Abstract
New complexes of type [M(HL)(CH3COO)(OH2)m]·nH2O (where M:Co, m = 2, n = 2; M:Ni, m = 2, n = 1.5; M:Zn, m = 0, n = 2.5 and M:Cd, m = 0, n = 0; H2L:5-bromo-N,N′-bis-(salicylidene)-o-tolidine) have been synthesized and characterized by microanalytical, IR, UV–Vis-NIR and magnetic data. Electronic spectra of Co(II) and Ni(II) complexes are characteristic for an octahedral stereochemistry. The IR spectra indicate a chelate coordination mode for mono-deprotonated Schiff base and a bidentate one for acetate ion. The thermal transformations are complex according to TG and DTA curves including dehydration, acetate decomposition and oxidative degradation of the Schiff base. The final product of decomposition is the most stable metallic oxide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multifunctional Schiff bases offer a versatile and flexible series of ligands capable to bind transition metal ions and generate complexes with suitable properties for theoretical studies or practical applications. As result, the complexes with multidentate Schiff bases derived from both aromatic amines and carbonylic derivatives have received considerable importance having in view their structural diversity, versatile bonding modes and promising biological implications [1]. Among these, complexes with tetradentate Schiff base derived from aromatic intermediates have been studied having in view properties such as their ability to bind reversibly the oxygen [2] and to catalyse several reactions like organic compounds oxygenation or oxidation [3–10], hydrogenation [10, 11], DNA oxidation [12–15] or several other processes [10].
Complexes with such ligands find also applications as structural or spectral models of certain metal enzymes involved in redox biological processes such dioxygen activation [16], decomposition of hydrogen peroxide [17, 18] or in hydrolytic processes [19, 20].
Important results of studies concerning syntheses, spectral, magnetic, redox and structural characteristics of the complexes with Schiff bases derived from bromosalicylaldehyde and several amines were also reported [1, 21–23]. Some of these derivatives present antimicrobial, cytotoxic [23, 24] as well as antiviral [1] activity.
Thermal stability of some complexes with bromosalicylaldimine derivatives was also investigated and indicated that decomposition follows several stages that comprise partial degradation of Schiff base ligand [25].
In this article, we report the physico-chemical as well as thermal characterisation of Co(II), Ni(II), Zn(II) and Cd(II) complexes with Schiff base bearing bromosalicyl and biphenyl moieties. The complexes have been characterized by different analytical, spectral and magnetic methods. The thermal decomposition investigated in synthetic air by thermal analysis confirmed the proposed formulas for complexes and provided information concerning the modifications during the heating and also the thermodynamic effects that accompany them.
Experimental
Materials
The high purity reagents were obtained commercially from Sigma-Aldrich (Co(CH3COO)2·4H2O, Ni(CH3COO)2·6H2O, Zn(CH3COO)2·2H2O, Cd(CH3COO)2·2H2O), Merck (5-bromosalicylaldehyde), ICN Biomedicals (3,3′-dimethylbenzidine) and Fluka (triethylamine) and were used as received without further purification.
Instruments
Chemical analysis of carbon, nitrogen and hydrogen has been performed using a Perkin Elmer PE 2400 analyzer.
IR spectra were recorded in KBr pellets with a Bruker Tensor 37 spectrometer in the range 400–4,000 cm−1.
Electronic spectra by diffuse reflectance technique, with spectralon as standard, were recorded in the range 200–2,000 nm, on a Jasco V670 spectrophotometer.
Magnetic measurements were done by Faraday’s method, at room temperature, using Hg[Co(NCS)4] as standard. The molar magnetic susceptibilities were calculated and corrected for the atomic diamagnetism. EPR spectra were recorded on microcrystalline samples at room temperature with a MiniScope MS200 Magnettech Ltd. (Germania) that operate in X band. The field was calibrated using crystalline diphenylpicrylhydrazyl (g = 2.0036).
1H NMR spectrum was recorded on a Varian Gemini 300 BB spectrometer (working frequency 300 MHz) at 25 °C by using DMSO-d6 solution. Chemical shifts were measured in parts per million from internal standard TMS. 13C NMR spectra were recorded on a Varian Gemini 300 BB spectrometer (working frequency 75 MHz) at 25 °C.
The heating curves (TG and DTA) were recorded using a Labsys 1200 SETARAM instrument, over the temperature range of 20–900 °C by using a heating rate of 10 °C/min. The measurements were carried out in synthetic air atmosphere (flow rate 16.66 cm3/min) by using alumina crucibles.
Synthesis of 5-bromo-N,N′-bis-(salicylidene)-o-tolidine
3,3′-Dimethylbenzidine (5 mmol) was dissolved in 50 mL of methanol containing few drops of acetic acid. A solution of 5-bromosalicylaldehyde (10 mmol) in 50 mL of methanol was added dropwise under continuous stirring, and the reaction mixture was then refluxed for 4 h. The orange solution was cooled at room temperature and the compound crystallises immediately. The microcrystalline species was filtered, washed with methanol and air-dried. Schiff base was recrystallized from benzene. Analysis found: C, 58.28; H, 3.72; N, 4.92, Br2C28H22N2O2 requires: C, 58.15; H, 3.83; N, 4.84%. 1H NMR (250 MHz, DMSO-d6) δ (ppm): 2.42 (s, 6H, CH3), 6.97 (d, J = 8.8 Hz, 2H, Ar–H), 7.43 (d, J = 8.2 Hz, 2H, Ar–H), 7.56 (dd, J = 8.8 (2.2) Hz, 2H, Ar–H), 7.66 (dd, J = 8.2 (2.2) Hz, 2H, Ar–H), 7.69 (d, J = 2.2 Hz, 2H, Ar–H), 7.90 (d, J = 2.5 Hz, 2H, Ar–H), 8.93 (s, 2H, CH=N); 13C NMR (250 MHz, DMSO-d6), δ (ppm): 117.62 (CH3), 118.90, 121.22, 125.04, 128.49, 132.12, 132.40, 135.30, 145.78, 155.10, 160.96 (C aromatic), 159.42 (N=CH).
Synthesis and analytical data of complexes
Co(HL)(CH3COO)·4H2O (1)
To a solution containing cobalt(II) acetate tetrahydrate (0.249 g, 1 mmol) in 40 mL ethanol was dropwise added a solution obtained by 5-bromo-N,N′-bis-(salicylidene)-o-tolidine (0.576 g, 1 mmol) dissolving in 30 mL benzene. The reaction mixture was magnetically stirred at 50 °C for 4 h until a sparingly soluble red coloured species was formed. The precipitate was filtered off, washed with hot benzene and air-dried. Analysis found: C, 46.78; H, 4.06; N, 3.72, CoBr2C30H32N2O8 requires: C, 46.96; H, 4.20; N, 3.65%.
Ni(HL)(CH3COO)·3.5H2O (2)
To a solution containing hydrated nickel(II) acetate tetrahydrate (0.249 g, 1 mmol) in 50 mL ethanol was dropwise added a solution obtained by 5-bromo-N,N′-bis-(salicylidene)-o-tolidine (0.576 g, 1 mmol) dissolving in 30 mL benzene in the presence of few drops of triethylamine. The reaction mixture was magnetically stirred at 60 °C for 4 h until the colour was modified and the solution volume was reduced to half. A sparingly soluble species, green coloured was formed after the solution was cooled at room temperature. The precipitate was filtered off, washed with hot benzene and air-dried. Analysis found: C, 47.32; H, 4.01; N, 3.92, NiBr2C30H31N2O7.5 requires: C, 47.53; H, 4.12; N, 3.70%.
Zn(HL)(CH3COO)·1.5H2O·(3)
To a hot solution containing hydrated zinc(II) acetate dihydrate (0.219 g, 1 mmol) in 40 mL ethanol was dropwise added a solution obtained by 5-bromo-N,N′-bis-(salicylidene)-o-tolidine (0.576 g, 1 mmol) dissolving in 30 mL benzene in the presence of few drops of triethylamine. The reaction mixture was refluxed for 6 h until and then solution was heated until the volume was reduced to half. A sparingly soluble species was formed after the solution was cooled at room temperature. The dark orange precipitate was filtered off, washed with hot benzene and air-dried. Analysis found: C, 47.93; H, 3.76; N, 4.08, ZnBr2C30H30N2O7 requires: C, 47.68; H, 4.00; N, 3.71%.
Cd(HL)(CH3COO) (4)
To a solution containing cadmium(II) acetate dihydrate (0.267 g, 1 mmol) in 40 mL ethanol was dropwise added a solution obtained by 5-bromo-N,N′-bis-(salicylidene)-o-tolidine (0.576 g, 1 mmol) dissolving in 30 mL benzene in the presence of few drops of triethylamine. The reaction mixture was refluxed for 6 h until the colour was modified and then solution was heated until the volume was reduced to half. A sparingly soluble species was formed after the solution was cooling at room temperature. The orange precipitate was filtered off, washed with hot benzene and air-dried. Analysis found: C, 48.23; H, 3.16; N, 3.92, CdBr2C30H24N2O4 requires: C, 48.12; H, 3.23; N, 3.74%.
Results and discussions
Condensation of 3,3′-dimethylbenzidine with 5-bromosalicylaldehyde in methanol under reflux afforded 5-bromo-N,N′-bis-(salicylidene)-o-tolidine (H2L). The complexes with formula [M(HL)(CH3COO)(OH2)m]·nH2O (where (1) M:Co, m = 2, n = 2; (2) M:Ni, m = 2, n = 1.5; (3) M:Zn, m = 0, n = 2.5 and (4) M:Cd, m = 0, n = 0; H2L: 5-bromo-N,N′-bis-(salicylidene)-o-tolidine) were synthesized by reaction of Schiff base (H2L) with metal(II) acetate for 1:1 molar ratio in triethylamine presence. The complexes were characterised on the basis of chemical and thermal analyses, IR and UV–Vis-NIR spectral data as well as magnetic moment at room temperature.
1H NMR and 13C NMR spectra of 5-bromo-N,N′-bis-(salicylidene)-o-tolidine
The 1H and 13C NMR spectra of 5-bromo-N,N′-bis-(salicylidene)-o-tolidine revealed the presence of signals at δ 8.93 and 159.42 ppm assigned to azomethine group together with characteristic resonances of methyl and aromatic groups provided both by 3,3′-dimethylbenzidine and 5-bromosalicylaldehyde intermediate for Schiff base structure (experimental part).
Infrared spectra
The major IR spectral features of complexes (Table 1) indicates that the Schiff base in monoanionic form coordinate as chelate and the acetate anion is involved also in coordination.
In the Schiff base and complexes spectra the bands characteristic for some fragments provided by both 5-bromosalicylaldehyde and 3,3′-dimethylbenzidine can be identified.
The Schiff base spectrum displays the stretching vibration of azomethine group ν(HC=N) at 1,617 cm−1 [26]. In the region characteristic for this vibration in the complexes spectra two bands appear; one slightly shifted to lower wavenumbers and the second one shifted also to lower wavenumbers by 23 cm−1. This behaviour indicates the Schiff base coordination through only one nitrogen atom of the azomethine group.
The band located in range 1,160–1,170 cm−1 is assigned to ν(C–O) vibration [25] and is slightly shifted to lower wavenumbers in the complexes spectra and/or their intensity is lowered. This behaviour indicates the coordination of phenolic oxygen to the metal ion. The broad bands that appear in the 3,200–3,400 cm−1 region are assigned to the stretching vibration of OH group from both Schiff base and water. For the Schiff base and complexes, the broad band in the 2,650–2,750 cm−1 range are assigned to the OH group vibration (ortho position) involved in intermolecular hydrogen bonds with the nitrogen atom of the CH=N group [27]. The presence of this band in the complexes spectra support the fact that only one phenol group is deprotonated. The presence of coordinated water molecules in the complexes (1) and (2) is further confirmed by appearance of the band characteristic for the wagging vibration mode in the region 640–650 cm−1 [28], information also supported by analytical and thermal analysis data.
The IR spectra of all complexes show the characteristic bands of carboxylate group in the region 1,515–1,565 cm−1 for the νas(COO) and 1,378–1,428 cm−1 for the νs(COO) vibration modes. In comparison with the free acetate, these bands are shifted in the ranges that indicate a bidentate coordination [29]. Furthermore the differences between the asymmetric and symmetric stretching frequencies of the coordinated carboxyl group lie about 150 cm−1 for Ni(II) and Zn(II) complexes and in the 128–137 cm−1 range for Co(II) and Cd(II) complexes. These indicate the acetate coordination as bridge in first case and as chelate in the second one [29].
In the small wavenumbers region two bands observed at 430–450 and 510–525 cm−1 are tentatively assigned to ν(M–N) and ν(M–O) vibration, respectively. These bands suggested that the Schiff base is coordinated through the azomethine nitrogen atoms and the oxygen of the phenol deprotonated group [27].
Electronic spectra and magnetic moments
Electronic spectra of ligand and its complexes have been recorded in the 5,000–50,000 cm−1 range in solid state and the corresponding data together with magnetic moments at room temperature are given in Table 2. The values of the crystal field splitting energy (10 Dq), Racach parameter (B) and nephelauxetic ratio (β) have been calculated and summarized in the same table.
The intense bands observed in the UV–Vis regions at 24,630 and 39,215 cm−1 in the electronic spectrum of the ligand are assigned to intraligand π → π* transitions of –C=C– (aromatic ring) and –C=N– (azomethine) groups, respectively. In the electronic spectra of the complexes, these bands are different shifted to higher or lower energy as result of coordination.
The electronic spectrum of the Co(II) complex (1) shows two additional bands at 9,175 and 19,610 cm−1, which can be assigned to 4T1g → 4T2g and 4T1g → 4T1g(P) spin allowed transitions in octahedral stereochemistry [30].
The magnetic moment of 4.79 B.M. at room temperature for this complex lies in the range accepted for octahedral species (4.3–5.2 B.M.) with three unpaired electrons and orbital contribution [31].
The magnetic moment values of Ni(II) octahedral complex generally lie between 2.9 and 3.4 B.M. depending on the degree of orbital contribution [31]. The experimental magnetic moment value Ni(II) complex (2) is found to be 3.20 B.M. in agreement with this geometry.
This complex exhibits three bands at 8,475, 15,380 and 21,280 cm−1 assigned to spin allowed 3A2g → 3T2g, 3A2g → 3T1g(F) and 3A2g → 3T1g(P) transitions, respectively, and confirm the octahedral environment around the Ni(II) ion [30]. The broad aspects of the bands can indicate the distortion from a regular octahedral stereochemistry.
A nephelauxetic parameter of 0.79/0.72 for complexes (1) and (2) indicate a high degree of covalency perhaps as result of acetate coordination as bidentate.
For Zn(II) and Cd(II) complexes the electronic spectra display supplementary intense bands at 23,810/22,730 and 19,600/19,200 cm−1, respectively, that may be assigned to metal to ligand charge transfer transitions [30]. As expected according to their electronic configuration, the last two complexes are diamagnetic. A tetrahedral stereochemistry was tentatively proposed for these compounds considering that both ligands are negatively charged and as result such a stereochemistry minimize their repulsions [31].
Thermal behaviour of complexes
The thermal behaviour of complexes was studied in air in 20–900 °C temperature range and the details will be discussed as follows.
Thermal decomposition of [Co(HL)(CH3COO)(OH2)2]·2H2O (1)
The TG and DTA curves corresponding to the complex (1) indicate that decomposition follows four steps (Fig. 1). Thus, the first two steps of compound thermal degradation consist in an endothermic and stepwise elimination of water (Table 3). Having in view the temperature interval that corresponds to these transformations it is obviously that two water molecules are retained in crystalline lattice of complex while other two are coordinated and more difficult to eliminate [32–34]. According to the mass loss in the third weak exothermic step the acetate into carbonate transformation occurs [35]. The carbonate formation is supported by IR spectrum of residue isolated at 220 °C [36]. The last step is not a single one being an overlapping of at least four processes as DTA curve indicates. This step corresponds to the oxidative degradation of Schiff base, Co(II) to Co(III) oxidation and carbonate decomposition, respectively. All these transformation lead to Co3O4 as final residue at 600 °C (found/calcd. overall mass loss: 89.3/89.5%).
Thermal decomposition of [Ni(HL)(CH3COO)(OH2)2]·1.5H2O (2)
Two steps can be noticed on the TG curve of complex (2) (Fig. 2). The stepwise elimination of water molecules is not well observed on TG curve [34] but considering the presence of Schiff base as chelate and of acetate as bridge it can be assumed that two water molecules are coordinated in order to realise the octahedral stereochemistry around of Ni(II) ion, stereochemistry indicated by spectral and magnetic data. Taking into account that water elimination starts at 50 °C the crystallisation nature of some of these molecules can be considered also. The last step is not a single one being an overlapping of at least three exothermic processes as DTA curve indicates. This step consists in both acetate and Schiff base oxidative degradation. The final product of decomposition is nickel (II) oxide according with overall mass loss (found/calcd. 90.1/90.1%).
Thermal decomposition of [Zn(HL)(CH3COO)]·2.5H2O (3)
For the complex (3) the water molecules are endothermic eliminated up to 90 °C as result of their crystallisation nature (Fig. 3) [37–40], and the anhydrous species is stable over a 103 °C temperature range. The second step is not a single one being an overlap of two exothermic processes as DTA curves indicates (Fig. 3). This step corresponds to the stepwise transformation of acetate into carbonate. Next step weak exothermic corresponds to the carbonate decomposition together with bromine elimination from the ligand. The lack of more effects on DTA curve within the 235–360 °C temperature range could be the result of endothermic effects associated with the bonds breaking in the Schiff base structure superposition with some exothermic ones, such as carbonate decomposition and rearrangement of bonds in the ligand molecule. The remaining organic part oxidative degradation take place in the last step and at least three exothermic, overlapped processes can be noticed on DTA curve. All these transformation at the heating lead to ZnO as final product (found/calcd. overall mass loss: 89.2/89.2%).
Thermal decomposition of [Cd(HL)(CH3COO)] (4)
The decomposition of complex (4) comprises three steps and as result of their anhydrous character the decomposition starts at 214 °C with two weak exothermic processes. The mass loss indicates the acetate into carbonate transformation in the first step (Fig. 4). The carbonate complex is stable over a temperature range of 40 °C and at 290 °C its decomposition is accompanied by a sharp exothermic effect. Schiff base undergoes an oxidative degradation in the last step and as result two exothermic processes can be observed on DTA curve. Finally, all these thermal transformation lead to CdO according to overall mass loss (found/calcd: 82.6/82.8%). As result of their high degree of covalency, this species decomposes slightly up to 900 °C as indicate the mass decrease and the exothermic effect noticed on DTA curve.
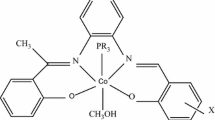
The coordination proposed for the new complexes based on these data are presented in Fig. 5.
Conclusions
New Co(II), Ni(II), Zn(II) and Cd(II) complexes with 5-bromo-N,N′-bis-(salicylidene)-o-tolidine (H2L) of type [M(HL)(CH3COO)(OH2)m]·nH2O have been synthesized and characterized by microanalytical, IR, UV–Vis-NIR and magnetic data at room temperature.
Electronic spectra of Co(II) and Ni(II) complexes are characteristic for an octahedral stereochemistry while the modifications in the IR spectra indicate the presence of the Schiff base as chelate, in mono-deprotonated form. The acetate is coordinated both as bridge and chelate depending on the metallic ion.
The thermal decomposition have confirmed the complexes formula and provided information concerning the presence of both crystallization and coordination water molecules. The thermal transformations are complex according to TG and DTA curves including dehydration, acetate decomposition and oxidative degradation of the Schiff base. The final product of decomposition was the most stable metallic oxide as overall mass loss indicates.
References
Vigato PA, Tamburini S. Advances in acyclic compartmental ligands and related complexes. Coord Chem Rev. 2008;252:1871–995.
Park S, Mathur VK, Planalp RP. Syntheses, solubilities and oxygen absorption properties of new cobalt(II) Schiff-base complexes. Polyhedron. 1998;17:325–30.
Lee NH, Byun JC, Baik JS, Han C-H, Han S-b. Development of Mn(III) (Schiff Base) complexes for the catalyst of olefin oxygenation to alcohols in the presence of NaBH4. Bull Korean Chem Soc. 2002;23:1365–6.
Mardani HR, Golchoubian H. Selective and efficient C–H oxidation of alkanes with hydrogen peroxide catalyzed by a manganese(III) Schiff base complex. J Mol Cat A Chem. 2006;259:197–200.
Kwiatkowski E, Romanowski G, Nowicki W, Kwiatkowski M, Suwińska K. Chiral dioxovanadium(V) complexes with single condensation products of 1,2-diaminocyclohexane and aromatic o-hydroxycarbonyl compounds: synthesis, characterization, catalytic properties and structure. Polyhedron. 2007;26:2559–68.
Maurya MR, Chandrakar AK, Chand S. Zeolite-Y encapsulated metal complexes of oxovanadium(VI), copper(II) and nickel(II) as catalyst for the oxidation of styrene, cyclohexane and methyl phenyl sulfide. J Mol Cat A Chem. 2007;274:192–201.
Ourari A, Baameur L, Khan MA, Bouet G. Is the electrocatalytic epoxidation of stilbene isomers using manganese (III) tetradentate Schiff bases complexes stereoselective? Electrochem Commun. 2008;10:1736–9.
Roy P, Nandi M, Manassero M, Riccó M, Mazzani M, Bhaumik A, Banerjee P. Four μ4-oxo-bridged copper(II) complexes: magnetic properties and catalytic applications in liquid phase partial oxidation reactions. Dalton Trans. 2009;43:9543–54.
Ourari A, Khelafi M, Aggoun D, Bouet G, Khan MA. Synthesis, characterization, and electrochemical study of tetradentate ruthenium-Schiff base complexes: dioxygen activation with a cytochrome P450 model using 1- or 2-methylimidazole as axial bases. Adv Phys Chem. 2011;. doi:10.1155/2011/157484.
Gupta KC, Sutar AK. Catalytic activities of Schiff base transition metal complexes. Coord Chem Rev. 2008;252:1420–50.
Krishnaraj S, Muthukumar M, Viswanathamurthi P, Sivakumar S. Studies on ruthenium(II) Schiff base complexes as catalysts for transfer hydrogenation reactions. Trans Met Chem. 2008;33:643–8.
Burrows CJ, Muller JG, Poulter GT, Rokita SE. Nickel-catalyzed oxidations: from hydrocarbons to DNA. Acta Chem Scand. 1996;50:337–44.
Firdausa F, Fatmaa K, Azama M, Khanb SN, Khanb AU, Shakir M. Template synthesis and physico-chemical characterization of 14-membered tetraimine macrocyclic complexes, [MLX2] [M=Co(II), Ni(II), Cu(II) and Zn(II)]. DNA binding study on [CoLCl2] complex. Spectrochim Acta Part A. 2009;72:591–6.
Shakira M, Azama M, Parveena S, Khanb AU, Firdaus F. Synthesis and spectroscopic studies on complexes of N,N′-bis-(2-pyridinecarboxaldimine)-1,8-diaminonaphthalene (L); DNA binding studies on Cu(II) complex. Spectrochim Acta Part A. 2009;71:1851–6.
Shakir M, Khanam S, Azam M, Aatif M, Firdaus F. Template synthesis and spectroscopic characterization of 16-membered [N4] Schiff-base macrocyclic complexes of Co(II), Ni(II), Cu(II), and Zn(II): in vitro DNA-binding studies. J Coord Chem. 2011;64:3158–68.
Ourari A, Ouari K, Khan MA, Bouet G. Dioxygen activation with a cytochrome P450 model. characterization and electrochemical study of new unsymmetrical tetradentate Schiff-base complexes with iron(III) and cobalt(II). J Coord Chem. 2008;61:3846–59.
Nakamura T, Niwa K, Fujiwara M, Matsushita T. Novel dinuclear manganese(III) complexes with tridentate and bridging tetradentate Schiff base ligands: preparation. properties and catalase-like function. Chem Lett. 1999;10:1067–8.
Dede B, Karipcin F, Cengiz M. Novel homo- and hetero-nuclear copper(II) complexes of tetradentate Schiff bases: synthesis, characterization, solvent-extraction and catalase-like activity studies. J Hazard Mat. 2009;163:1148–56.
Halcrow MA, Christou G. Biomimetic chemistry of nickel. Chem Rev. 1994;94:2421–81.
Zhang J, Xie J-q, Tang Y, Li J, Li J-z, Zeng W, Hu C-w. Hydrolysis of phosphate diester catalysed by transition metal complexes of a salicylaldimine Schiff base bearing dibenzo-18-crown-6. J Chem Res. 2005;2005:130–4.
Szłyk E, Wojtczak A, Surdykowski A, Gozdzikiewicz M. Five-coordinate zinc(II) complexes with optically active Schiff bases derived from (1R,2R)-(-)cyclohexanediamine: X-ray structure and CP MAS NMR characterization of [cyclohexylenebis(5-chlorosalicylideneiminato)zinc(II)pyridine] and [cyclohexylenebis(5-bromosalicylideneiminato)zinc(II)pyridine]. Inorg Chim Acta. 2005;358:467–75.
Mitra K, Biswas S, Lucas CR, Adhikary B. Manganese(III) complexes of N2O2 donor 5-bromosalicylideneimine ligands: combined effects of electron withdrawing substituents and chelate ring size variations on electrochemical properties. Inorg Chim Acta. 2006;359:1997–2003.
El-Sherif AA, Eldebss TMA. Synthesis, spectral characterization, solution equilibria, in vitro antibacterial and cytotoxic activities of Cu(II), Ni(II), Mn(II), Co(II) and Zn(II) complexes with Schiff base derived from 5-bromosalicylaldehyde and 2-aminomethylthiophene. Spectrochim Acta Part A. 2011;79:1803–14.
Garoufis A, Hadjikakou SK, Hadjiliadis N. Palladium coordination compounds as anti-viral, anti-fungal, anti-microbial and anti-tumor agents. Coord Chem Rev. 2009;253:1384–97.
Khalaji AD, Rad SM, Grivani G. Nickel(II) and copper(II) complexes with an asymmetric bidentate Schiff-base ligand derived from furfurylamine Synthesis, spectral, XRD, and thermal studies. J Therm Anal Calorim. 2011;103:747–51.
Avsar G, Altinel H, Yilmaz MK, Guzel B. Synthesis, characterization, and thermal decomposition of fluorinated salicylaldehyde Schiff base derivatives (salen) and their complexes with copper(II). J Therm Anal Calorim. 2010;101:199–203.
Dolaz M, Tumer M. Synthesis, spectroscopic characterization and properties of new metal complexes. Trans Met Chem. 2004;29:516–22.
Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. New York: Wiley; 1986.
Deacon GB, Philips JR. Relationships between the carboneoxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord Chem Rev. 1980;33:227–50.
Lever ABP. Inorganic Electronic Spectroscopy. Amsterdam, London, New York: Elsevier; 1986.
Gispert JB. Coordination Chemistry. Weinheim: Wiley-VCH; 2008.
Pethe G, Yaul A, Aswar A. Synthetic, spectroscopic and thermal studies of some complexes of unsymmetrical Schiff base ligand. J Therm Anal Calorim. 2012;107:97–103.
Badea M, Olar R, Marinescu D, Vasile G. Thermal behavior of some new complexes bearing ligands with polymerisable groups. J Therm Anal Calorim. 2006;85:285–8.
Olar R, Badea M, Marinescu D, Mardale R. Thermal behaviour of new Cu(II) complexes with Schiff bases functionalised with 1,3,5-triazine moieties as potential antibacterial agents. J Therm Anal Calorim. 2011;105:553–7.
Olar R, Badea M, Cristurean E, Parnau C, Marinescu D. Thermal behaviour of new N,N-dimethylbiguanide complexes having selective and effective antibacterial activity. J Therm Anal Calorim. 2006;84:53–8.
Oldham C. Carboxylates, squarates and related species. In: Wilkinson G, Gillard RD, McCleverty JA, editors. Comprehensive coordination chemistry. Oxford: Pergamon Press; 1986. p. 435–56.
Rotaru A, Constantinescu C, Mândruleanu A, Rotaru P, Moldovan A, Győryová K, Dinescu M, Balek V. Matrix assisted pulsed laser evaporation of zinc benzoate for ZnO thin films and non-isothermal decomposition kinetics. Thermochim Acta. 2010;498:81–91.
Tătucu M, Rotaru P, Rău I, Spînu C, Kriza A. Thermal behaviour and spectroscopic investigation of some methyl 2-pyridyl ketone complexes. J Therm Anal Calorim. 2010;100:1107–14.
Badea M, Olar R, Marinescu D, Uivarosi V, Aldea V, Nicolescu TO. Thermal stability of new vanadyl complexes with flavonoid derivatives as potential insulin-mimetic agents. J Therm Anal Calorim. 2010;99:823–7.
Badea M, Olar R, Uivarosi V, Marinescu D, Aldea V, Barbuceanu SF, Nitulescu GM. Thermal behavior of some vanadyl complexes with flavone derivatives as potential insulin-mimetic agents. J Therm Anal Calorim. 2011;105:559–64.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alan, I., Kriza, A., Badea, M. et al. Synthesis and characterisation of Co(II), Ni(II), Zn(II) and Cd(II) complexes with 5-bromo-N,N′-bis-(salicylidene)-o-tolidine. J Therm Anal Calorim 111, 483–490 (2013). https://doi.org/10.1007/s10973-012-2249-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2249-y