Abstract
Betula platyphylla is a native tree species in northern China that has high economic and medicinal value. We developed an efficient protocol for the induction of somatic embryogenesis in B. platyphalla from immature zygotic embryos and assessed the effects of explant type, genotype, and plant growth regulators (PGRs) on embryogenic callus induction. Among the various explants evaluated, embryogenic callus was only produced from mature and immature zygotic embryos on medium with added 2,4-dichlorophenoxyacetic acid (2,4-D). Supplementation of 2,4-D-containing medium with cytokinins increased the frequency of embryogenic callus induction. On the 20 days after pollination, immature zygotic embryos that had been collected in mid-May yielded embryogenic tissue at the highest frequency (16.8%) when cultured on half-strength MS medium supplemented with 2.0 mg L−1 2,4-D and 0.2 mg L−1 6-benzylaminopurine (6-BA). The process of proliferation of embryogenic callus, somatic embryo formation, and subsequent plantlet conversion occurred under optimal culture conditions. When regenerated plants were transplanted to soil, 95% of them developed normally and grew vigorously. This somatic embryogenesis system required 3–4 months for the regeneration of B. platyphalla plantlets from immature zygotic embryos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Betula platyphylla var. japonica (Miquel) Hara (Betulaceae), commonly known as Asian white birch, is widely distributed in many Asian countries, including Korea, Japan, China, and eastern Siberia. The white birch is a fast-growing tree with a fine horticultural characteristics and strong vitality. In response to ecological degradation, the demand for tree planting is growing, and white birch trees are playing an increasingly important role in urban greening. Most importantly, the bark of B. platyphylla has been used as a traditional medicine for the treatment of inflammatory diseases such as choloplania, pneumonia, nephritis, and chronic bronchitis (Matsuda et al. 1998; Cho et al. 2014; Eom et al. 2016, 2017), as well as for the relief of fever and coughs (Huh et al. 2018). Currently, there is increased interest in propagation techniques for birch. Traditional birch propagation techniques include seed cultivation and cutting technology. But seed preservation is difficult, and seed germination rates decline with increasing storage time. Cutting technology also has a number of disadvantages, including high labor costs and strict requirements for soil, temperature, and environmental conditions. Therefore, tissue culture is the most suitable method for large-scale birch propagation.
Despite its potential environmental and medicinal benefits, few studies have reported tissue culture protocols suitable for large-scale clonal propagation of birch. Such protocols would shorten the time period for breeding and improve the uniformity and quality of clonal propagation (Stasolla and Yeung, 2003; Yang et al. 2011). There are two major systems of tissue culture and plant regeneration: organogenesis and somatic embryogenesis. Compared with the former, somatic embryogenesis is regarded as more appropriate for large-scale micropropagation of woody plants because it requires a single hormonal signal to induce a bipolar structure. By contrast, organogenesis requires two different hormonal signals to induce a shoot organ and a root organ (Phillips et al. 1995). Somatic embryogenesis was studied in a large number of species, ranging from monocots to dicots and from herbaceous to woody/forestry species (Bonga 1995). Somatic embryogenesis is regarded as a particularly feasible system for in vitro regeneration and has many advantages in woody species. Traditional breeding programs impose significant limitations on the propagation of forest trees like B. platyphalla because of its long-life cycle and the difficulties of seed conservation and vegetative reproduction. To successfully produce somatic embryos (SEs), many factors have been tested: auxin types, induction medium components, and the age and type of explant, including both vegetative organs and immature and mature zygotic embryos (Pais 2019). Most plant transformation requires a highly efficient system for gene transfer and for the selection and regeneration of transgenic plants. Therefore, somatic embryogenesis is an attractive plant regeneration system for genetic transformation (Gaj 2001).
Although somatic embryogenesis has been successfully applied in regeneration systems for many woody species (Pais 2019), there is, to our knowledge, only one published report on birch somatic embryogenesis and plant regeneration from seedling and mature leaf tissue of B. pendula (Kurtén et al. 1990). Here, we describe a detailed investigation of the factors that influence high-frequency somatic embryogenesis from immature zygotic embryos, including the effects of plant growth regulators (PGRs) and sucrose on embryogenic callus induction, SE development, and plant conversion. Our work establishes a large-scale micropropagation protocol based on somatic embryogenesis.
Materials and methods
Plant materials
In early May of the first year, axillary buds, flower buds, young leaf blades, young petioles, young stems, and mature seeds were collected from Betula platyphylla of more than 10 years grown in the greenhouse of Northeast Forestry University. These tissues were used as explants for embryogenic callus induction on half-strength MS medium (Murashige and Skoog 1962, ½ MS). The medium contained 4.0% sucrose, 2 g L−1 agar (Duchefa Biochemie, Haarlem, Netherlands), and 1 g L–1 casein hydrolysate (Sigma, MO, USA) at pH 5.8 and was supplemented with 2.0 mg L–1 2,4-dichlorophenoxyacetic acid (2,4-D; Sigma, MO, USA) and 0.5 mg L–1 kinetin (KT; Sigma, MO, USA). In the second year, we selected different developmental stages of immature zygotic embryos (10–40 d after pollination) from mother tree (06) 1‒39 for callus induction. Prior to callus induction, the mature seeds were cut in half. In the third year, we randomly selected immature zygotic embryos (20 d after pollination) from multiple B. platyphylla mother trees, including (06) 1‒28, (06) 1‒32, (06) 1‒33, (06) 1‒34, (06) 1‒38, (06) 1‒39, (06) 1‒41, (12) 1‒38, (12) 1‒39, and (10) 1‒33, to investigate callus induction frequency. All explants were agitated in 70% ethanol for 1 min, surface sterilized using a 0.8% sodium hypochlorite solution containing 0.02% Tween 20 for 10 min, and rinsed five times with sterile distilled water.
Embryogenic callus induction
To optimize PGRs for embryogenic callus induction, surface-sterilized immature zygotic embryos from mother tree (06) 1‒28 (20 d after pollination, 20 explants per flask) were placed on ½ MS medium containing 4.0% sucrose, 2 g L−1 agar, and 1 g L−1 casein hydrolysate and supplemented with 1.0, 2.0, or 4.0 mg L−1 2,4-D alone or in combination with 0.2 mg L−1 6-benzylaminopurine (6-BA; Sigma, MO, USA) or 0.2 mg L−1 KT. The cultures were maintained in the dark at 25 ± 1 °C. The induction frequency (%) was measured after 2-month culture. Squashed callus sections were observed under a light microscope (Docuval, Carl Zeiss, Germany). All PGRs were purchased from Sigma, Steinheim, Germany.
Somatic embryo development and plantlet regeneration
For SE development, the friable and fast-growing embryogenic calluses were transferred to ½ MS medium without PGRs, supplemented with 6-BA or 1-naphthaleneacetic acid (NAA) alone, or supplemented with 0.1 or 0.2 mg l−1 6-BA combined with 0.1 mg L−1 NAA. The medium used for the development of SEs to plant conversion was the same medium described above. Conversion frequency (%) was measured after 4-week culture.
Transplantation
Well-developed, regenerated B. platyphylla plantlets with normal leaf and root systems were transferred to pots that contained an autoclaved 3:1 soil and sand mixture. Polythene bags covered the pots to maintain high humidity and were removed after 3 weeks when the plants had initiated several newer leaves. Survival rates (%) were recorded 8 weeks after transplantation.
Culture conditions
In this study, all media were adjusted to pH 5.8 before the addition of plant agar (Duchefa Biochemie, Haarlem, Netherlands) and then sterilized by autoclaving at 1.1 kg cm−2 (121 °C) for 20 min. The induction of embryogenic callus to proliferation occurred in 150 mL Erlenmeyer flasks with 50 mL medium, and the calluses were subcultured every 4 weeks in the dark. For SE development, plantlet conversion, and regeneration, the plants were cultured at 25 ± 1 °C with a 16 h d−1 photoperiod using cool white fluorescent tubes (36 μmol s−1 m−2) and subcultured at 4-week intervals. All experiments were replicated three times.
Data analysis
One-way analysis of variance (ANOVA) and LSD tests were performed with SPSS 16.0 (SPSS Inc., Chicago, IL, USA) to calculate the significance of differences. Means were compared using Duncan’s multiple range test at P = 0.05.
Results and discussion
Effect of explant type on embryogenic callus induction
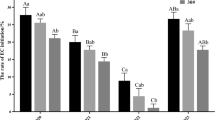
The selection of explant type plays a key role in the successful induction of somatic embryogenesis (Manoharan et al. 2016; Lu et al. 2017). Consistent with a previous study (Kurtén et al. 1990), induced callus was readily observed from all explants cultured on ½ MS medium supplemented with 2.0 mg L−1 2,4-D, 0.5 mg l−1 KT, and 1 g L−1 casein hydrolysate after 4 weeks. After 2-month culture, the callus formation rate remained stable, although the callus weight continued to increase. Figure 1 shows that friable callus was directly initiated from the cut wound of dormant buds (Fig. 1a), leaves (Fig. 1b), stems (Fig. 1c) and mature seeds (Fig. 1d) after about 4-week culture in the dark. The callus induction frequency of mature seeds was significantly higher than that of leaves and stems. However, not all of the induced calluses were embryogenic. Only mature seeds produced embryogenic callus, and their induction frequency was very low (0.7%) (Table 1). Analysis of variance indicated that explant type had a significant effect on the frequency of both callus induction (P = 0.003) and embryogenic callus induction (P = 0.000).
Callus induction from different Betula platyphalla explant types from the mother tree (06) 1–39 after 4-week culture in ½ MS medium supplemented with 2.0 mg L−1 2,4-D, 0.5 mg L−1 KT, and 1 g L−1 casein hydrolysate. The callus was initiated from dormant bud (a), leaf (b), stem (c), and mature seed (d) tissue after 4-week culture at 25 °C in the dark. Bars: a–c 200 μm, d 1 cm
Based on these results, we tried to use immature embryos to induce callus. The developmental stage had a significant effect on the frequency of callus induction (P = 0.000) and embryogenic callus induction (P = 0.000). Immature zygotic embryo explants showed significantly higher formation of callus than mature seeds. They had a higher callus induction frequency compared with mature seeds, especially for immature embryos 20 d after pollination (97.4%) (Table 2). The induction frequency of embryogenic callus was much lower than that of callus induction for all explant types. Similar results for the induction frequency of embryogenic callus using different explant types was reported in Eucalyptus camaldulensis (Prakash and Gurumurthi 2010) and Quercus alba (Corredoira et al. 2012). Currently, there is only one study on the induction of somatic embryogenesis in B. pendula (Kurtén et al. 1990). These authors also tested the effect of explant type on callus induction, including seeds, seedlings and leaves. Only seedlings and leaves produced embryogenic callus, and they did so with a very low frequency.
Immature embryos 20 days after pollination had the highest induction frequency of embryogenic callus (5.6%), followed by embryos 25 days after pollination (3.1%) (Table 2). Somatic embryos induced from immature zygotic embryos of different developmental stages have been used as initial explants to induce SE in many plant species, such as Schisandra chinensis (Chen et al. 2010), Cunninghamia lanceolata (Hu et al. 2017), and Oocarpa pine (Lara-Chavez et al. 2011). The improved callus induction frequency may be owing to the higher embryogenic potential of immature zygotic embryo explants, which are conducive to cell differentiation and division.
Genotype differences in somatic embryogenesis
The genotype effect is known to be important for somatic embryogenesis and to influence the morphogenic response in vitro (Pais 2019). Genotype specificity during embryogenic induction has been reported in numerous species: Gentiana spp. (Fiuk and Rybczyński 2008), E. guineensis (Thawaro and Te-chato 2009), and Zea mays (Anami et al. 2010). For example, Thawaro and Te-chato (2009) investigated differences in callus formation among zygotic embryos from six E. guineensis genotypes. Among the genotypes we tested, between 81.5 and 92.1% formed callus. Analysis of variance indicated that the frequency of callus induction and embryogenic callus induction differed significantly among genotypes (P = 0.000). We also tested whether immature zygotic embryos (Fig. 2a, b) from different genotype family trees differed in embryogenic callus induction. We used the optimal developmental stage (20 d after pollination, Fig. 2c) for explants. All immature zygotic embryos from 10 genotypes cultured on callus induction medium produced callus or embryogenic callus. However, the induction frequency differed significantly among the various genotypes. The induction frequency from the (06) 1–28 family tree (Fig. 2d) was the highest and reached 5.6% (Table 3). Therefore, (06) 1–28 was used to conduct the following assay.
Effect of PGRs on callus induction
The initiation of callus induction is significantly influenced by the optimization of PGR supplementation (Zhao et al. 2011; Zhou et al. 2012). A concentration series of 1, 2, and 4 mg L−1 2,4-D combined with 0.2 mg L−1 6-BA or KT was used to optimize PGR conditions for the induction of translucent callus. 6-BA and 2,4-D concentrations significantly affected the induction frequency of embryogenic callus (P = 0.000). Supplementation with different concentrations of 2,4-D alone resulted in embryogenic callus induction frequencies lower than those obtained with the other two combinations, indicating that 2,4-D alone was not the best protocol for embryogenic callus induction. Among all the 0.2 mg L−1 6-BA supplementation treatments with different concentrations of 2,4-D, the treatment with 2 mg L−1 2,4-D produced the highest embryogenic callus induction frequency, reaching 16.8% (Table 4). At higher concentrations of 2,4-D (4 mg L−1), somatic embryogenesis was inhibited (Table 4). This result was similar to that of a previous study, which indicated that higher auxin concentrations inhibit subsequent embryo development, although they are important for the induction of somatic embryogenesis (Khilwani et al. 2016). The combination of different concentrations of 2,4-D with KT produced lower embryogenic callus induction frequencies (Table 4). Therefore, the optimal PGR combination for embryogenic callus induction was ½ MS medium supplemented with 0.2 mg L−1 6-BA and 2 mg L−1 2,4-D, a result which differed from that of Kurtén et al. (1990).
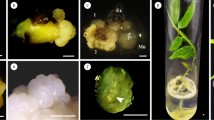
At the early stage of the culture period, the proliferation frequency of embryogenic callus was very low. Following successive culture for two or three time periods, the multiplication frequency increased substantially. We found that two different types of callus existed. Fine embryogenic callus was yellow in color with a loose structure (Fig. 3b), whereas non-embryogenic callus was white in color, strongly hard, and vigorously growing (Fig. 3a). Microscopy observations showed that non-embryogenic callus cells were large with a low cell content (Fig. 3c), whereas fine embryogenic callus cells were compact with an abundant cell content (Fig. 3d). Therefore, Fig. 3b with the cellular characteristics of Fig. 3d was classified as embryogenic callus.
Induction of embryogenic callus from immature zygotic embryos using the mother tree (06) 1–28. a Non-embryogenic callus of Betula platyphalla induced after 4 weeks of culture in ½ MS medium supplemented with 2.0 mg L−1 2,4-D, 0.5 mg L−1 KT, and 1 g L−1 casein hydrolysate. b Embryogenic callus of Betula platyphalla after 4-week culture in ½ MS medium supplemented with 2.0 mg L−1 2,4-D, 0.5 mg L−1 KT, and 1 g L−1 casein hydrolysate. Histological observation of c non-embryogenic callus and d embryogenic callus. a 1 cm, b 500 μm, c, d 50 μm
Development of SEs
After 2 months, the embryogenic callus was transferred to embryo development medium and produced SEs (Fig. 4a) of different developmental stages, including globular, heart, and cotyledonary embryos (Fig. 4b–d). The combination of 6-BA and NAA significantly affected the germination (P = 0.000) and development (P = 0.000) of SEs (Table 5). Always, hormone-free medium or lower concentrations of hormones have been shown to benefit the development of SEs (Li et al. 2011; Lu et al. 2017). This is probably because high hormone concentrations can inhibit SE tissue differentiation and affect the normal development of apical meristems (Halperin and Wetherell 1964). In the present study, we also found that hormone-free medium produced the highest germination frequency (88.2%) and conversion frequency (95.4%) (Table 5). Conversely, as concentrations of 6-BA and/or NAA increased, germination frequency and conversion frequency of germinated SEs decreased. The combination of 0.2 mg L−1 6-BA and 0.1 mg L−1 NAA produced the lowest germination frequency (46.7%) and conversion frequency of germinated SEs (65.4%) (Table 5). The effects of 6-BA alone or in combination with other PGRs on somatic embryo development or increasing plantlet conversion frequency has also been investigated in many species, such as Carica papaya L. (Solórzano-Cascante et al. 2018) and Phragmites communis (Yang et al. 2003). Germination of the embryos is a key or main limiting developmental stage in somatic embryogenesis regeneration, and germination frequency varies among different plant species (Sezgin and Dumanoğlu 2014; Manoharan et al. 2016; Lu et al. 2017). However, conversion was not synchronous in B. platyphylla. SEs may not have been sufficiently mature or the PGR combination may not have been suitable, causing shoots and roots to be incapable of synchronous development.
Development of SE and plantlet conversion after 4-week culture in ½ MS medium supplemented with 0.5 mg L−1 BA and 0.1 mg l−1 NAA. a Somatic embryos produced on embryogenic callus. b Early-stage cotyledonary embryos. c Middle-stage cotyledonary embryos. d Late-stage cotyledonary embryos. e Regenerated plantlet
Conversion of somatic embryos to plantlets and transplantation
Generally, SEs induced from callus in initial explant proliferation were isolated and maintained as clonal embryogenic lines. Plantlet regeneration efficiency and further growth characteristics were investigated for 4 weeks after the germinated single SEs were transferred to the regeneration medium. The isolated cotyledonary embryos were cultured in the same medium described above. Finally, a total of 85 regenerated plants grew well and were obtained after conversion (Fig. 4e). Approximately 95% of regenerated plantlets survived after acclimatization to greenhouse conditions.
Conclusion
We report a high-frequency direct somatic embryogenesis of B. platyphylla. The result showed the explants, genotypes and PGRs affect the embryogenic callus induction frequency significantly. The immature embryos that pollinate after 20 days was the best explants for embryogenic callus induction among all the tested explants, and the induction frequency from (06) 1–28 family tree was the highest. For all the different PGRs combination, 2 mg L−1 2,4-D and 0.2 mg L−1 produced the highest embryogenic callus induction frequency BA. After somatic embryos development, conversion and final transplantation, 95% of regenerated plantlets survived after acclimatization to greenhouse conditions. Up to now, there is no report concerning B. platyphylla somatic embryogenesis. The protocol established in this study will improve large-scale vegetative propagation, and also applicable to genetic modification of B. platyphylla via transformation.
References
Anami SE, Mgutu AJ, Taracha C, Coussens G, Karimi M, Hilson P, Van Lijsebettens M, Machuda J (2010) Somatic embryogenesis and plant regeneration of tropical maize genotypes. Plant Cell Tiss Org Cult 102:285–295
Bonga JM (1995) A comparative evaluation of the application of somatic embryogenesis, rooting of cuttings, and organogenesis of conifers. Can J For Res 45:379–383
Chen AH, Yang JL, Niu YD, Yang CP, Liu GF, Yu CY, Li CH (2010) High-frequency somatic embryogenesis from germinated zygotic embryos of Schisandra chinensis and evaluation of the effects of medium strength, sucrose, GA3, and 6-BA on somatic embryo development. Plant Cell Tiss Organ Cult 102:357–364
Cho N, Lee HK, Jeon BJ, Kim HP, Lee JH, Kim YC, Sung SH (2014) The effects of Betula platyphylla bark on amyloid beta-induced learning and memory impairment in mice. Food Chem Toxicol 74:156–163
Corredoira E, San-José MC, Vieitez AM (2012) Induction of somatic embryogenesis from different explants of shoot cultures derived from young Quercus alba trees. Trees 26:881–891
Eom HJ, Kang HR, Choi SU, Kim KH (2017) Cytotoxic triterpenoids from the barks of Betula platyphylla var. japonica. Chem Biodivers 14:e1600400
Eom HJ, Kang HR, Kim HK, Jung EB, Park HB, Kang KS, Kim KH (2016) Bioactivity-guided isolation of antioxidant triterpenoids from Betula platyphylla var. japonica bark. Bioorg Chem 66:97–101
Fiuk A, Rybczyński JJ (2008) Genotype and plant growth regulator-dependent response of somatic embryogenesis from Gentiana spp. leaf explants. Vitro Cell Dev Biol-Plant 44:90–99
Gaj MD (2001) Direct somatic embryogenesis as a rapid and efficient system for in vitro regeneration of Arabidopsis thaliana. Plant Cell Tissue Org Cult 64:39–46
Halperin W, Wetherell DF (1964) Adventive embryony in tissue cultures of the wild carrot. Daucus Carota Am J Bot 51(3):274–283
Hu R, Sun Y, WuB Duan H, Zheng H, Hu D, Lin H, Tong Z, Xu J, Li Y (2017) Somatic embryogenesis of immature cunninghamia lanceolata (Lamb) hook zygotic embryos. Sci Rep 7(1):56
Huh JY, Lee S, Ma EB, Eom HJ, Baek J, Ko YJ, Kim KH (2018) The effects of phenolic glycosides from Betula platyphylla var japonica on adipocyte differentiation and mature adipocyte metabolism. J Enzyme Inhib Med Chem 33(1):1167–1173
Khilwani B, Kaur A, Ranjan R, Kumar A (2016) Direct somatic embryogenesis and encapsulation of somatic embryos for in vitro conservation of Bacopa monnieri (L.) Wettst. Plant Cell Tiss Organ Cult 127:433–442
Kurtén U, Nuutila AM, Kauppinen V, Rousi M (1990) Somatic embryogenesis in cell cultures of birth (Betula pendula Roth.). Plant Cell Tiss Organ Cult 23:101–105
Lara-Chavez A, Flinn BS, Egertsdotter U (2011) Initiation of somatic embryogenesis from immature zygotic embryos of Oocarpa pine (Pinus oocarpa Schiede ex Schlectendal). Tree Physiol 31(12):1422
Li M, Wang S, Feng D (2011) The advance of plant somatic embryogenesis and development. Chin Agric Sci Bull 27(03):237–241
Lu D, Wei W, Zhou W, McGuigan LD, Ji F, Li X, Xing Y, Zhang Q, Fang K, Cao Q, Qin L (2017) Establishment of a somatic embryo regeneration system and expression analysis of somatic embryogenesis-related genes in Chinese chestnut (Castanea mollissima Blume). Plant Cell Tiss Organ Cult 130:601–616
Manoharan R, Tripathi JN, Tripathi L (2016) Plant regeneration from axillary bud derived callus in white yam (Dioscorea rotundata). Plant Cell Tiss Organ Cult 126:481–497
Matsuda H, Ishikado A, Nishida N, Ninomiya K, Fujiwara H, Kobayashi Y, Yoshikawa M (1998) Hepatoprotective, superoxide scavenging, and antioxidative activities of aromatic constituents from the bark of Betula platyphylla var. japonica. Bioorg Med Chem Lett 8:2939–2944
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Pais MS (2019) Somatic embryogenesis induction in woody species: the future after OMICs data assessment. Front Plant Sci 10:240
Phillips GC, Hubstenberger JF, Hansen EE (1995) Plant regeneration from callus and cell suspension cultures by somatic embryogenesis. In: Gamborg OL, Phillips GC (eds) Plant cell tiss organ cult: fundamental methods. Springer, Heidelberg, pp 81–90
Prakash MG, Gurumurthi K (2010) Effects of type of explant and age, plant growth regulators and medium strength on somatic embryogenesis and plant regeneration in Eucalyptus camaldulensis. Plant Cell Tiss Organ Cult 100:13–20
Sezgin M, Dumanoģlu H (2014) Somatic embryogenesis and plant regeneration from immature cotyledons of European chestnut (Castanea sativa Mill). Vitro Cell Dev Biol-Plant 50:58–68
Solórzano-Cascante P, Sánchez-Chiang N, Jiménez VM (2018) Explant type, culture system, 6-benzyladenine, meta-topolin and encapsulation affect indirect somatic embryogenesis and regeneration in Carica papaya L. Front Plant Sci 9:1769
Stasolla C, Yeung EC (2003) Recent advances in conifer somatic embryogenesis: improving somatic embryo quality. Plant Cell Tiss Org Cult 74:15–35
Thawaro S, Te-Chato S (2009) Effect of genotypes and auxins on callus formation from mature zygotic embryos of hybrid oil palms. J Agric Tech 5:167–177
Yang JL, Niu YD, Yang CP, Liu GF, Li CH (2011) Induction of somatic embryogenesis from female flower buds of elite Schisandra chinensis. Plant Cell Tiss Organ Cult 106:391–399
Yang YG, Guo YM, Guo Y, Guo ZC, Lin JX (2003) Regeneration and large-scale propagation of Phragmites communis through somatic embryogenesis. Plant Cell Tiss Organ Cult 75:287–290
Zhao W, Zheng S, Ling HQ (2011) An efficient regeneration system and Agrobacterium-mediated transformation of Chinese upland rice cultivar Handao297. Plant Cell Tiss Organ Cult 106(3):475–483
Zhou HC, Li M, Zhao X, Fan XC, Guo AG (2012) Plant regeneration from in vitro leaves of the peach rootstock ‘Nemaguard’ (Prunus persica×P. davidiana). Plant Cell Tiss Organ Cult 101(1):79–87
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This research was supported by the National Key Research and Development Programme of China (No. 2017YFD0600603), the 111 Project (No. B16010), and the Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team).
The online version is available at https://www.springerlink.com
Corresponding editor: Yu Lei.
Rights and permissions
About this article
Cite this article
Yang, J., Yang, D., Lü, W. et al. Somatic embryogenesis and plant regeneration in Betula platyphalla. J. For. Res. 32, 937–944 (2021). https://doi.org/10.1007/s11676-020-01131-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-020-01131-9