Abstract
Cerastigma willmottianum Stapf (Plumbaginaceae, Cerastigma), a native Chinese plant, has decorative and medicinal values and is used in green areas and landscapes. However, wild plants have small flowers and scattered, pendulous branches with lower compactness, making them inferior as ornamentals. Polyploidization can improve ornamental properties and carbon sequestration capacity, providing novel germplasms with higher carbon dioxide assimilation abilities based on plant-based C biosequence. This study induced polyploidy in apical buds in vitro through tissue culturing and application of colchicine, trifluralin, or pendimethalin; in the most efficient protocol, explants were cultured for 7 days in MS medium supplemented with 1200.0 μM trifluralin and 2% dimethyl sulfoxide (DMSO), with a survival rate of 74.45% and polyploidization rate of 17.78%. After 5–8 successive generations obtained by cutting aseptic apical buds for purification, ploidy level was effectively and precisely identified by morphological observation, stomatal measurement, DNA content analysis, and chromosome counting. According to our breeding goal, the morphology and photosynthetic capacity of tetraploids were screened. Compared to diploids, tetraploids have improved ornamental properties, including greater compactness, larger flowers and heart-shaped leaves, and a greater net photosynthetic rate, with higher CO2 assimilation efficiency. We developed an integrated protocol for the induction, purification, screening and identification of tetraploid C. willmottianum plants. Moreover, the newly developed tetraploid germplasm can be utilized to breed new cultivars. We hope that our research will lead to novel garden plants with improved ornamental value and high carbon sequestration ability, contributing to carbon neutrality.
Key message
An optimal integrated protocol for the polyploidization, purification, screening and identification of C. willmottianum was developed. A new tetraploid germplasm with improved ornamental value and high carbon sequestration ability was obtained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After the first Industrial Revolution, the emission of CO2, a representative greenhouse gas, has caused increasingly severe global warming. Therefore, countries around the world have proposed a concept called carbon neutrality, which aims to neutralize CO2 emissions by afforestation and reductions in air pollution emission (Ravilious 2020). Plants absorb CO2 and release O2 through the process of photosynthesis. Green vegetation plays a critical role in offsetting atmospheric CO2. Many flowering plants have multifunctional ecological and pharmaceutical values. As an increasingly environmentally friendly industry, the use of flowering plants has formed an intricate industrial chain, linking environmental protection, medical treatment and health care, which are predominant in the construction of human settlements and wellness (Kalluri et al. 2020; Niazian and Nalousi 2020; Tuna et al. 2020; Zhang et al. 2020a). China, known as the ‘Mother of Gardens’, has bred unique and valuable varieties of wild plant resources, including Cerastigma willmottianum Stapf (Huang 2011; Li et al. 2014).
C. willmottianum Stapf (family: Plumbaginaceae; genus: Cerastigma), commonly known as purple lotus, is a perennial deciduous subshrub that is a native wild plant in China. It was first found in the Minjiang River Valley in Sichuan Province. Due to its strong resistance, extensive management, elegant purple flowers and long flowering period (from May to December), it is regarded as a valuable plant among endemic wild plants applied in landscaping. However, the branches of mature diploids (2n = 2x = 12) tend to be scattered and pendulous with lower compactness, which decreases the ornamental value of the plant. The roots of C. willmottianum are rich in some chemically active ingredients, such as plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone), which has anticancer, antibacterial and other properties. Therefore, this species has been applied in folk medicine in China, India and other countries. Moreover, it is considered a Bach flower remedy plant and is made into a ‘Natural Therapy’ healthcare product that can soothe negative emotions (Li et al. 2014; Li 2016). Taken together, these results indicate that C. willmottianum is a multifunctional plant with ornamental, health care and medicinal values. However, to date, researchers have focused on its medicinal ingredients, tissue cultures and biological characteristics (Yue et al. 1997; Li 2016; Hu et al. 2019; Gao et al. 2021). Owing to the lack of germplasm innovation and research and the development of new cultivars, the promotion and application of C. willmottianum germplasm resources are limited.
The artificial induction of polyploidy is an effective and feasible breeding method to modify plant traits. It can cause changes in morphology (e.g., the ‘giga effect’, which causes gigantism of organs), physiological structure (stomatal size), biochemical substances in vivo (the contents of chlorophyll and photosynthetic active enzymes) and photosynthetic capacity. It has been reported that after polyploidization, the general capacity for CO2 assimilation improves, and the photosynthetic capacity of polyploid plants is higher than that of the original diploid plants (Warner and Edwards 1989; Vyas et al. 2007; Iannicelli et al. 2020; Martin et al. 2019; Niazian and Nalousi 2020). Therefore, polyploidization is a useful strategy for absorbing excess CO2 from the atmosphere through the photosynthetic process to achieve carbon neutrality.
Induction, purification and identification are all indispensable parts of polyploid breeding. For induction, it is important to determine the best induction system, explant type and size and mutagen concentration and exposure duration (Eng and Ho 2019). To date, aside from the longest and most widely used chemical colchicine, herbicides such as pendimethalin and trifluralin have been increasingly used due to their small required doses and high effectiveness (Dhooghe et al. 2011; Eng and Ho 2019; Jiang et al. 2019). DMSO is a common penetrating agent and mutagen adjuvant. It can promote rapid cell penetrations by mutagens, significantly increasing the polyploidization rate and decreasing the chimerism rate (Dhooghe et al. 2011; Eng and Ho 2019). Moreover, to accelerate the process of polyploid breeding, it is important to understand how to purify induced seedlings and quickly identify their ploidy levels after induction. Currently, direct adventitious shoot regeneration and serial generation by cutting organs are commonly used methods for purification. Finally, polyploids are identified by stomatal measurement, flow cytometry (FCM) and chromosome counting (Dhooghe et al. 2011; Eng and Ho 2019; Iannicelli et al. 2020; Niazian and Nalousi 2020).
Based on the ability of polyploidization to modify plant traits and enrich the diversity of germplasm resources, this study induced polyploidy in C. willmottianum in vitro with a combination of tissue cultures and chemical mutagens (pendimethalin, trifluralin and colchicine). The objectives are threefold: (1) to develop a target-oriented protocol for polyploidization with efficient induction, fast purification and precise identification by comparing the survival rate and tetraploid induction rate achieved when using mutagens, (2) to evaluate the agronomic traits of C. willmottianum in the field and achieve tetraploid strains with greater compactness and larger flowers, and (3) to assess the photosynthetic capacity to test the development of tetraploid strains with higher CO2 assimilation efficiency. Our results can fill a gap by creating a protocol for polyploidization of the genus Cerastigma based on tissue cultures and chemical mutagenesis. Moreover, they can also be applied to enrich the varieties of multifunctional plants in cities and provide a scientific basis for selecting plants that capture CO2 efficaciously to achieve carbon neutrality.
Materials and methods
Plant materials
Sterile apical shoots with buds and leaves were collected from our previously established aseptic seedlings of C. willmottianum (2n = 2x = 12). The experiment was conducted in the laboratory of the Landscape Architecture Institute at Sichuan Agricultural University, Chengdu, China.
The mutagens included in this study were colchicine (Shanghai Meryer Chemical Technology Co., Ltd., China), trifluralin (Zhenjiang Jiansu Pesticide Chemical Co., Ltd., China) and pendimethalin (India United Phosphates Co., Ltd.).
Polyploid induction based on tissue cultures and chemical mutagenesis
Filter-sterilized 2% DMSO was first added to the prepared medium (4.42 g L−1 MS + 30 g L−1 sucrose + 7 g L−1 agar; pH = 5.80; high-pressure sterilization), and filter-sterilized colchicine, trifluralin and pendimethalin were then added. For the sake of clarity, medium supplemented with the mutagens is referred to as mutagenic medium. Then, cut apical buds of aseptic seedlings were inoculated on solid mutagenic medium on an ultraclean bench. The concentrations and durations of the three mutagen treatments were determined by a secondary saturated D-optimal design (Table S1). There was a total of 18 treatments with three mutagens and 2 controls, which were referred to as Control 1 (2% DMSO) and Control 2 (the blank control). Each treatment contained 30 explants, and the experiment was repeated 3 times. After induction, the explants were rinsed in sterile water 4–5 times; then, the portion of the stem that was in contact with the mutagenic medium was excised, and the rest of the plant material was transferred to and continuously incubated in solid rooting medium (2.21 g L−1 MS + 30 g L−1 sucrose + 7 g L−1 agar + 0.5 mg L−1 IBA; pH 5.80; high-pressure sterilization) (referring to methods described by Hu et al. 2019) for 45 days. During this period, the survival rate and morphological variation rate were observed and recorded. In particular, to obtain purified plantlets, we cut plant used the method of serial generation by cutting plant organs. That is, the apical buds of explants with observed morphological variations were cut and subcultured in solid rooting medium every 15–20 days for 5–8 generations. The plants were held in the culture media at room temperature (25 ± 1 °C), with humidity maintained at 70 ± 5%, a photoperiod of 14 h/day, and a light intensity of 1500–2000 lx.
Tetraploid identification
Stomatal measurement
Preliminarily, plantlets with stable morphological variations were screened from the purifications, and stomata were observed and measured under a microscope (Olympus BX53 + DP80, Tokyo, Japan) with a 40 × objective lens (referring to methods described by Zhang and Gao (2021) with slight modifications). In particular, three leaves from each plantlet were measured, and each leaf was measured 3 times, three randomly sampled microscopic-field areas of each production were measured, for a total of 27 samples. In comparison to diploid plants, there were plantlets with morphological variations (named potential plantlets and abbreviated PP); these plantlets, which had enlarged and relatively low numbers of stomata, were regarded as having stomatal variation and were selected for the next test.
Flow cytometry analysis and chromosome counting
The nuclear DNA content of the PP, compared with that of diploid plants, i.e., the internal reference, was estimated using FCM (BD Accuri® C6 Plus, USA). PPs having a doubled nuclear DNA content were selected to conduct chromosome counting. The experiments were conducted following the methods described by Jiang et al. (2019) with slight modifications.
Anatomical characteristics and ornamental traits of the tetraploids
To compare diploids with tetraploids, we first analyzed their anatomical characteristics. The anatomical structures of roots, stems and leaves of plants with the two ploidy levels (10 of each) were compared by examination under an Olympus BX53 + DP80 microscope. Then, we measured the stomata of plants with the two ploidy levels (30 of each); the method used for this analysis was the same as that applied for stomatal measurement (referring to methods described by Zhang and Gao (2021) with slight modifications). To clarify, the ‘two ploidy levels’ mentioned in this paragraph refer to uniformly grown aseptic diploids and tetraploids (90 days old).
To quantify ornamental characteristics, first, traits such as plant height of potted diploids and tetraploids (60 of each) were measured (referring to methods described by Zhang et al. (2020b) with slight modifications). Then, the values of the LAI (leaf area index, equal to total leaf area/the ground shadow of the plant canopy, abbreviated S1/S2) for plants with the two ploidy levels (30 of each) was determined following the methods described by ASEF et al. (2020) and Silva et al. (2019) with slight modifications. To clarify, the ‘two ploidy levels’ mentioned in this paragraph refer to uniformly grown potted diploids and tetraploids (180 days old).
CO2 fixation ability of tetraploids
According to the morphological data, we randomly chose ten tetraploids with greater compactness and larger flowers and ten diploids; these plants were potted during the same growth period and were used for the following experiments.
Diurnal gas exchange patterns under field conditions
Parameters such as the net photosynthetic rate (Pn), intercellular carbon dioxide concentration (Ci), stomatal conductance (Gs), transpiration (Tr), water use efficiency (WUE) and vapor pressure deficit (VPD) were measured by a CIRAS-3 portable photosynthesis system (American PP Systems Co., Ltd.) (referring to methods described by García-García et al. (2020) with slight modifications).
Chlorophyll fluorimeter measurements
The minimal values of chlorophyll a fluorescence (Fo), maximum fluorescence (Fm) and variable fluorescence of PS II (Fv, equal to (Fm-Fo)/Fm) were measured by a Handy PEA fluorimeter (Hansatech, UK). In the present study, the third to fifth leaves of each plantlet from top to bottom were measured from 9:00 to 11:00 on a sunny morning (referring to the protocols described by Jiang et al. (2019) and García-García et al. (2020), with slight modifications).
Photosynthetic pigments
The chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Chl a + b) and chlorophyll a/chlorophyll b (Chl a/b) contents were measured at 646 nm and 663 nm by a full wavelength Multiskan GO microplate reader (Thermo Fisher Science Oy, Ratastie 2, FI-01620 Vantaa, Finland) (referring to methods described by Warner and Edwards (1989), with slight modifications).
Statistical analysis
In this study, the data were analyzed by IBM SPSS statistics 22.0. The data were assessed by ANOVA with the HSD-Tukey test or Pearson correlation analysis. A P value less than 0.05 was considered a significant difference or significant correlation. The tables and graphs were made by Microsoft Excel 2010 and Adobe Photoshop CC 2018.
Results
Tetraploid induction by trifluralin, pendimethalin and colchicine
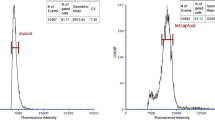
In this study, a total of 109 PPs were screened from 469 surviving aseptic seedlings by a combination of morphological observation and stomatal measurement. Then, we conducted an analysis with FCM. As shown in Fig. 1, the fluorescence intensity of the main peak of the tetraploids was approximately twice as strong as that of the diploids. Next, the chromosome numbers were determined (Fig. 2).
During a period of normal culture for 45 days after induction, there were differences in the survival rate between the two controls and treatments with three mutagens (Table 1). The analysis performed with SPSS showed that there was no significant difference in the survival rate between Control 1 (supplemented with 2% DMSO) and Control 2 (the blank control) (P = 0.601). Furthermore, there was a significant difference in the survival rate between Control 1 and the colchicine-treated group (P = 0.000). Combined with observations, these results indicated that colchicine was extremely harmful to the explants and that they experienced difficultly rooting, leading to curly leaves and brown leaf margins after treatment. The average survival rate of all the explants treated with colchicine was as low as 13.16%. Furthermore, colchicine failed to induce tetraploidy (Table 2). As shown in Table 2, the polyploidization rate of trifluralin was higher than that of pendimethalin. Further multifactor variance analysis showed that treatment with trifluralin, the treatment concentration, the treatment duration and their interaction all led to significant differences (P = 0.000, P = 0.001 and P = 0.003, respectively). The effect of the concentration was greater than that of the duration (partial Eta squared values: concentration (0.740) > duration (0.691)). Taken together, the results of this study indicated that an optimal protocol for tetraploid induction of C. willmottianum was treating aseptic apical buds with 1200.0 μM trifluralin and 2% DMSO for 7 days, with a survival rate of 74.45% and a polyploidization rate of 17.78%.
Anatomical and morphological features of the tetraploids
To compare the diploids with tetraploids, we first observed and analyzed their anatomical structures (Tables 3, 4; Fig. 3a-j). For the tetraploid plants, except for the stomatal density which decreased, increases were seen in all of the structures, including the diameters of the root and stem; thicknesses of the leaf, leaf epidermal cells, palisade tissue, spongy tissue, and main vein; and the lengths and widths of the stomata and guard cells. Apart from the thickness of the leaf epidermal cells (P = 0.112), the other parameters were all significantly different (spongy tissue: P = 0.002; remaining structures: P = 0.000).
Comparison of anatomical structures of diploids (left) and tetraploids (right). Root cross-section (a, b); Stem cross-section (c, d); Leaf cross-section(e, f, g, h. SA, stomata; UE, upper epidermal; LE, lower epidermal; MV, main vein; PT, palisade tissue; ST, spongy tissue); Guard cells and stomata (i, j)
Among the morphological characteristics of the tetraploids (Table 5; Fig. 4a-f), plant height, stem diameter, canopy diameter, leaf length, leaf shape index and internode length were reduced; however, leaf width, leaf thickness, flower diameter, cylinder length and leaf area index increased. Except for the stem diameter, leaf thickness, internode length and leaf area index (P = 0.343, P = 0.490, P = 0.061 and P = 0.384, respectively), significant differences were observed for all the parameters (all P = 0.000). In addition, we found that white granules (Fig. 4g-j) on the leaf surface and epidermal hairs (Fig. 4k, l) of the leaf margin were more obvious for tetraploids than for diploids.
CO2 fixation ability of tetraploids
As shown in Fig. 5, the contents of Chl a, Chl b and total chlorophyll were higher in the tetraploids than in the diploids, and there were significant differences in the contents between ploidy levels (all P = 0.000). Chl a/b was slightly lower in the tetraploids than in the diploids but showed no significant difference (P = 0.079). Furthermore, Fm/Fo, Fv/Fo and Fv/Fm, which were determined by chlorophyll fluorimeter measurement, were not significantly different between the diploid and tetraploid plants (P = 1.000, P = 1.000 and P = 0.742, respectively) (Table 5). For the parameters of the diurnal gas exchange patterns between the two ploidy levels (Fig. 6), first, the same trend (a bimodal curve) was observed for Pn, Gs and Ci, which increased to a maximum value and then decreased gradually. The Ci values of the diploids and tetraploids both peaked at 9:30 (420.44 ± 14.06 μmol·mol−1 and 377.44 ± 13.09 μmol·mol−1, respectively). However, throughout the day, the maximum Pn and Gs values of the tetraploid plants (8.24 ± 1.19 μmol·m−2·s−1 and 179.78 ± 5.99 mmol·m−2·s−1, respectively) were reached 1 h later than those of the diploid plants (6.37 ± 0.35 μmol·m−2·s−1 and 229.556 ± 57.82 mmol·m−2·s−1, respectively). Except from 13:30 to 15:30, the value of Ci in the tetraploids was always lower than that in the diploids. From the correlation coefficients of the daily pattern of photosynthetic characteristic parameters (Table 6), we found that there was an extremely significant positive correlation of the Pn and Gs between the diploids and tetraploids.
Comparison of photosynthetic pigment contents of diploids and tetraploids (Chl a, Chl b, Chl a + b, Chl a/b). Value representing the mean ± SE (standard error) of ten samples of each ploidy. Different capital letters based on Duncan’s test at P ≤ 0.05 indicating significant differences between diploids and tetraploids by two-sample t-test
Discussion
An optimal protocol for tissue culture and chemical mutagenesis for the induction and identification of tetraploids
Artificial polyploidization with chemical mutagens is the most effective and important way to acquire polyploids of garden plants, and it has many advantages, such as a low cost, simplicity, strong specificity and ability to produce a wide spectrum of mutations (Eng and Ho 2019). Currently, colchicine is widely used as an antimitotic agent. However, this chemical is more toxic than alternatives, and its ability to depolymerize plant tubulins is not as good as that of dinitroaniline herbicides, including pendimethalin and trifluralin (Castillo et al. 2009; Eng and Ho 2019). In this study, the colchicine treatment produced explants with curly leaves, brown leaf margins and very low rooting rates, with an average survival rate that was lower than those of pendimethalin and trifluralin. Therefore, in terms of explant damage, the colchicine treatment resulted in the most damage, followed by trifluralin and pendimethalin; this result is consistent with those of previous studies (Aslam et al. 2017; Ren et al. 2018; Jiang et al. 2019). Tetraploids were induced successfully by colchicine in maize (Zea mays L.) (Aslam et al. 2017) and tillered onion (Allium cepa L. var. aggregatum G. Don) (Ren et al. 2018), but the application of this chemical failed to produce tetraploid plants in this study. We speculate that plants of different genotypes and explant types have different sensitivities and tolerances to different mutagens (Eng and Ho 2019; Niazian and Nalousi 2020). Our research suggests that aseptic apical buds may not be suitable for colchicine treatment, that the treatment time at low concentrations was too short to double the number of chromosomes in C. willmottianum, or that colchicine was so harmful to the explants exposed to higher concentration for a long time that many dead explants may be polyploids (Eng and Ho 2019).
DMSO is a sulfur-containing organic compound used as a penetrating agent. It has been reported that adding 1%-2% DMSO to treatments can promote the absorption of mutagenic solutions by plant cells, causing a higher polyploidization rate. However, the toxicity of DMSO may affect the survival rate of plants (Eng and Ho 2019). Hamill et al. (1992) found that treatment with 2% DMSO increased the mortality rate of banana (Musa nana Lour., 'SH-3362'). In our study, Control 1 was treated with 2% DMSO, and the survival rate of plants in Control 1 was nearly equal to that in Control 2, which did not contain DMSO. DMSO had few side effects on the survival rate in this study, which differs from previously reported results.
The identification of polyploids is vital to determining polyploid induction efficiency. To date, polyploid identification methods have included morphological observation, stomatal measurement, FCM and chromosome counting (Eng and Ho 2019). In general, for the tetraploids, the lengths and widths of the stomata were greater than those of the diploids, while the stomatal density was lower in the tetraploids; similar findings were observed for Chinese privet (Ligustrum sinense Lour.) (Fetouh et al. 2020) and cultivated gerbera (Gerbera hybrida; Asteraceae) (Bhattarai et al. 2021). Stomatal measurements can be used as a preliminary screening method to determine whether plantlets are polyploids. However, stomatal mutations and environmental factors can also cause changes in stomata. In short, additional analyses are needed to confirm the presence of polyploidy (Eng and Ho 2019). FCM is a fast and effective method to estimate the nuclear DNA content of samples and determine the ploidy level. However, the results will be affected by the freshness of the samples and the cytoplasmic compounds. Thus, FCM is used only as an indirect method for screening large numbers of explants after induction with its time-saving and increased efficiency of identification (Ochatt et al. 2011; Talebi et al. 2017; Eng and Ho 2019; Zhang and Gao 2020). Although chromosome counting is direct and intuitive, it is necessary to conduct chromosome preparation. Therefore, using this method to identify the polyploidy level is difficult on a large scale (Ochatt et al. 2011).
To date, four methods have been applied for the identification of ploidy level (Madani et al. 2015; Ravandi et al. 2013; Hannweg et al. 2016; Talebi et al. 2017; Fetouh et al. 2020; Zhang and Gao 2020, 2021; Zhang et al. 2020b); however, these methods are relatively time-consuming and laborious, and there are no obvious advantages among them. Ren et al. (2018) combined the four methods in the following order to identify polyploids: morphological observation, stomatal size measurement, chromosome counting, and FCM. However, this process requires a certain amount of time, and fresh roots are required to count the number of chromosomes. Chromosome production requires highly skilled experimentalists and easily fails. Large-scale chromosome identification is time-consuming. FCM can be performed with fresh materials obtained at any time and is carried out in a stepwise manner under an experimental protocol with demonstrated success; furthermore, application of this method can save time (Eng and Ho 2019). Therefore, we believe that it is more efficient and practical to conduct FCM analysis before chromosome counting for polyploidy identification. In this study, we developed an efficiently integrated method for screening and identifying polyploids that includes morphological observation, stomatal measurement, DNA content analysis, and chromosome counting.
Assessment of ornamental features and CO2 fixation ability of tetraploids
Generally, polyploidization increases the size of cells, causing changes in their anatomical structure and morphological characteristics. It also affects the photosynthetic process (Vyas et al. 2007; Coate et al. 2012; Eng and Ho 2019). In this study, we observed and analyzed the anatomical structures of plants at two ploidy levels. We found that the sizes of the cells and tissues in the roots, stems and leaves of the tetraploids increased in comparison to those of the diploids, which is consistent with the findings of Jiang et al. (2019). Morphologically, polyploidization causes a ‘giga’ effect, resulting in larger organs (leaves and flowers) and changes in leaf shape (Hannweg et al. 2016; Mariana et al. 2016; Niazian and Nalousi 2020). This study found that tetraploids had shortened internodes, dwarf stature and canopy diameters that were reduced by half. The C. willmottianum tetraploids were transformed into more compact plants than the diploids. Furthermore, there was a significant difference in the leaf shape index, and the leaf shape changed from an obovate rhombus to a heart shape. These results are similar to the results of Ravandi et al. (2013), Fetouh et al. (2016), Hannweg et al. (2016), and Mo et al. (2020).
It has been reported that CO2 fixation in leaves is proportional to the LAI (Doughty and Goulden 2008; Choi et al. 2014). Field measurements of the LAI of the tetraploids were higher than those of the diploids, which provides preliminarily evidence that the tetraploids absorbed more CO2 for fixation. Photosystem II (PS II) and photosystem I (PS I), included in the photoreaction stage, represent the ability of plants to fix CO2 and synthesize carbohydrates. Furthermore, plants use chlorophyll to collect and transmit light energy to the reaction center for the photoreaction stage of photosynthesis, synthesizing NADPH and ATP, which are used to fix CO2 in the carbon reaction stage. Thus, the chlorophyll content can, to some extent, represent and is generally positively correlated with the photosynthetic capacity of a plant (Jajoo et al. 2001; Cenzano et al. 2013; Chen et al. 2019; Eng and Ho 2019). In this study, the contents of total chlorophyll, Chl a and Chl b in the tetraploids were all significantly higher than those in the diploids. This demonstrated that the tetraploids had a higher ability to collect and transmit light energy to PS II and PS I, which is consistent with the results of Madani et al. (2015) and Cao et al. (2018). Fo, Fm and Fv in PS II are measured by a chlorophyll fluorimeter to analyze their state of reactivity and reflect the physiological conditions of photosynthesis (Demmig-Adams et al. 1989; Cai and Feng 2020). This study found that Fm/Fo, Fv/Fo and Fv/Fm were almost equal between the diploid and tetraploid plants. In other words, the transportation of electrons of PS II, the activity of PS II and the maximal quantum yield of PS II were almost equal to each other. The Pn, determined by both PS II and PS I, is proportional to the CO2 assimilation ability of a plant (Jajoo et al. 2001). This study found that the Pn of the tetraploids was always higher than that of the diploids, which meant that there was more CO2 fixation in the tetraploids. Therefore, we speculate that the PS I of the tetraploids had stronger reactivity and thus could synthesize more NADPH and ATP to be used in the carbon reaction stage or that the tetraploids had a greater ability to fix CO2 in the carbon reaction stage. Moreover, some studies have shown that when Pn and Gs are positively correlated, a higher Gs causes a higher Ci with more CO2 available for assimilation (Yuan et al. 2018; Chen et al. 2019, 2021). In this study, we found that there was an extremely significant positive correlation of the Pn and Gs between the diploids and tetraploids. The Gs and Ci of the tetraploids on most of the days were both lower than those of the diploids, according to the measurements of the diurnal gas exchange patterns. In other words, the stomatal conductance and intercellular CO2 concentrations of the tetraploids were both lower than those of the diploids. However, the Pn of the tetraploids was higher than that of the diploids on most of the days. Further analysis indicated that tetraploids have a higher efficiency of CO2 fixation. In conclusion, for the tetraploids, the related results of greater LAI, higher chlorophyll content, and higher Pn with a positive correlation with Gs verified that the efficiency of CO2 assimilation by the tetraploids was higher than that by the diploids.
Conclusions
In this study, we have unprecedentedly developed an efficiently integrated protocol for the polyploidization, purification, screening and identification of C. willmottianum (Fig. 7). Compared with diploids, tetraploids have unique characteristics, including their morphology, anatomy and photosynthetic ability. Tetraploid plants have larger flowers, heart-shaped leaves and greater compactness than diploid plants, greatly increasing their ornamental value. They have a greater ability to fix CO2, with a higher efficiency of CO2 assimilation, during the photosynthetic process. Taken together, these results indicate that the novel tetraploid germplasm can not only enrich the supply of novel plants in green areas and landscapes but also absorb excess CO2 from the atmosphere through the photosynthetic process to achieve carbon neutrality.
Change history
06 April 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11240-022-02302-8
Abbreviations
- CO2 :
-
Carbon dioxide
- DMSO:
-
Dimethyl sulfoxide
- FCM:
-
Flow cytometry
- LAI:
-
Leaf area index
- PS I:
-
Photosystem I
- PS II:
-
Photosystem II
- Pn:
-
Net photosynthetic rate
- Ci:
-
Intercellular carbon dioxide concentration
- Gs:
-
Stomatal conductance
- Tr:
-
Transpiration
- WUE:
-
Water use efficiency
- VPD:
-
Vapor pressure deficit
- Fm:
-
Maximum fluorescence
- Fo:
-
Minimal value of chlorophyll a fluorescence
- Fv:
-
Variable fluorescence of PS II
- Fv/Fm:
-
Maximal quantum yield of PS II
- Fv/Fo:
-
Maximum primary yield of photochemistry of PS II
- Fm/Fo:
-
The transportation of electrons of PS II
References
Asef SM, Tolba O, Fahmy A, (2020) The effect of leaf area index and leaf area density on urban microclimate. J Eng Appl Sci 67(2):427–446
Aslam M, Farid B, Khakwani K, Maqbool MA, Zou HW (2017) In vivo maternal haploid seed production and chromosome doubling with different anti-microtubular agents in maize. Int J Agric Biol 19(1):114–120
Bhattarai K, Kareem A, Deng ZA (2021) In vivo induction and characterization of polyploids in gerbera daisy. Sci Hortic 282:110054. https://doi.org/10.1016/j.scienta.2021.110054
Cai LJ, Feng YY (2020) Effects of exogenous ABA on the physiological characteristics and chlorophyll fluorescence of Gynura cusimbua seedlings under drought stress. IOP Conf Ser Earth Environ Sci 615:012089. https://doi.org/10.1088/1755-1315/615/1/012089
Cao QZ, Zhang XQ, Gao X, Wang LJ, Jia GX (2018) Effects of ploidy level on the cellular, photochemical and photosynthetic characteristics in Lilium FO hybrids. Plant Physiol Bioch 133:50–56. https://doi.org/10.1016/j.plaphy.2018.10.027
Castillo AM, Cistué L, Vallés MP., Soriano M (2009) Chromosome Doubling in Monocots. In: Alisher Touraev, Brain P. Forster, S. Mohan Jain (Eds) Advances in Haploid Production in Higher Plants. Springer, Dordrecht. PP: 329–338
Cenzano AM, Varela MC, Bertiller Mó, nica B, Luna MV, (2013) Effect of drought on morphological and functional traits of Poa ligularis and Pappostipa speciosa, native perennial grasses with wide distribution in Patagonian rangelands. Argentina Aust J Bot 61(5):383–393. https://doi.org/10.1071/BT12298
Chen S, Wang ZC, Guo XP, Rasool G, Zhang J, Xie Y, Hamoud YA, Shao GC (2019) Effects of vertically heterogeneous soil salinity on tomato photosynthesis and related physiological parameters. Sci Hortic 249:120–130. https://doi.org/10.1016/j.scienta.2019.01.049
Chen ZY, Chai SF, Qi HH, Wei YG, Cen HF (2021) Diurnal variation of photosynthetic physiological characteristics of Kadsura coccinea (Lem.) A. C. Smith. Agri Biotechnol 10(4):48–50
Choi EG, Jin BO, Kim CH, Kim MH, Baek GY, Moon BE, Lee SY, Kim HT (2014) Estimate of the LAI to calculate the CO2 consumption of lettuce leaf. Acta Hortic 1037(1037):801–806
Coate JE, Luciano AK, Seralathan V, Minchew KJ, Owens TG, Doyle JJ (2012) Anatomical, biochemical, and photosynthetic responses to recent allopolyploidy in Glycine dolichocarpa (Fabaceae). Am J Bot 99(1):55–67. https://doi.org/10.3732/ajb.1100465
Demmig-Adams B, Adams WW, Winter K, Meyer A, Schreiber U, Pereira JS, Krü A, ger, Czygan FC, Lange OL, (1989) Photochemical efficiency of photosystem II, photon yield of O2 evolution, photosynthetic capacity, and carotenoid composition during the midday depression of net CO2 uptake in Arbutus unedo growing in Portugal. Planta 177(3):377–387. https://doi.org/10.1007/BF00403596
Dhooghe E, Laere K, Eeckhaut T, Leus L, Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tiss Org 104(3):359–373. https://doi.org/10.1007/s11240-010-9786-5
Doughty CE, Goulden ML (2008) Seasonal patterns of tropical forest leaf area index and CO2 exchange. J Geophys Res-Biogeo. https://doi.org/10.1029/2007JG000590
Eng WH, Ho WS (2019) Polyploidization using colchicine in horticultural plants: A review. Sci Hortic 246(1):604–617. https://doi.org/10.1007/s11240-010-9786-5
Fetouh MI, Kareem A, Knox GW, Wilson SB, Deng ZA (2016) Induction, identification, and characterization of tetraploids in Japanese rivet (Ligustrum japonicum). HortScience 51(11):1371–1377. https://doi.org/10.21273/HORTSCI11138-16
Fetouh MI, Deng ZA, Wilson SB, Adams CR, Gary WK (2020) Induction and characterization of tetraploids in Chinese Privet (Ligustrum sinense Lour.). Sci Hortic 271:109482. https://doi.org/10.1016/j.scienta.2020.109482
Gao SP, Li WJ, Hong MT, Lei T, Shen P, Li JN, Jiang MY, Duan YF, Shi LS (2021) The nonreciprocal heterostyly and heterotypic self-incompatibility of Ceratostigma willmottianum. J Plant Res 134(3):543–557. https://doi.org/10.1007/s10265-021-01269-5
García-García AL, Grajal-Martín MJ, González-Rodríguez ÁM (2020) Polyploidization enhances photoprotection in the first stages of Mangifera indica. Sci Hortic 264(109):198. https://doi.org/10.1016/j.scienta.2020.109198
Geng XS, Xiao SQ, Ge XG (2010) Midday depression of photosynthesis of plant. Biology Teaching 35(12):59
Hamill SD, Smith MK, Dodd WA (1992) In vitro induction of banana autotetraploids by colchicine treatment of micropropagated diploids. Aust J Bot 40(6):887–896. https://doi.org/10.1071/BT9920887
Hannweg K, Visser G, de Jager K, Bertling, (2016) In vitro-induced polyploidy and its effect on horticultural characteristics, essential oil composition and bioactivity of Tetradenia riparia. S Afr J Bot 106:186–191. https://doi.org/10.1016/j.sajb.2016.07.013
Hu J, Gao SP, Liu SL, Hong MT, Zhu Y, Wu YC, Hu D, Zhang L, Lei T (2019) An aseptic rapid propagation system for obtaining plumbagin of Ceratostigma willmottianum Stapf. Plant Cell Tiss Org 137(2):369–377. https://doi.org/10.1007/s11240-019-01577-8
Huang HW (2011) Plant diversity and conservation in China: planning a strategic bioresource for a sustainable future. Bot J Linn Soc 166(3):282–300. https://doi.org/10.1111/j.1095-8339.2011.01157.x
Iannicelli J, Guariniello J, Tossi VE, Regalado JJ, Di Ciaccio L, van Baren CM, Pitta Á, lvarez SI, Escandó, n AS, (2020) The “polyploid effect” in the breeding of aromatic and medicinal species. Sci Hortic 260:108854. https://doi.org/10.1016/j.scienta.2019.108854
Jajoo A, Bharti S, Mohanty P (2001) Evaluation of the specific roles of anions in electron transport and energy transfer reactions in photosynthesis. Photosynthetica 39(3):321–337. https://doi.org/10.1023/A:1015125008028
Jiang YL, Liu SL, Hu J, He GT, Liu YQ, Chen X, Lei T, Li Q, Yang LJ, Li WJ, Hu D, Li JN, Gao SP (2019) Polyploidization of Plumbago auriculata Lam. in vitro and its characterization including cold tolerance. Plant Cell Tiss Org. https://doi.org/10.1007/s11240-019-01729-w
Kalluri UC, Yang XH, Wullschleger SD (2020) Plant biosystems design for a carbon-neutral bioeconomy. BioDesign Res 1:48–52
Li Z (2016) Preliminary study on the geographical distribution and seed germination of medical ornamental plants Ceratostigma willmottianum Stapf. Dissertation, Sichuan Agricultural University.
Li Z, Bai W, Tang XH, Wang H, Shang D, Liu YS, Liu J (2014) Research status on medical ornamental plants Ceratostigma willmottianum Srapf. J Anhui Agric Sci 42:8951–8953
Liu FL, Jensen CR, Shahanzari A, Andersen MN, Jacobsen S-E (2004) ABA regulated stomatal control and photosynthetic water use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Sci 168(3):831–836. https://doi.org/10.1016/j.plantsci.2004.10.016
Madani H, Hosseini B, Dehghan E, Rezaei-chiyaneh E (2015) Enhanced production of scopolamine in induced autotetraploid plants of Hyoscyamus reticulatus L. Acta Physiol Plant 37(3):1–11. https://doi.org/10.1007/s11738-015-1795-x
Mariana S, Carlos C, Wellington C (2016) The polyploidy and its key role in plant breeding. Planta 243(2):281–296. https://doi.org/10.1007/s00425-015-2450-x
Martin Č, Radka S, Martin W, Jan S, Filip K (2019) Ploidy-altered phenotype interacts with local environment and may enhance polyploid establishment in Knautia serpentinicola (Caprifoliaceae). New Phytol 221(2):1117–1127. https://doi.org/10.1111/nph.15426
Mo L, Chen JH, Lou XZ, Xu QW, Dong RH, Tong ZK, Huang HH, Lin E (2020) Colchicine-induced polyploidy in rhododendron fortunei lindl. Plants (basel, Switzerland) 9(4):424. https://doi.org/10.3390/plants9040424
Niazian M, Nalousi AM (2020) Artifcial polyploidy induction for improvement of ornamental and medicinal plants. Plant Cell Tiss Org 142:447–469. https://doi.org/10.1007/s11240-020-01888-1
Ochatt SJ, Patat-Ochatt EM, Moessner A (2011) Ploidy level determination within the context of in vitro breeding. Plant Cell Tiss Org 104(3):329–341. https://doi.org/10.1007/s11240-011-9918-6
Ravandi EG, Rezanejad E, Zolala J, Dehghan E (2013) The effects of chromosome-doubling on selected morphological and phytochemical characteristics of Cichorium intybus L. J Hortic Sci Biotech 88(6):701–709. https://doi.org/10.1080/14620316.2013.11513027
Ravilious K (2020) Life in a carbon-neutral world. Phys World 33(4):32–37
Ren J, Wu XX, Song C, Liang Y, Gao WZ, Wang Y (2018) Induction of polyploid tillered onion using colchicine and pendimethalin. Sains Malays 47(11):2617–2624. https://doi.org/10.17576/jsm-2018-4711-04
Silva JRd, Heldwein AB, Puhl AJ, Amarante AAd, Salvadé DM, Santos CJOGd, Leonardi M (2019) Leaf area estimation in chamomile. J Agr Sci 11(2):429. https://doi.org/10.5539/jas.v11n2p429
Talebi SF, Saharkhiz MJ, Kermani MJ, Sharafi Y, Raouf Fard F (2017) Effect of different antimitotic agents on polyploid induction of anise hyssop (Agastache foeniculum L.). Caryologia 70(2):184–193. https://doi.org/10.1080/00087114.2017.1318502
Tuna A, Ay BH, Karakuş Ş (2020) Integration of medicinal and aromatic plants in an urban landscape as a living hBhattaeritage: an example in Malatya City (Turkey). Environ Monit and Assess 192(8):548. https://doi.org/10.1007/s10661-020-08498-6
Vyas P, Bisht MS, Miyazawa SI, Yano S, Noguchi K, Terashima I, Funayama-Noguchi S (2007) Effects of polyploidy on photosynthetic properties and anatomy in leaves of Phlox drummondii. Funct Plant Boil 34(8):673–682. https://doi.org/10.1071/FP07020
Wang LJ, Zhang Q, Cao QZ, Gao X, Jia GX (2020) An efcient method for inducing multiple genotypes of tetraploids Lilium rosthornii Diels. Plant Cell Tiss Org 141:499–510. https://doi.org/10.1007/s11240-020-01807-4
Warner DA, Edwards GE (1989) Effects of polyploidy on photosynthetic rates, photosynthetic enzymes, contents of DNA, chlorophyll, and sizes and numbers of photosynthetic cells in the C(4) Dicot Atriplex confertifolia. Plant Physiol 91(3):1143–1151. https://doi.org/10.1104/pp.91.3.1143
Yuan YG, Ge LT, Yang HS, Ren WZ (2018) A meta-analysis of experimental warming effects on woody plant growth and photosynthesis in forests. Journal of Forestry Research 29(3):727–733. https://doi.org/10.1007/s11676-017-0499-z
Yue JM, Xu J, Zhao Y, Sun HD, Lin ZW (1997) Chemical Components from Ceratostigma willmottianum. J Nat Prod 60(10):1031–1033. https://doi.org/10.1021/np970044u
Zhang XQ, Gao JY (2020) In vitro tetraploid induction from multigenotype protocorms and tetraploid regeneration in Dendrobium officinale. Plant Cell Tiss Org 141(2):289–298. https://doi.org/10.1007/s11240-020-01786-6
Zhang XQ, Gao JY (2021) Colchicine-induced tetraploidy in Dendrobium cariniferum and its effect on plantlet morphology, anatomy and genome size. Plant Cell Tiss Org 144(2):409–420. https://doi.org/10.1007/s11240-020-01966-4
Zhang J, Fu ZL, Chu ZX, Song BW (2020a) gastroprotective activity of the total flavones from abelmoschus manihot (L.) medic flowers. Evid-Based Compl Alt 2020:6584945. https://doi.org/10.1155/2020/6584945
Zhang YS, Chen JJ, Cao YM, Duan JX, Cai XD (2020b) Induction of tetraploids in “Red Flash” caladium using colchicine and oryzalin: Morphological, cytological, photosynthetic and chilling tolerance analysis. Sci Hortic 272:109524. https://doi.org/10.1016/j.scienta.2020.109524
Acknowledgements
This work was supported by the Sichuan Science and Technology Program (No. 2021YFYZ0006). Special thanks to American Journal Experts (AJE) for editing the language of the manuscript.
Funding
This article was funded by Sichuan Province Science and Technology Support Program, NO.2021YFYZ0006, Suping Gao.
Author information
Authors and Affiliations
Contributions
LSS, SPG and TL designed the research. SPG, TL and LJY supervised the whole research. LSS performed most of the experiments, analyzed the data and wrote the manuscript. YFD, QL conducted part of the experiments. JNL and ZAZ proposed helpful suggestions and collected resources. Moreover, thanks to people such as YML, WJL, LP, SCJ helped in the research.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Klaus Eimert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article has been revised: Table 2 has been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, L., Gao, S., Lei, T. et al. An integrated strategy for polyploidization of Cerastigma willmottianum Stapf based on tissue culture and chemical mutagenesis and the carbon dioxide fixation ability of tetraploids. Plant Cell Tiss Organ Cult 149, 767–782 (2022). https://doi.org/10.1007/s11240-022-02277-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02277-6