1247 (C–O), 1409 (C=C), 520 (M–O), 439 (M–N); LC–MS (m/z): calcd 447.47, obs 447.3; Anal. calcd for C27H35MnN2O4 (%): C, 64.02, H, 6.96, N, 5.53, Found: C, 64.98, H, 6.00, N, 5.51; μ eff: 4.65 B.M.; Conductance (ΛM, Ω−1 cm2 mol−1) in DMF: 9.60.
Complex 2
Co(II) was prepared by dropwise addition of 1 mmol of cobalt acetate tetrahydrate dissolved in ethanol to a stirred solution of the ligand (1 mmol) in ethanol. The reaction mixture was refluxed after completion of the reaction, the solution obtained was evaporated and cthe ollected product was washed with cold ethanol and then diethyl ether. Spectral data for (2) Co(II) complex: Color: reddish-brown; Yield: 70%; UV–Vis (DMF) λ max(nm): 269, 401, 622; FT-IR (KBr, cm−1) νmax: 1562 (C=N), 1230 (C–O), 1454 (C=C), 542 (M–O), 439 (M–N); LC–MS (m/z): calcd 451.47, obs 451.4; Anal. calcd for C25H32CoN2O2(%): C, 66.51, H, 7.14, N, 6.20, Found: C, 68.06, H, 6.57, N, 5.90;μ eff: 3.48 B.M.; Conductance (ΛM, Ω−1 cm2 mol−1) in DMF: 11.68.
Complex 3
Ni(II) was prepared by dropwise addition of a 1-mmol ethanolic solution of nickel chloride hexahydrate to the stirred solution of the ligand (1 mmol) in ethanol. A few drops of triethylamine were added to the solution and the color of solution changed instantly. The reaction mixture was refluxed, and, after completion of the reaction, the precipitate obtained was collected and washed with cold ethanol and then diethyl ether. The dark green single crystals of (3) Ni(II)suitable for X-ray crystallography were obtained by slow evaporation of ethanol. The crystals were isolated and washed with hexane. Spectral data for (3) Ni(II) complex: Color: dark green; Yield: 68%; UV–Vis (DMF) λ max(nm): 271, 354, 418, 579; FT-IR (KBr, cm−1) ν max: 1560 (C=N), 1234 (C–O), 1452 (C=C), 474 (M–O), 432 (M–N); 1H-NMR (CDCl3, 400 MHz) (δ, ppm): 7.58 (s, 2H, HC=N), 6.98 (d, 2H, Ar–H, J = 7.2 Hz), 6.33 (d, 2H, Ar–H, J = 7.2 Hz), 3.46 (t, 4H, CH2), 3.02 (t, 2H, CH), 2.13 (s, 6H, 2(CH3)), 1.98 (t, 2H, CH2), 1.21–1.63 (d, 12H, 4(CH3)); LC–MS (m/z): calcd 451.23, obs 451.0; Anal. calcd for C25H32N2NiO2 (%): C, 66.54, H, 7.15, N, 6.21, Found: C, 64.35, H, 6.94, N, 6.10; Conductance (ΛM, Ω−1 cm2 mol−1) in DMF: 12.72.
Complex 4
Cu(II) was prepared by dropwise addition of 1 mmol of cupric acetate monohydrate dissolved in ethanol to the stirred solution of the ligand (1 mmol) in ethanol. Triethylamine was added as 2–3 drops, and the color of solution changed instantly. The reaction mixture was refluxed, and, after completion of the reaction, shining dark green crystals were collected by filtration and washed with cold ethanol and then diethyl ether. Spectral data for 4.Cu(II) complex: Color: shining dark green; Yield: 78%. UV–Vis: (DMF) λ max(nm): 284, 379, 598; FT-IR (KBr, cm−1) ν max: 1556 (C=N), 1224 (C–O), 1446 (C=C), 520 (M–O), 422 (M–N); LC–MS (m/z): calcd 456.08, obs 456.30; Anal. calcd for C25H32CuN2O2(%): C, 65.84, H, 7.07, N, 6.14, Found: C, 68.04, H, 7.20, N, 6.43; μ eff: 1.85 B.M.; Conductance (ΛM Ω−1 cm2 mol−1): 12.67; g ‖ = 2.2317, g ⊥ = 2.0472.
Bioassay
Antibacterial activity
The antibacterial activity of the compounds was performed by enumerating the viable number of cells in the nutrient broth containing various concentrations of the compounds. The viable number is represented by the colony count method. The antibacterial activity was performed on Bacillus subtilis (NCIM-2063), Pseudomonas aeruginosa (NCIM-2036) and Staphylococcus aureus (NCIM-2901). In this method, the cells of the test organisms were grown in nutrient broth until the mid-log phase and used as an inoculum for performing the antimicrobial test. Approximately, 1 × 106 cells/mL of the test organisms were each separately inoculated with 0–500 µg/mL concentrations of different compounds and incubated for 16–18 h at 37 °C. During this incubation, the cells tended to grow and multiply in number. However, if the compounds interfered with the growth of the cells, the number of cells decreased. After 16–18 h, viable numbers of cells were recorded by spreading an aliquot from the broth inoculated with the test organisms and compounds as colony-forming units per milliliter (CFU/mL). Minimum inhibitory concentrations (MIC) were determined using the standard agar method. Dimethyl sulfoxide was used as solvent control. Ciprofloxacin, Ampicillin and Streptomycin were used as standards for the comparison of antibacterial activity [17].
Antioxidant activity
The hydrogen atom or electron-donating ability of the compounds was measured from the bleaching of the purple-colored methanol solution of 1,1-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity [18]. The spectrophotometric assay uses the stable radical DPPH as a reagent to 4 mL of 0.004% (w/v) methanol solution of DPPH, and 1 mL of various concentrations of the test compound (5, 10, 25, 50 and 100 μg/mL) in methanol was added. After 30 min incubation at room temperature, the absorbance was measured against blank at 517 nm. The percent inhibition (I %) of free radical production from DPPH was calculated by the following equation:
where ‘A control’ is the absorbance of the control reaction (containing all reagents except the test compound) and ‘A sample’ is the absorbance of the test compound. Tests were carried out in triplicate.
Result and discussion
The symmetrical Schiff base was prepared by simple condensation of 2-hydroxy-3-methyl-benzaldehyde [19] with propane-1,3-diamine in a 2:1 molar ratio (Scheme 1). The (1) Mn(III), (2) Co(II), (3) Ni(II) and (4) Cu(II) mononuclear complexes were synthesized by the direct reaction of the ligand with an equimolar amount of metal(II) salt in ethanol (Scheme 2 ). All the complexes are soluble in polar solvents like ethanol, DMSO and DMF. The elemental and spectral analyses agree well with the proposed structure of the compounds.
The ligand HL and their (1) Mn(III), (2) Co(II), (3) Ni(II) and (4) Cu(II) complexes were subjected to in vitro antibacterial activity on the selected species such as Bacillus subtilis, Pseudomonas aeruginosa and Staphylococcus aureus by using Ciprofloxacin, Ampicillin and Streptomycin as standards for the comparison. From Fig. 1. The Schiff base exhibits better activity as compared to the Ampicillin drug. (1) Mn(III) and (2) Co(II) complexes possess better activity against the Bacillus subtilis and Staphylococcus aureus as compared to the Ampicillin standard drug. The (3) Ni(II)complex shows superior antibacterial activity against all the bacterial strains of Bacillus subtilis, Pseudomonas aeruginosa and Staphylococcus aureus as compared to the Ciprofloxacin and Ampicillin standard drugs. (4) Cu(II) complex shows moderate to poor activity against all the bacterial strains as compared to all the standards. The increased activity of the metal chelates can be explained by Overtone’s concept and Tweedy’s theory [20, 21].
All the title compounds were also subjected to antioxidant activity by using standard drugs, butylated hydroxyl toluene (BHT), ascorbic acid and α-tocopherol. From Fig. 2. the results indicate that free ligand showed moderate activity; however, upon complexation with metal ions, the activity was found to vary significantly. The (2) Co(II) complex shows good anti-oxidant activity as compared to the butylated hydroxyl toluene (BHT) standard drug.
Single crystal X-ray crystallography studies
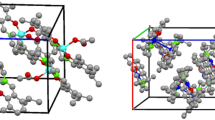
Single crystals suitable for X-ray analysis of (3) Ni(II) complex were obtained by the slow evaporation of the ethanolic solution of the Ni(II) complex at room temperature. A dark green-colored crystal of suitable size 0.400 mm × 0.200 mm × 0.100 mm was mounted on a Bruker axs kappa apex2 CCD Diffractometer equipped with Mo-Kα radiation at the wavelength 0.71073 Å. The cell refinement and data reduction were performed by using an APEX2/SAINT(Bruker 2004) and a SAINT/XPREP (Bruker 2004), respectively [22]. The multi-scan absorption corrections were applied to the data using the SADABS (Bruker [23]) program. The structure was solved by using SIR92 [24] and the structure was refined by full-matrix least squares refinement on F 2. The molecular structure, Packing diagrams and hydrogen bonding were generated by using ORTEP 3.0 software [25]. Crystals suitable for X-ray analysis of Cu(II) complex were obtained by slow evaporation of a chloroform–ethanol (1:1 v/v) at room temperature. A suitable crystal size 0.26 mm × 0.2 mm5 × 0.24 mm was selected on a Xcalibur, Eos, Nova diffractometer. The crystal was kept at 298 K during data collection. Using Olex2 [26], the structure was solved with the ShelXT [27] structure solution program using Direct Methods and refined with the ShelXT [28] refinement package using Least Squares minimization. The pertinent details of the crystallographic parameters, data collection and refinement for both the (3) Ni(II) and (4) Cu(II) complexes are given in Table 1. Additional details regarding the data collections, structure solution and refinement are included in the supporting data. The CCDC No. for Ni(II) complex is 1451939 and for the Cu(II) complex is 1443333. The crystal structures of the (3) Ni(II) and (4) Cu(II) complexes are presented in Fig. 3. The selected bond lengths and bond angles are listed in Table 2. The presented structures illustrate that the (3) Ni(II) and (4) Cu(II) complexes are formed by the deprotonation of a symmetrical tetradentate ligand. The crystal structures indicate that two nitrogen and two oxygen atoms are coordinated to the central metal ion. Both the complexes show a square planar structure. The Cu–O and Cu–N distances are in the ranges of 1.87–1.91 and 1.92–1.95 Å reported for Cu–O and Cu–N distances, respectively, and normally observed in N2O2 donor square-planar Cu(II) complexes [29–31]. Similarly, the Ni–O and Ni–N distances are within the limit ranges of 1.83–1.87 and 1.83–1.89 Å for Ni–O and Ni–N distances reported for N2O2 square-planar Ni(II) complexes [32, 33]. Selected bond lengths and bond angles for the (3) Ni(II) and (4) Cu(II) crystal structures are listed in Table 2.
Spectral characterization
Electronic spectra
The electronic spectra of the synthesized ligand and its metal complexes were recorded in DMSO solutions at different concentrations in the range 200–800 nm. The spectral data of the Schiff base shows two bands at 267 nm and 333 nm due to π → π* and n → π* transitions [34]. The electronic spectra of the (1) Mn(III) complex shows absorption bands having a λ max 266 nm assigned to the π → π* of the aromatic ring and 327 nm assigned to the n → π* transition of –C=N–. The (2) Co(II) complex shows absorption bands having a λ max 269 nm band assigned π → π* of the aromatic ring, the 401 nm band assigned to ligand to metal ion charge transfer and the d–d transition at 622 nm assigned to the 1A1g → 1B1g transition of the square planar complex formation [5]. The electronic spectra of the (3) Ni(II) complex shows absorption bands having λ max 271 nm assigned to the π → π* of the aromatic ring and 354 nm assigned to the n → π* transition of –C=N–, the 418 nm band assigned to ligand to metal ion charge transfer and the d–d transition at 579 nm of the complex indicating square planar geometry [35–37]. The electronic spectra of the (4) Cu(II) complex shows an absorption band having a λ max 284 nm band assignable to the π → π* of the aromatic ring and the band around 379 nm due to the ligand to metal charge transfer and the 598 nm band assigned to the 2B1g → 2A1g transition showing the square planar geometry [38, 39] (Fig. 4).
FT-IR Specta
FTIR spectroscopy is an important tool to probe various structural ordering phenomena, and this procedure provides the information about the position of ions in the crystal and about the interatomic vibration modes. The absorption of specific wavelength IR radiation depends on the natural frequency of the vibration of the functional group/moiety [40]. The IR spectra of the ligand and complexes are compared to confirm the coordination of the ligand. The band at 1610 cm−1 attributed to the –C=N– in the Schiff base ligand was observed to shift to a lower frequency region, 1610–1556 cm−1, in all the complexes illustrating participation of the azomethine nitrogen atom in coordination.
The peak at 3221 cm−1 appears for the phenolic –OH group. The peak appearing in the ligand at 1259 cm−1 due to C–O was shifted to 1247–1340 cm−1upon complexation.The weak- and low-frequency bands appearing in the range 400–600 cm−1 correspond to the presence of M–O and M–N in coordination with metal, respectively [41].
Magnetic susceptibility
The manganese complex shows the magnetic moment 4.65 B.M. which is lower than the expected value 5.92 B.M. of the Mn(II) d 5 configuration which may be attributed to the oxidation to Mn(III). The (2) Co(II) complex exhibited the magnetic moment 3.48 B.M. indicating the square planar configuration [36–42]. In general, Ni(II) complexes are square planar and diamagnetic, while tetrahedral complexes have magnetic moments in the range 3.20–4.10 B.M. [43]. In the present investigation, the (3) Ni(II) complex represented the diamagnetic nature and represented square planar geometry around Ni(II) as confirmed from the crystal structure. The (4) Cu(II) complex has the magnetic moment in the range 1.85 representing square planar geometry showing one unpaired electron [44, 45].
The molar conductivity
The molar conductivities Λ M of the metal complexes dissolved in DMF at 10−3 Mol concentration were found to be in the range of 8–15 (Ω−1 cm2 mol−1). These low values indicate that all these complexes are non-electrolyte in nature due to the absence of any counter ions in their structures [44].
Thermogravimetric analysis
Thermogravimetric analysis is a very useful method for investigating the thermal decomposition of solid substances involving simple metal salts as well as complex compounds [46]. Thermogravimetric analysis also gives information about the presence of water molecules inside or outside the inner coordination sphere of the central metal ion [47]. Thermogravimetric studies were performed for the (1) Mn(III), (2) Co(II), (3) Ni(II) and (4) Cu(II) complexes, in which (1) Mn(III) complex showed decomposition in two stages: the first shows the decomposition of uncoordinated water molecules below 100°C, and second decomposition of ligand with the acetate group from 498°C resulting in the formation of manganese oxide. In the cases of the (2) Co(II), (3) Ni(II) and (4) Cu(II) complexes, thermograms showed only major single decomposition for the loss of ligand moiety and the formation of corresponding oxides above 600 °C [48].
FE-SEM analysis
Scanning electron microscopy (SEM) was used to evaluate the morphology and particle size of the synthesized (1) Mn(III), (2) Co(II), (3) Ni(II) and (4) Cu(II) schiff base metal complexes as shown in Fig. 5. From the results of the SEM photographs, we note that there is a uniform matrix of the synthesized complexes, which leads to dealing with homogeneous phase material. All the complexes have particles taking agglomerization and average grain size was found between 50 nm and 10 μm with different morphologies. A (2) Co(II) complex shows single phase formation having ball-shape morphology with particle size between 1 and 10 μm and there was single phase formation of the (1) Mn(III), (2) Ni(II) and (3) Cu(II) complexes having morphologies with particle sizes of 1–10 μm.
ESR spectra
The X-band ESR spectrum of a copper complex at liquid nitrogen temperature was recorded in DMF. The ESR spectrum of the complex gives information about hyperfine and super hyperfine structures, which is important in the study of the metal ion coordination in the complexes, i.e., the shape, the nature of the coordination sites of the ligand and the degree of covalency of the metal–ligand bond. From the spectrum, the observed values are g ‖ = 2.2317, g ⊥ = 2.0472 and g = 2.0023 which indicate that they follow the trend g ‖ > g ⊥ > g indicating the square planar geometry [38] and also supporting that the unpaired electron lies in the \(d_{x}^{2} -_{y}^{2}\) orbital of the Cu(II) ion having 2B1g as ground state. The present EPR result shows that g‖ is less than 2.3, suggesting that the copper complex has a covalent nature; it also shows g ‖ in the range 2.24–2.29, which is in conformity with the presence of mixed copper–nitrogen and copper–oxygen bonds in these chelates [49–52]. The empirical factor f = 135.23 is close to the range of 105–135 cm for a square planar complex [53, 54]. The geometric parameter G is a measure of the exchange interaction coupling constant by the expression G = (g ‖ − 2.0023)/(g ⊥/2.0023) for axial spectra. If G > 4, exchange interaction is negligible, and if G < 4, considerable exchange interaction is indicated in the solid complex. G value is 5.10 which is in the range 1.68–5.14, consistent with a \(d_{x}^{2} -_{y}^{2}\) ground state in square planar geometry [55, 56]. The value of the in-plane sigma banding parameter α 2 was estimated from the following expression, α 2 = −(A ‖/0.036) + (g ‖ − 2.0023) +3/7(g ⊥ − 2.0023) + 0.04.
The value of α 2 = 0.5 indicates complete covalent bonding, while the value of α 2 = 1.0 suggests complete ionic bonding. The observed value of α 2 (0.77) is <1, which indicates that the complex has some covalent character in the ligand environment [57].
Electrochemical properties
The electrochemical behavior of the ligand and metal complexes was examined in DMF and TBAP as supporting electrolyte. Figure 6 shows the electrochemical cyclic voltammograms of curves of the ligand, (1) Mn(III), (2) Co(II), (3) Ni(II) and (4) Cu(II) complexes in DMF, and the concentration of complexes (10−3 M) in DMF containing 0.1 M tetrabutyl ammonium perchlorate (TBAP) as electrolyte with Ag/AgCl as reference electrode at scan rate 100 mVs−1. The electrochemical data are summarized in Table 3.
For the ligand, a cyclic sweep in the −1.5 to +0.50 V range shows a cathodic peak at −1.03 V and an anodic peak at −1.146 V. Electrochemical properties of the manganese complex show that the one-electron reduction peak (Epc) corresponding to the Mn (III/II) occurs at −0.944 V and, during the reverse scan, the reoxidation peak (Epa) is observed at −1.200 V. The electrochemical data of the cobalt and nickel complexes have similar Epc in the potential range given in Table 3, attributed to the Co (II/I) and Ni (II/I), the sweep potential shows the reoxidation peak (Epa) for both complexes with the potential values −1.030 and −1.116 V, respectively, while the copper complex shows the Epc corresponding to the Cu (II/I), at −0.971 V and in the reverse scan the Epa is observed at −1.019 V [58].
Conclusion
In this investigation, we are reporting the synthesis and characterization of a symmetrical salen-based ligand and the (1) Mn(III), (2) Co(II), (3) Ni(II) and (4) Cu(II) complexes. The single crystal structures of (3) Ni(II) and (4) Cu(II) have been solved by X-ray crystallography which indicated square planar geometry around the metal centers. The ligand and metal complexes were screened for their biological activities such as in vitro antibacterial and anti-oxidant activity. The results indicated that the (2) Co(II) complex shows good anti-oxidant activity which is close to the standard drug BHT. The Ni(II) complex has the superior antibacterial activity against all the bacterial strains Bacillus subtilis, Pseudomonas aeruginosa and Staphylococcus aureus as compared to standard ampicilin.The complexes (2) Co(II) and (3) Mn(III) exhibited the higher antibacterial activity against Bacillus subtilis and Staphylococcus aureus as compared to the standard drug ampicillin, while the ligand presented better activity against Bacillus subtilis, Pseudomonas aeruginosa and Staphylococcus aureus as compared to ampicillin.
References
S.S. Djebbar, B.O. Benali, J.P. Deloume, Synthesis, characterization and electrochemical behaviour of some copper (II) complexes with linear and tripodaltetradentate ligands derived from Schiff bases. Polyhedron 16, 2175–2182 (1997)
L. He, S. Gou, Q. Shi, M. Qian, C. Duan, The formation of a Schiff base intermediate: a nickel (II) complex of an asymmetric tripodal ligand. J. Chem. Crystallogr. 29, 207–210 (1999)
A.O. Sobola, G.M. Watkins, complexes of Schiff bases derived from o-aminophenol and salicylaldehyde derivatives. J. Chem. Pharm. Res. 5, 147–154 (2013)
D.A. Atwood, M.J. Harvey, Group 13 compounds incorporating salen ligands. Chem. Rev. 101, 37–52 (2001)
N. Raman, C. Thangaraja, S. Johnsonraja, Synthesis, spectral characterization, redox and antimicrobial activity of Schiff base transition metal (II) complexes derived from 4-aminoantipyrine and 3-salicylideneacetylacetone. Open Chem. 3, 537–555 (2005)
J. Liu, B. Zhang, B. Wu, K. Zhang, S. Hu, The direct electrochemical synthesis of Ti(II), Fe(II), Cd (II), Sn (II), and Pb(II) complexes with N, N-bis (Salicylidene)-o-phenylenediamine. Turk. J. Chem. 31, 623–629 (2007)
S. Akine, W. Dong, T. Nabeshima, Octanuclear zinc (II) and cobalt (II) clusters produced by cooperative tetrameric assembling of oxime chelate ligands. Inorg. Chem. 45, 4677–4684 (2006)
S.A. Patel, S. Sinha, A.N. Mishra, B.V. Kamath, R.N. Ram, Olefin epoxidation catalysed by Mn(II) Schiff base complex in heterogenised–homogeneous systems. J. Mol. Catal. A Chem. 192, 53–61 (2003)
M. Salehi, M. Kubicki, G. Dutkiewicz, A. Rezaei, M. Behzad, S. Etminani, Synthesis, characterization, electrochemical studies, and antibacterial activities of cobalt (III) complexes with Salpn-Tipe Schiff base ligands. Crystal structure of trans-[CoIII (L1)(Py) 2] ClO4. Rus. J. Coord. Chem. 39, 716–722 (2013)
S. Routier, J.L. Bernier, M.J. Waring, P. Colson, C. Houssier, C. Bailly, Synthesis of a functionalized salen-copper complex and its interaction with DNA. J. Org. Chem. 61, 2326–2331 (1996)
S. Lin et al., A G-quadruplex-selective luminescent probe with an anchor tail for the switch-on detection of thymine DNA glycosylase activity. Biosens. Bioelectron. 86, 849–857 (2016)
L. Lu et al., Development of a luminescent G-quadruplex-selective iridium (III) complex for the label-free detection of adenosine. Sci. Rep. 6, 19368 (2016)
Z. Mao et al., An Ir(III) complex chemosensor for the detection of thiols. Sci. Technol. Adv. Mater. 17(1), 109–114 (2016)
W. Wang et al., A long lifetime luminescent iridium (iii) complex chemosensor for the selective switch-on detection of Al3+ ions. Chem. Commun. 52(18), 3611–3614 (2016)
N. Chatterjee et al., A highly selective and sensitive “Turn-On” fluorescence chemosensor for the Cu2+ ion in aqueous ethanolic medium and its application in live cell imaging. J. Photochem. Photobiol. A Chem. 330, 110–116 (2016)
M. Schatz, M. Becker, F. Thaler, F. Hampel, S. Schindler, R.R. Jacobson, Z. Tyeklar, N.N. Murthy, P. Ghosh, Q. Chen, J. Zubieta, Copper (I) complexes, copper (I)/O2 reactivity, and copper (II) complex adducts, with a series of tetradentatetripyridylalkylaminetripodal ligands∇. Inorg. Chem. 40, 2312–2322 (2001)
D. Greenwood, R.C.B. Slack, J.F. Peutherer, Medical Microbiology, vol. 1, 14th edn. (ELBS, London, 1992)
M. Burits, F. Bucar, Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 14, 323–328 (2000)
J.D. Rajput, S.D. Bagul, S.K. Tadavi, P.S. Karandikar, R.S. Bendre, Design, synthesis and biological evaluation of novel class diindolyl methanes (DIMS) derived from naturally occurring phenolic monoterpenoids. Med. Chem. 6, 123–1228 (2016)
N. Dharmaraj, P. Viswanathamurthi, K. Natarajan, Ruthenium (II) complexes containing bidentate Schiff bases and their antifungal activity. Transit. Met. Chem. 26(1–2), 105–109 (2001)
B.G. Tweedy, Plant extracts with metal ions as potential antimicrobial agents. Phytopathology 55, 910–914 (1964)
A.P. Bruker, SAINT (Version 7.03), SADABS (Version 2.11), SHELXTL (Version 6.12) (Bruker AXS, Madison, 2004)
S.A.I.N.T. Bruker, Blok Version 6.02 includes XPREP and SADABS (Bruker AXS, Madison, 1999)
A. Altomare, G. Cascarano, C. Giacovazzo, A. Guagliardi, Completion and refinement of crystal structures with SIR92. J. Appl. Crystallogr. 26, 343–350 (1993)
(a) L.J. Farrugia, ORTEP-3 for Windows-a version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 30, 565 (2002). (b) I.J. Bruno, J.C. Cole, P.R. Edgington, M. Kessler, C.F. Macre, P. McCabe, J. Pearson, R. Taylor, New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr. B 58, 389–397 (2002)
O.V. Dolomanov, L.J. Bourhis, R.J. Gildea, J.A. Howard, H. Puschmann, A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009)
G.M. Sheldrick, SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A. 71, 3–8 (2015)
G.M. Sheldrick, A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008)
M. Lashanizadegan, S. Seraj, Synthesis and characterization of non-symmetric tetradentate complexes of Zn (II), Co (II), and Cu (II). Turk. J. Chem. 34, 263–268 (2010)
N. Matsumoto, T. Akui, H. Murakami, J. Kanesaka, A. Ohyoshi, H. Ōkawa, Synthesis, characterization, and X-ray crystal structures of mononuclear copper (II) complexes with unsymmetrical quadridentate ligands involving an imidazole moiety and discrete imidazolate-bridged binuclear copper (II) complexes with non-identical co-ordination geometries in the binuclear unit. J. Chem. Soc. Dalton Trans. 4, 1021–1035 (1988)
S. Meghdadi, M. Amirnasr, M. Bagheri, F.A. Najafabadi, K. Mereiter, K.J. Schenk, F. Ziaee, Synthesis, crystal structure, and electrochemical properties of Ni (II) and Cu (II) complexes with two unsymmetrical N2O2 Schiff base ligands. J. Iran. Chem. Soc. 11, 985–991 (2014)
M. Lashanizadegan, D.M. Boghaei, Synthesis and X-ray structural characterization of unsymmetrical tetradentate complexes of Ni (II) and Cu (II). Synth. React. Inorg. Met. Org. Chem. 32, 345–355 (2002)
A. Mederos, A. Medina, E. Medina, F.G. Manrique, P. Nuńez, M.L. Rodriguez, Synthesis, structure and spectroscopic studies of ethylenediamine-N, N-bis [1′-methyl-3′-oxo-3′-(2′′-hydroxy-5′′-methylphenyl) propenyl] and its mono and homobinuclear complexes with nickel (II) and Copper (II). J. Coord. Chem. 15, 393–404 (1987)
H.H. Hammud, A. Ghannoum, M.S. Masoud, Spectral regression and correlation coefficients of some benzaldimines and salicylaldiminesin different solvents. Spectrochim. Acta Part A. 63, 255–265 (2006)
P.M. Alex, K.K. Aravindakshan, Synthesis, characterization, thermal decomposition and antifungal studies of Cr(III), Mn(II), Fe(III), Co (II), Ni (II) and Cu (II) complexes of N, N′-bis [1, 3-benzodioxol-5ylmethylene] ethane-1, 2-diamine. Synth. React. Inorg. Met. Org. Nano Met. Chem. 39, 718–733 (2009)
N. Raman, V. Muthuraj, S. Ravichandran, A. Kulandaisamy, Synthesis, characterisation and electrochemical behaviour of Cu (II), Co (II), Ni (II) and Zn (II) complexes derived from acetylacetoneandp-anisidine and their antimicrobial activity. J. Chem. Sci. 115, 161–167 (2003)
N. Raman, Y.P. Raja, A. Kulandaisamy, Synthesis and characterisation of Cu (II), Ni (II), Mn(II), Zn (II) and VO (II) Schiff base complexes derived fromo-phenylenediamine and acetoacetanilide. J. Chem. Sci. 113, 183–189 (2001)
S. Chattopadhyay, M.S. Ray, S. Chaudhuri, G. Mukhopadhyay, G. Bocelli, A. Cantoni, A. Ghosh, Nickel (II) and copper (II) complexes of tetradentate unsymmetrical Schiff base ligands: first evidence of positional isomerism in such system. Inorg. Chim. Acta 359, 1367–1375 (2006)
M.S. Ray, R. Bhattacharya, S. Chaudhuri, L. Righi, G. Bocelli, G. Mukhopadhyay, A. Ghosh, Synthesis, characterisation and X-ray crystal structure of copper (II) complexes with unsymmetrical tetradentate Schiff base ligands: first evidence of Cu (II) catalysed rearrangement of unsymmetrical to symmetrical complex. Polyhedron 22, 617–624 (2003)
V. Jagadeesha Angadi et al., Observation of enhanced magnetic pinning in Sm 3+ substituted nanocrystalline Mn Zn ferrites prepared by propellant chemistry route. J. Alloy. Compd. 682, 263–274 (2016)
N. Raman, Y.P. Raja, A. Kulandaisamy, Synthesis and characterisation of Cu (II), Ni (II), Mn(II), Zn (II) and VO (II) Schiff base complexes derived fromo-phenylenediamine and acetoacetanilide. J. Chem. Sci. 113(3), 183–189 (2001)
R.L. Dutta, A. Syamal, Elements of Magnetochemistry, 2nd edn. (Elsevier, New Delhi, 1992)
A.A. Osowole, G.A. Kolawole, O.E. Fagade, Synthesis, characterization and biological studies on unsymmetrical Schiff-base complexes of nickel (II), copper (II) and zinc (II) and adducts with 2, 2′-dipyridine and 1, 10-phenanthroline. J. Coord. Chem. 61, 1046–1055 (2008)
N.S. Youssef, E. A. NEl-Zahany, B. N. Barsoum, A. M. El-Seidy, Synthesis and characterization of copper (II), cobalt (II), nickel (II), and iron (III) complexes with two diamine Schiff bases and catalytic reactivity of a chiral diamine cobalt (II) complex. Transit. Met. Chem. 34, 905–914 (2009)
M.S. Nair, R.S. Joseyphus, Synthesis and characterization of Co (II), Ni (II), Cu (II) and Zn (II) complexes of tridentate Schiff base derived from vanillin and DL-α-aminobutyric acid. Spectrochim. Acta Part A. 70, 749–753 (2008)
S.A. AbdelLatif, Y.M. Issa, Spectroscopic, thermal and magnetic properties of some transition metal complexes derived from 1-Phenyl-3-Substituted-4-Nitroso-5-Pyrazolones. Nat. Sci. 2, 1035 (2010)
G.G. Mohamed, M.M. Omar, A.M. Hindy, Metal complexes of Schiff bases: preparation, characterization, and biological activity. Turk. J. Chem. 30, 361–382 (2006)
G.G. Mohamed, M.M. Omar, A.M. Hindy, Synthesis, characterization and biological activity of some transition metals with Schiff base derived from 2-thiophene carboxaldehyde and aminobenzoic acid. Spectrochim. Acta Part A. 62(4), 1140–1150 (2005)
M.A. Neelakantan, F. Rusalraj, J. Dharmaraja, S. Johnsonraja, T. Jeyakumar, M.S. Pillai, Spectral characterization, cyclic voltammetry, morphology, biological activities and DNA cleaving studies of amino acid Schiff base metal (II) complexes. Spectrochim. Acta Part A. 71, 1599–1609 (2008)
S.S. Kandil, G.Y. Ali, A. El-Dissouky, Cobalt (II, III) and copper (II) complexes of 3-(2-furylidene) hydrazino-5, 6-diphenyl-1, 2, 4-triazine. Transit. Met. Chem. 27, 398–406 (2002)
Z.L. You, H.L. Zhu, W.S. Liu, Solvolthermal syntheses and crystal structures of three linear trinuclear Schiff base complexes of zinc (II) and cadmium (II). Zeitschriftfüranorganische und allgemeine Chemie 630, 1617–1622 (2004)
Z.L. You, H.L. Zhu, Syntheses, crystal structures, and antibacterial activities of four Schiff base complexes of copper and zinc. Zeitschriftfüranorganische und allgemeine Chemie 630(15), 2754–2760 (2004)
B.B. Raj, M.P. Kurup, E. Suresh, Synthesis, spectral characterization and crystal structure of N-2-hydroxy-4-methoxybenzaldehyde-N′-4-nitrobenzoyl hydrazone and its square planar Cu (II) complex. Spectrochim. Acta Part A. 71, 1253–1260 (2008)
R. Pogni, M.C. Baratto, A. Diaz, R. Basosi, EPR characterization of mono (thiosemicarbazones) copper (II) complexes. Note II. J. Inorg. Biochem. 79, 333–337 (2000)
E. Manoj, M.P. Kurup, A. Punnoose, Preparation, magnetic and EPR spectral studies of copper (II) complexes of an anticancer drug analogue. Spectrochim. Acta Part A. 72, 474–483 (2009)
B. Hathaway, D.E. Billing, The electronic properties and stereochemistry of mono-nuclear complexes of the copper (II) ion. Coord. Chem. Rev. 5, 143–207 (1970)
N. Raman, S. Ravichandran, S.C. Thangaraja, Copper (II), cobalt (II), nickel (II) and zinc (II) complexes of Schiff base derived from benzil-2, 4-dinitrophenylhydrazone with aniline. J. Chem. Sci. 116, 215–219 (2004)
E. Tas, M. Aslanoglu, M. Ulusoy, H. Temel, Synthesis, spectral characterization and electrochemical studies of copper (II) and cobalt (II) complexes with novel tetradentatesalicylaldimines. J. Coord. Chem. 57, 677–684 (2004)
Acknowledgements
Samina K. Tadavi gratefully thanks UGC, New Delhi, for Rajiv Gandhi National Fellowship for ST Candidates. We are also grateful to SAIF, IIT-Madras, and Prof. T. N. Guru Row, IISC, Bangalore, for help in solving of the crystal structures of nickel and copper, respectively.
Funding was provided by University Grants Commmission (Grant No. F1-17.1/2013-14/RGNF-2013-14-ST-MAH-45781/(SA-III/WEBSITE).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tadavi, S.K., Rajput, J.D., Bagul, S.D. et al. Synthesis, crystal structures, biological screening and electrochemical analysis of some salen-based transition metal complexes. Res Chem Intermed 43, 4863–4879 (2017). https://doi.org/10.1007/s11164-017-2917-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2917-4