Abstract
Birch (Betula platyphylla Suk.) is a deciduous tree with medicinal and ornamental value. During the process of genetic transformation, somatic embryos do not easily develop into transgenic plants, which is a limitation in genetic breeding. The Arabidopsis thaliana WUSCHEL (AtWUS) gene, which is a transcription factor, plays an important role in maintaining and regulating stem cell characteristics, which determines whether the stem cell population is differentiated. To explore methods for inducing somatic embryogenesis (SE) in birch, we overexpressed the AtWUS gene and transferred it to birch. The expression of AtWUS increased the SE rate from 101.4 to 717.1%. The expression of the AtWUS gene led to the downregulation of BpWUS gene expression in both calli and globular embryos as well as bud meristems. The expression of a few genes, i.e., BpLEC1 (LEAFY COTYLEDON 1), BpLEC2 (LEAFY COTYLEDON 2) and BpFUS3 (FUSCA 3), was upregulated during both embryogenesis and bud meristem development. However, BpABI3 (ABSCISIC ACID INSENSITIVE 3) gene expression was upregulated only in calli embryos, while BpSTM (SHOOT MERISTEMLESS) and BpCUC2 (CUP-SHAPED COTYLEDON 2) gene expression was upregulated only in bud meristems. This result indicated that overexpression of the AtWUS gene promoted SE by increasing the expression of SE-related genes. In conclusion, this study focused on the role of the AtWUS gene in birch SE and the molecular mechanism by which SE was promoted.
Key message
This work indicates that overexpression of the WUSCHEL gene from Arabidopsis thaliana in birch can promote somatic embryogenesis and increase the development of lateral branches and buds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Birch (Betula platyphylla Suk.) is one of the most important hardwood trees in northern China. Its wide distribution, high adaptability, and rapid growth make birch a popular timber species for crafts, boards and furniture manufacture. Somatic embryogenesis (SE) is the key to asexual plant reproduction and regeneration, and SE is controlled by the coregulation of various transcription factors, small RNAs and hormones (Moon and Hake 2011; Jha et al. 2020; Tian et al. 2020; Wojcik et al. 2020; Salaun et al. 2021). The breeding and improvement of birch depend on the development of a large number of plant embryos, and the development of plant embryos depends on the expression of the WUS gene. Promoting SE has been reported in many plants, such as Panax ginseng (Kim et al. 2019), Carica papaya (Solorzano-Cascante et al. 2018), Gossypium hirsutum (Zheng et al. 2014), Holm oak (Martinez et al. 2019) and Medicago truncatula Gaertn (Rose 2019; Kadri et al. 2021).
In many plants, various genes involved in SE have been identified. These include LEAFY COTYLEDON1 (LEC1), LEAFY COTYLEDON2 (LEC2), FUS3 (AP2/B3-like transcription factor family protein, FUSCA3), ABSCISIC ACID INSENSITIVE3 (ABI3), AGAMOUS-LIKE 15 (AGL15) and BABY BOOM (BBM). FUS3 and LEC1 jointly regulate the differentiation of stem cells and play a role in the formation of cotyledons (Gaj et al. 2005). The BBM protein directly acts upstream of the LAFL (LEC1, ABI3, FUS3 and LEC2) gene to activate the LAFL network to induce SE (Roscoe et al. 2015; Horstman et al. 2017). The LAFL/AGL15 protein is necessary for the promotion of SE by BBM because the overexpression of BBM in lec1, lec2, fus3 and agl15 mutants reduces or eliminates the ability of seedlings to form somatic embryos. The abi3 mutant exhibits the same maturation defects as other LAFL mutants (Parcy 1994; Gazzarrini et al. 2004; To 2006; Jia et al. 2014).
WUSCHEL (WUS), SHOOT MERISTEMLESS (STM) and CLAVATA3 (CLV3) are essential for the stem cell division and differentiation of SAM (Reddy 2008). The expression of WUS is limited to the region composed of 500 cells below the central region of the apical meristem, WUS interacts with CLV, and regulation of WUS gene expression determines the identity of the meristem (Endrizzi et al. 1996; Mayer et al. 1998; Brand et al. 2000; Schoof et al. 2000). In wus mutants, the apical meristem cannot maintain the characteristics of stem cells (Laux et al. 1996). In Arabidopsis, ectopic expression of the WUS gene promotes the formation of meristems in roots (Gallois et al. 2004; Negin et al. 2017). GhWUS promotes the regeneration of embryoids and buds of Gossypium hirsutum (Xiao et al. 2018).
Both WUS and STM encode homeodomain proteins that control the rate of cell division and differentiation in meristematic regions (Long et al. 1996). STM is an important component for maintaining the characteristics of stem cells in meristematic tissues, preventing cells from prematurely committing to the differentiation pathway. A study of strong stm (shoot meristemless) mutants found that meristems disappear in an early stage of embryogenesis and that stem cell characteristics cannot be maintained in meristematic tissues (Barton and Poethig 1993). Ectopic expression of the WUS gene was observed in Arabidopsis thaliana, indicating the formation of somatic embryos without the induction of exogenous hormone expression (Zuo et al. 2002). In Coffea canephora, heterologous expression of the transcription factor WUS increases SE, induces callus formation, and increases somatic embryo yields by 400% (Arroyo-Herrera et al. 2008). However, no reports have described the expression of the Arabidopsis WUSCHEL gene in birch to increase the SE rate. CUP-SHAPED COTYLEDON 1 and 2 (CUC1 and CUC2) are genes that are essential for SAM maintenance during embryo development or SAM maintenance after embryo development (Takada et al. 2001). stm and wus (wuschel) double mutants or cuc1 and cuc2 double mutants cannot form normal SAMs. CUC1 and CUC2 are thought to act upstream of STM and are localized to cells at the top of the globular embryo to determine the identity of SAM. Overexpression of CUC1 itself induces ectopic bud formation in transgenic plants (Aida 1997; Takada et al. 2001). Overexpression of the WUS gene in Arabidopsis is sufficient to induce SE in shoots and root tips (Chatfield et al. 2013).
Birch is an economically important tree species. The efficient in vitro regeneration of embryogenic calli contributes to the development of somatic embryos in birch (Yang et al. 2021). There are no reports on lateral branch development in birch. Previous studies have reported that the AtWUS gene can promote the SE of other plants. We assume that the overexpression of the AtWUS gene can significantly change the SE of birch callus and form a large number of bud tissues. Therefore, the AtWUS gene promotes the development of somatic embryos, lateral branches and bud formation.
Materials and methods
Plant materials and growth conditions
Birch seeds were collected from Northeast Forestry University (Harbin, China, 45°43′45.83″ northern latitude, 126°38′11.14″ eastern longitude). Birch seeds were sterilized in 75% ethanol for 2 min, washed with sterile water, and seeded on basal medium consisting of MS (Murashige and Skoog 1962), sucrose (30 g L−1), and agar (Macklin, product code: A800728) (6 g L−1), MS medium adjusted to pH = 5.8. The seedlings were grown in a culture chamber at 22 °C ± 2 °C with six LED lights that generated a light intensity of 30 μmol m−2 s−1. When the birch seedlings grew to 6 cm, 4–5 mm stem segments were cut for explants and cultured on woody plant medium (WPM) to yield calli. The medium was supplemented with 6-BA (6-benzylaminopurine, 0.8 mg L−1) and NAA (naphthalene acetic acid, 0.6 mg L−1). The calli were transferred to WPM differentiation medium supplemented with 6-BA (1.0 mg L−1) (Zeng et al. 2010). Finally, the samples were transferred to WPM rooting medium supplemented with IBA (indole-3-butyric acid, 0.5 mg L−1). All the media used in our experiments were adjusted to pH 5.8 and autoclaved at 115 °C for 20 min. Leaves were selected for DNA and RNA isolation for the identification of transgenes. Birch buds were fixed with FAA for 24 h, embedded in paraffin and sectioned for observation.
Gene cloning and vector construction
RNA was extracted from Columbia Arabidopsis thaliana, and the full-length CDS of the AtWUS gene was cloned. The overexpression vector pH7WG2D-WUS was prepared using a Gateway clone series (Nakagawa et al. 2007) (http://gateway.psb.ugent.be), and the gene was expressed under the control of the CaMV35S promoter. The cloned fragments in all the vectors were confirmed by PCR. The plasmids were transformed into Agrobacterium GV3101 cells. Details of the primers used for this purpose are provided in Table S2. The full-length sequence of the AtWUS gene was derived from NCBI (Accession No. At2g17950).
RNA extraction and quantitative real-time PCR (qRT–PCR)
Plant material was collected and frozen in liquid nitrogen, and total RNA and DNA were extracted according to the CTAB method (Murray and Thompson 1980; Gambino et al. 2008). First strand cDNA was synthesized using the Takara™ First Strand cDNA Synthesis Kit (Takara, Dalian, China, product code: RR047A). The PCR amplification reaction mix included 2 μL of cDNA, which was equivalent to 50 ng total RNA, and 1X PCR buffer, 0.2 mM dNTPs, 1.5 mM MgCl2, 0.05 U Taq DNA polymerase (Invitrogen®) and 0.2 μM of each primer in a final volume of 50 μL (Table S2). qRT–PCR was performed in 96-well plates with each reaction volume (20 µl) including 10 µl 2 × SYBR Premix Ex Taq™, 6.8 μL PCR-grade water, 2 μL cDNA template, 0.4 μL 50 × ROX reference dye I and 0.4 μL each of the forward and reverse primer (10 μM). The thermal cycling conditions were as follows: denaturation at 95 °C for 5 min followed by 40 cycles of amplification at 95 °C for 8 s, 58 °C for 30 s and 72 °C for 20 s. The gene sequences analyzed by qRT–PCR were obtained from the birch transcriptome database (http://birch.genomics.cn/page/species/index.jsp). The specific primers are listed in the attached table S2. Three biological replicates and three technical replicates were performed for each of the analyzed genes. The relative transcript levels of each gene were calculated with the comparative cycle threshold (ddCt) method (Livak and Schmittgen 2001).
Genetic transformation of birch
In the present study, genetic transformation was performed as per the procedure prescribed by Zeng et al. 2010. Birch seeds were inoculated on 1/2 MS medium after disinfection. The stem segments (Fig. 1b) of birch (approximately 2 cm) were used as explants for inoculation on WPM (Woody Plant Medium) supplemented with 6-BA (0.8 mg L−1) and NAA (0.6 mg L−1) for 30 days to allow callusing. The excess bacterial solution on the surface of the callus was dried with sterile filter paper and inoculated on the coculture medium, which was cocultured in the dark at 28 °C for 2 days. To remove bacterial growth, cocultured calli were incubated in sterile water containing 700 mg L−1 cephalosporin. The explants were separated from Agrobacterium by light shaking, and the surface liquid of the explants was removed with sterile filter paper. Furthermore, these explants were inoculated on transgenic plant selection medium comprising WPM supplemented with 6-BA (0.8 mg L−1), NAA (0.6 mg L−1), hygromycin (50 mg L−1) and cephalomycin (500 mg L−1). After 60 days, the explants were transferred to the medium to screen transgenic plants, namely, induction medium comprising WPM supplemented with 6-BA (1.0 mg L−1), hygromycin (50 mg L−1) and cephalomycin (500 mg L−1).
DNA extraction and identification of transgenic plants
A plant DNA extraction kit (Takara, Dalian, China, product code: 9765) was used to extract total genomic DNA from leaves according to the manufacturer's instructions to analyze the putative AtWUS gene. AtWUS gene-specific primers were used to amplify the full-length AtWUS-CDS in the present study to identify transgenic plants, and the sequences are listed in Table S2. We used AtWUS gene-specific primers to amplify the 882-bp WUS fragment. Primers were also designed for the identification of the 35S promoter fusion with the AtWUS gene, which had an expected amplicon length of 1200 bp 35S:WUS fusion region. The PCR products were analyzed by electrophoresis on a 1% agarose gel. We used a fluorescence microscope to detect GFP expression in the roots of transgenic plants under excitation light.
Southern blotting analysis
The genomic DNA samples (10 µg/sample) of wild-type and transgenic plants were digested with EcoRI or BamHI, resolved on a 0.7% agarose gel, and imprinted on a charged nylon membrane. Probe labeling of the AtWUS gene with digoxigenin and southern hybridization were performed according to the manufacturer’s instructions (Roche, http://www.rocheapplied-science.com). Blocking reagent, anti-DIG-AP and NBT/BCIP were purchased from Sigma. Digoxigenin-labeled probes were used for Southern hybridization and detection. AtWUS gene probe binding was analyzed by electrophoresis (Figure S5).
Statistical analysis
All the experiments, including germination rate and branching number statistical analyses, were completely randomized and repeated three times. In each treatment, 30 calli explants or transgenic plants were used, and the EC rate in the table indicates the number of somatic embryos formed by each callus/ the number of calli. After 40 days of culture in SIM (shoot inducing medium), we repeatedly measured the weight of the embryos derived from the calli in 35S:WUS and CK with three techniques. We conducted a t test to determine significant differences (p < 0.05 or p < 0.01, depending on the experiment). The average number of branches was determined by statistically analyzing the results of 30 transgenic plants (Fig. 4d). To statistically analyze plant height, plant height was measured upward from the upper part of the root (Fig. 4e). The average number of meristems of each branch of 150-day-old plants was calculated based on the number of branch meristems in the stem region 2 cm from the ground (Fig. 4 h). SPSS software v 19.0 was used to analyze the data.
The sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: WUS (At2g17950), ABI3 (At3g24650), LEC1 (At1g21970), FUS3 (At3g26790), STM (At1g62360), PIN1 (At1g73590), LEC2 (At1g28300), and CUC2 (At5g53950). We compared the nucleotide sequence of Arabidopsis thaliana with the Betula platyphylla database at http://birch.genomics.cn/page/species/index.jsp.
Results
SE process of birch
The SE process of birch was determined. First, sterile birch seedlings were cultured (Fig. 1a), and 1–2 cm stem nodal explants of birch were inoculated into callus formation medium (Fig. 1b). SE was induced in differentiation medium after callus formation (Fig. 1c). Globular embryos (Fig. 1d, h), heart-shaped embryos (Fig. 1e, i), torpedo embryos (Fig. 1f, j) and cotyledon embryos (Fig. 1g, k) were observed by scanning electron microscopy (h–k is the model figure). The structure of birch during embryonic development is clearly visible (arrows).
Overexpression of AtWUS resulted in abnormal SE
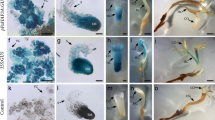
Transgenic birch plants overexpressing (OEx) AtWUS were established, and the results are shown in Fig. 2. High levels of AtWUS expression were observed in the transgenic lines (Fig. 2b, d–f, h–j). WT calli exhibited difficulty in SE and bud formation (Fig. 2a, c, g). However, the explants overexpressing the AtWUS gene showed increased SE (Fig. 2d, e, f), and clustered buds were also formed on the callus (Fig. 2g, h, i). This phenomenon indicated that overexpression of heterologous AtWUS promoted the development of somatic embryos and formation of buds. In addition, there were no obvious differences in other parts of the transgenic plants compared with the WT plants, except for a higher SE rate.
Somatic embryogenesis in wild-type and AtWUS-overexpressing tissues. Wild-type (a) and AtWUS-overexpressing tissues (b) in the callus stage. Wild-type c and AtWUS-overexpressing tissues in the L1d, L2e, and L3f stages of somatic embryogenesis. Wild-type (g) and AtWUS-overexpressing tissues in the L1h, L2i, and L3j stages of plant regeneration. (Scale bar = 1 mm)
The SE rate was investigated in explants. We observed that transgenic line 3 (L3) exhibited the highest SE rate, reaching 717.1%, which was approximately 7 times higher than the 101.4% of wild-type plants (Table 1). The SE rates of L1 and L2 were 615.2% and 371.6%, respectively (Table 1), which were higher than those of the wild type. These results suggested that overexpression of AtWUS can increase the SE rate in birch.
AtWUS gene overexpression promotes vegetative-to-embryogenic transition
We examined the expression patterns of 8 genes (including BpLEC1, BpLEC2, BpFUS3, BpABI3, BpSTM, BpPIN, BpWUS and BpCUC2) in calli and globular embryos in WT and transgenic birch. We characterized the effects of AtWUS overexpression on SE development in birch somatic embryos. We analyzed the overexpression of AtWUS in different transgenic lines, and the expression of L3 was higher than that of L1 and L2 (Fig. S1a). We analyzed the expression of several candidate genes in transgenic and wild-type AtWUS L3 plants, including the callus and globular embryo stages of SE (Fig. 3a–d). This observation suggested that the expression of the BpWUS gene in calli and globular embryos was lower than that in WT samples, which may be due to the high homology and similar structure of BpWUS and AtWUS. The expression of the endogenous BpWUS gene was inhibited (the amino acid sequence alignment of the gene in this paper is shown in Fig. S3). The BpSTM gene was highly expressed during the formation of globular embryos, but there was no significant difference between the wild type and the callus. This indicated that the overexpression of the AtWUS gene and BpSTM gene interacted to control the formation of the meristem and promote the generation of somatic embryos.
Overexpression of the AtWUS gene promotes embryonic development and increases SE. Morphology of wild-type and AtWUS L3-overexpressing plants in the callus (a, b) and globular embryo (c, d) stages. e Gene expression analysis of transgenic calli and globular embryos expressing AtWUS. (Scale bar = 1 mm; P values were calculated by Student’s t test, * is < 0.05, and ** is P < 0.01)
LEC1, LEC2, ABI3 and FUS3 are essential for SE. In calli and globular embryos, the expression of AtWUS induced the high expression of BpLEC1 and BpLEC2 and further induced the high expression of BpABI3 and BpFUS3. This explains the abnormal increase in SE in transgenic birch. CUC2 is the gene required for STM expression. However, there was no difference in the BpSTM expression of transgenic birch in the calli, but BpCUC2 expression in transgenic birch was higher than that in WT birch, and the gene expression in globular embryos showed the opposite trend. This may be due to the disordered endogenous gene regulation caused by overexpression of the AtWUS gene. However, the auxin transport gene BpPIN1 was highly expressed in globular embryos, but there was no difference in the calli. The reason was that after SE development began, the auxin concentration gradient activated the expression of the BpPIN1 gene (Fig. S1b).
After 40-day quality statistical analysis of abnormally increased calli in transgenic and WT birch, it was found that SE exhibited significant quality differences in these samples. These poor qualities represent increased somatic embryos (Table S1).
Overexpression of AtWUS led to an abnormal increase in the number of lateral branches and bud meristems of birch
Through the cultivation of transgenic plants, it was found that transgenic plants had developed lateral branches (Fig. 4a). The average lateral branch number was 5.38 ± 0.49. During 150 days of culture, we monitored plant height every 10 days and performed statistical analysis (Fig. 4a–c, e). In the first 50 days, there was little difference in plant height between wild-type plants and transgenic plants. With the growth and development of plants, the plant height of wild-type plants increased faster than that of transgenic plants. This may be caused by nutritional limitations. More lateral branches require more nutrition, which limits the development of transgenic plants.
Overexpression of AtWUS increased the number of lateral branches and bud meristems in birch. Morphological characteristics of transgenic birch at 30 days (a), 50 days (b), 150 days (c). d Number of lateral branches quantified as the mean ± SE. e Plant height of wild-type and transgenic plants within 150 days. f, g Wild-type and AtWUS L3 cells were cultured for 150 days, and the branch bud meristem number of the plant stems 2 cm from the ground. h Number of bud meristems are presented as the mean ± SE. (Scale bar in a, b = 1 cm; c = 10 cm; f, g = 5 mm; P values were calculated by Student’s t test, * is < 0.05, and ** is P < 0.01)
We counted the number of buds 2 cm above the stem; in the WT plants, the number was 1.29 ± 0.69, and in the transgenic birch plants, the number was 5.28 ± 1.25. This suggests that overexpression of AtWUS resulted in increased bud numbers (Fig. 4f–h). Interestingly, the buds change from single to axisymmetric (Fig. 4f, g).
To further investigate the expression of AtWUS, we identified transgenic birch. The PCR results of the AtWUS transgenic plants showed that they had a 882 bp product (Fig. 5a). We used 35S:WUS gene fusion PCR detection to detect a 1200 bp fusion amplification fragment (Fig. 5b). We used a digoxigenin-labeled AtWUS probe for southern blotting (Fig. S5). Southern blotting of AtWUS L3 expression showed that this gene was successfully transferred into the plants (Fig. 5c). The identification of GFP (green fluorescent protein) in birch roots showed that AtWUS was successfully expressed (Fig. 5d).
Identification of transgenic birch plants. a Specific amplified 882 bp fragment of the AtWUS gene. b The 1200 bp amplification fragment was the result of the detection of the 35S:WUS gene fusion gene. c Southern blotting results of AtWUS L3. d Identification of GFP in birch roots showed successful expression of AtWUS. (Scale bar in c, d = 10 cm; and b = 1 cm)
Bud structure and expression of SE-related genes in birch
To further explain the abnormal increase in bud numbers on the branches of transgenic birch, the characteristics of the shoot meristem in WT and transgenic plants were observed in frozen section (Fig. 6a–d). No difference was found in buds (Fig. S4). Further analysis of the expression of genes related to the AtWUS gene in buds showed that the expression of the BpWUS gene was downregulated, but the expression of BpSTM was upregulated. The expression levels of BpLEC1, BpLEC2, BpFUS3, BpPIN1 and BpCUC2 were upregulated, and the expression of BpABI3 was not significantly different (Fig. 6e). The above experimental results showed that the overexpression of AtWUS activated BpWUS-related regulatory genes and promoted the increase in bud numbers by upregulating BpLEC2 expression (Fig. S2).
Overexpression of the AtWUS gene promotes embryonic development-related gene expression and promotes increased bud meristem. Morphology of wild-type and AtWUS L3-overexpressing plants in resting buds (a, b) and buds (c, d) stages. e Gene expression analysis of transgenic buds carrying AtWUS. (Scale bar = 300 μm)
Discussion
In this study, AtWUS transgenic plants were generated to elucidate the molecular mechanism underlying SE. Regulation of embryonic development and bud meristem formation by plant stem cells is a complex process. Environmental signals, auxin, cytokinin (CKs), ethylene, abscisic acid (ABA) and epigenetic mechanisms that are involved in chromatin remodeling have become the key factors of SE ( Mendez-Hernandez et al. 2019; Wojcik et al. 2020; Jha et al. 2020; Salaun et al. 2021). The WUS gene exerted an antagonistic effect on STM and was negatively associated with CLV3 (Schoof et al. 2000; Gallois et al. 2002; Reddy 2005; Yadav et al. 2010). Our results showed that AtWUS promoted the expression of BpSTM (Fig. 3e and Fig. 6e). Overexpression of the WUS gene has been performed to promote SE in many plants, such as Gossypium hirsutum (Zheng et al. 2014), Coffea (Arroyo-Herrera et al. 2008), Panax ginseng (Kim et al. 2019) and Medicago truncatula Gaertn (Kadri et al. 2021). We also observed the same results.
The plant SAM (shoot apical meristem) plays a role in controlling tissue differentiation and corresponding external developmental signals, and the differentiation rate is regulated by multiple genes (Lenhard et al. 2002; Carles and Fletcher 2003; Baurle 2005; Kieffer 2006). The WUS gene promotes transformation from the vegetative stage to the embryonic stage and promotes the formation of somatic embryos. Overexpression of the AtWUS gene in Arabidopsis promotes the development of somatic embryos and increases the size of embryos (Brand et al. 2000; Schoof et al. 2000; Zuo et al. 2002; Arroyo-Herrera et al. 2008). Our results were more exciting (Fig. 2 and Table 1). Expression of the AtWUS gene in plants increased the SE rate of birch, resulting in the formation of multiple lateral branches and multiple buds. The great difference in SE development quality between transgenic plants and wild-type plants may be due to the increase in the SE rate of birch induced by AtWUS gene transfer, and transgenic plants had more buds than WT plants.
In birch, PIN plays critical roles in SE. The globular embryo moves auxin from the tip to the bottom of PIN1 (PIN-FORMED 1) to establish an auxin concentration gradient. During somatic embryo development, auxin accumulates in apical cells, is carried to cotyledon primordia, and is finally detected in the radicle (Friml et al. 2003; Wisniewska et al. 2006; Su et al. 2009). Overexpression of AtWUS promotes PIN gene expression and further promotes SE.
In our study, the AtWUS gene induced BpLEC2 and BpLEC1 gene expression in the calli and buds of birch and further induced BpFUS3 gene expression. The high expression of these genes affected the growth and development of plants through AUXIN, GA and ABA signaling (Fig. 7). LEC plays an important role in embryonic development (Braybrook and Harada 2008). The BBM protein directly targets the upstream activation of the LAFL network of the LAFL (LEC1, ABI3, FUS3 and LEC2) gene to induce SE (Roscoe et al. 2015; Horstman et al. 2017). The expression of AtLEC2 and AtIPTs in Arabidopsis promotes tobacco embryogenic callus formation and bud regeneration (Guo et al. 2013; Li, et al. 2019). Changes in the expression of LEC1 and FUS3 affect microspore embryogenesis in Brassica napus (Elahi et al. 2016). LEC2 is an important WUS response factor that promotes SE in cocoa (Fister, et al. 2018). FUS3 and LEC1 play roles in cotyledon formation, and BpPIN1 expression is related to the formation of cleft leaves (Gaj et al. 2005; Qu et al. 2020). Our results showed that the expression of the AtWUS gene increased the expression of the BpPIN1 gene during SE. BpCUC1 or BpCUC2 act upstream of BpSTM and are expressed in cells at the top of globular embryos; these genes determine whether the identity of SAM is closely related to plant SE (Aida 1997; Takada et al. 2001; Ikeda-Iwai et al. 2002; Su et al. 2009).
In summary, our study provides a new method for the genetic transformation of birch. increasing SE by AtWUS gene transformation. Similarly, the lateral branches of transgenic birch developed abnormally, which changed the normal morphology of birch and transformed it from an arbor morphology to shrub morphology. This method provides new varieties for landscaping in northern China.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SAM:
-
Shoot apical meristem
- 6-BA:
-
6-Benzylaminopurine;
- NAA:
-
Naphthalene acetic acid
- IBA:
-
Indole-3-butyric acid
- CIM:
-
Callus induction medium
- SIM:
-
Shoot induction medium
- ABA:
-
Abscisic acid
- GA:
-
Gibberellic acid
- ABI3:
-
Abscisic acid insensitive 3
- AGL15:
-
Agamous-like 15
- BBM:
-
Baby boom
- FUS3:
-
Fusca 3
- LEC1:
-
Leafy cotyledon 1
- LEC2:
-
Leafy cotyledon 2
- SE:
-
Somatic embryogenesis
- WUS:
-
Wuschel
- STM:
-
Shoot meristemless
- CLV3:
-
Clavata3
- CUC1:
-
Cup-shaped cotyledon 1
- CUC2:
-
Cup-shaped cotyledon 2
- PIN1:
-
Pin-formed 1
References
Aida M (1997) Genes involved in organ separation in Arabidopsis: an Analysis of the cup-shaped cotyledon mutant. The Plant Cell Online 9:841–857
Arroyo-Herrera A, Ku Gonzalez A, Canche Moo R, Quiroz-Figueroa FR, Loyola-Vargas VM, Rodriguez-Zapata LC, Burgeff D’Hondt C, Suárez-Solís VM, Castaño E (2008) Expression of WUSCHEL in Coffea canephora causes ectopic morphogenesis and increases somatic embryogenesis. Plant Cell, Tissue Organ Cult 94:171–180
Barton MK, Poethig RS (1993) Formation of the shoot apical meristem in arabidopsis thaliana: an analysis of development in the wild type and in the shoot meristemless mutant. Development 119:823–831
Baurle I (2005) Regulation of WUSCHEL transcription in the stem cell niche of the Arabidopsis shoot meristem. The Plant Cell Online 119:823–831
Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R (2000) Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289:617–619
Braybrook SA, Harada JJ (2008) LECs go crazy in embryo development. Trends Plant Sci 13:624–630
Carles CC, Fletcher JC (2003) Shoot apical meristem maintenance: the art of a dynamic balance. Trends Plant Sci 8:394–401
Chatfield SP, Capron R, Severino A, Penttila PA, Alfred S, Nahal H, Provart NJ (2013) Incipient stem cell niche conversion in tissue culture: using a systems approach to probe early events in WUSCHEL-dependent conversion of lateral root primordia into shoot meristems. Plant J 73:798–813
Elahi N, Duncan RW, Stasolla C (2016) Effects of altered expression of LEAFY COTYLEDON1 and FUSCA3 on microspore-derived embryogenesis of Brassica napus L. J Genet Eng Biotechnol 14:19–30
Endrizzi K, Moussian B, Haecker A, Levin JZ, Laux T (1996) The SHOOT MERISTEMLESS gene is required for maintenance of undifferentiated cells in Arabidopsis shoot and floral meristems and acts at a different regulatory level than the meristem genes WUSCHEL and ZWILLE. Plant J 10:967–979
Feher A (2015) Somatic embryogenesis - stress-induced remodeling of plant cell fate. Biochim Biophys Acta 1849:385–402
Fister AS, Landherr L, Perryman M, Zhang Y, Guiltinan MJ, Maximova SN (2018) Glucocorticoid receptor-regulated TcLEC2 expression triggers somatic embryogenesis in Theobroma cacao leaf tissue. PLoS One 13:e0207666
Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426:147–153
Gaj MD, Zhang S, Harada JJ, Lemaux PG (2005) Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta 222:977–988
Gallois JL, Woodward C, Reddy GV, Sablowski R (2002) Combined SHOOT MERISTEMLESS and WUSCHEL trigger ectopic organogenesis in Arabidopsis. Development 129:3207–3217
Gallois JL, Nora FR, Mizukami Y, Sablowski R (2004) WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes Dev 18:375–380
Gambino G, Perrone I, Gribaudo I (2008) A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem Anal 19:520–525
Gazzarrini S, Tsuchiya Y, Lumba S, Okamoto M, McCourt P (2004) The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the hormones gibberellin and abscisic acid. Dev Cell 7:373–385
Guo F, Liu C, Xia H, Bi Y, Zhao C, Zhao S, Hou L, Li F, Wang X (2013) Induced expression of AtLEC1 and AtLEC2 differentially promotes somatic embryogenesis in transgenic tobacco plants. PLoS One 8:e71714
Horstman A, Li M, Heidmann I, Weemen M, Chen B, Muino JM, Angenent GC, Boutilier K (2017) The BABY BOOM Transcription Factor Activates the LEC1-ABI3-FUS3-LEC2 Network to Induce Somatic Embryogenesis. Plant Physiol 175:848–857
Ikeda-Iwai M, Satoh S, Kamada H (2002) Establishment of a reproducible tissue culture system for the induction of Arabidopsis somatic embryos. J Exp Bot 53:1575–1580
Jha P, Ochatt SJ, Kumar V (2020) WUSCHEL: a master regulator in plant growth signaling. Plant Cell Rep 39:431–444
Jia H, Suzuki M, Mccarty DR (2014) Regulation of the seed to seedling developmental phase transition by the LAFL and VAL transcription factor networks. Wiley Interdiscip Rev Dev Biol 3:135–145
Kadri A, Grenier De March G, Guerineau F, Cosson V, Ratet P (2021) WUSCHEL overexpression promotes callogenesis and somatic embryogenesis in Medicago truncatula Gaertn. Plants (basel) 10:715
Kieffer M (2006) Analysis of the transcription factor WUSCHEL and its functional homologue in antirrhinum reveals a potential mechanism for their roles in meristem maintenance. The Plant Cell Online 18:560–573
Kim JY, Adhikari PB, Ahn CH, Kim DH, Chang Kim Y, Han JY, Kondeti S, Choi YE (2019) High frequency somatic embryogenesis and plant regeneration of interspecific ginseng hybrid between Panax ginseng and Panax quinquefolius. J Ginseng Res 43:38–48
Laux T, Mayer KF, Berger J, Jürgens G (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development (cambridge, England) 122:87–96
Lenhard M, Jurgens G, Laux T (2002) The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary roles in Arabidopsis shoot meristem regulation. Development 129:3195–3206
Li K, Wang J, Liu C, Li C, Qiu J, Zhao C, Xia H, Ma C, Wang X, Li P (2019) Expression of AtLEC2 and AtIPTs promotes embryogenic callus formation and shoot regeneration in tobacco. BMC Plant Biol 19:314
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) method. Methods 25:402–408
Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379:66–69
Martinez MT, San-Jose MDC, Arrillaga I, Cano V, Morcillo M, Cernadas MJ, Corredoira E (2019) Holm Oak somatic embryogenesis: current status and future perspectives. Front Plant Sci 10:239
Mendez-Hernandez HA, Ledezma-Rodriguez M, Avilez-Montalvo RN, Juarez-Gomez YL, Skeete A, Avilez-Montalvo J, De-la-Pena C, Loyola-Vargas VM (2019) Signaling overview of plant somatic embryogenesis. Front Plant Sci 10:77
Moon J, Hake S (2011) How a leaf gets its shape. Curr Opin Plant Biol 14:24–30
Murashige T, Skoog F (1962) A Revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:42–44
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326
Nakagawa T, Suzuki T, Murata S, Nakamura S, Hino T, Maeo K, Tabata R, Kawai T, Tanaka K, Niwa Y, Watanabe Y, Nakamura K, Kimura T, Ishiguro S (2007) Improved gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci Biotechnol Biochem 8:2095–2100
Negin B, Shemer O, Sorek Y, Eshed Williams L (2017) Shoot stem cell specification in roots by the WUSCHEL transcription factor. PLoS One 12:e0176093
Parcy F (1994) Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. The Plant Cell Online 6:1567–1582
Qu C, Bian X, Han R, Jiang J, Yu Q (2020) Liu G (2020) Expression of BpPIN is associated with IAA levels and the formation of lobed leaves in Betula pendula ‘Dalecartica.’ J For Res 31(1):87–97
Reddy GV (2005) Stem-cell homeostasis and growth dynamics can be uncoupled in the Arabidopsis shoot apex. Science 310:663–667
Reddy GV (2008) Live-imaging stem-cell homeostasis in the Arabidopsis shoot apex. Curr Opin Plant Biol 11:88–93
Roscoe TT, Guilleminot J, Bessoule JJ, Berger F, Devic M (2015) Complementation of seed maturation phenotypes by ectopic expression of ABSCISIC ACID INSENSITIVE3, FUSCA3 and LEAFY COTYLEDON2 in arabidopsis. Plant Cell Physiol 56:1215–1228
Rose RJ (2019) Somatic embryogenesis in the medicago truncatula model: cellular and molecular mechanisms. Front Plant Sci 10:267
Salaun C, Lepiniec L, Dubreucq B (2021) Genetic and molecular control of somatic embryogenesis. Plants (basel) 10:1467
Schoof H, Lenhard M, Haecker A, Mayer KFX, Jürgens G, Laux T (2000) The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100:635–644
Solorzano-Cascante P, Sanchez-Chiang N, Jimenez VM (2018) Explant type, culture system, 6-Benzyladenine, meta-topolin and encapsulation affect indirect somatic embryogenesis and regeneration in Carica papaya L. Front Plant Sci 9:1769
Su YH, Zhao XY, Liu YB, Zhang CL, O’Neill SD, Zhang XS (2009) Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J 59:448–460
Takada S, Hibara K, Ishida T, Tasaka M (2001) The CUP-SHAPED COTYLEDON1 gene of Arabidopsis regulates shoot apical meristem formation. Development 128:1127–1135
Tian R, Paul P, Joshi S, Perry SE (2020) Genetic activity during early plant embryogenesis. Biochem J 477:3743–3767
To A (2006) A network of local and redundant gene regulation governs Arabidopsis seed maturation. The Plant Cell Online 18:1642–1651
Wisniewska J, Xu J, Brewer PB, Blilou L, Scheres B, Friml J (2006) Polar PIN localization directs auxin flow in plants. Science 312:883
Wojcik AM, Wojcikowska B, Gaj MD (2020) Current perspectives on the auxin-mediated genetic network that controls the induction of somatic embryogenesis in plants. Int J Mol Sci 21:1333
Xiao Y, Chen Y, Ding Y, Wu J, Wang P, Yu Y, Wei X, Wang Y, Zhang C, Li F, Ge X (2018) Effects of GhWUS from upland cotton (Gossypium hirsutum L.) on somatic embryogenesis and shoot regeneration. Plant Sci 270:157–165
Yadav RK, Tavakkoli M, Reddy GV (2010) WUSCHEL mediates stem cell homeostasis by regulating stem cell number and patterns of cell division and differentiation of stem cell progenitors. Development (cambridge, England) 137:3581–3589
Yang J, Yang D, Lü W, Zhang X, Ma M, Liu G, Jiang J, Li C (2021) Somatic embryogenesis and plant regeneration in Betula platyphalla. J Forestry Res 32:937–944
Zeng F, Qian J, Luo W, Zhan Y, Xin Y, Yang C (2010) Stability of transgenes in long-term micropropagation of plants of transgenic birch (Betula platyphylla). Biotechnol Lett 32:151–156
Zheng W, Zhang X, Yang Z, Wu J, Li F, Duan L, Liu C, Lu L, Zhang C, Li F (2014) AtWuschel promotes formation of the embryogenic callus in Gossypium hirsutum. PLoS One 9:e87502
Zuo J, Niu QW, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30:349–359
Acknowledgements
The author thanks the Fundamental Research Fund of Heilongjiang Province.
Funding
This work was supported by the Science Foundation of Heilongjiang Province, China (No. C2018002), Modern Agricultural Industrial Technology System Funding of Shandong Province, China (No. SDAIT-04–03), Agricultural Variety Improvement Project of Shandong Province, China (No. 662–2316109), and The Fundamental Research Funds for the Central Universities (No. 2572020DY15).
Author information
Authors and Affiliations
Contributions
All the authors read and approved the final manuscript. HL, LS and QJX designed the experiments and wrote the manuscript. HL, YTH, WZW and ZYC analyzed these data. Others participated in the experiments. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Paloma Moncaleán.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lou, H., Huang, Y., Wang, W. et al. Overexpression of the AtWUSCHEL gene promotes somatic embryogenesis and lateral branch formation in birch (Betula platyphylla Suk.). Plant Cell Tiss Organ Cult 150, 371–383 (2022). https://doi.org/10.1007/s11240-022-02290-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02290-9