Abstract
A simple and efficient procedure was established for in vitro propagation of lattice henbane (Hyoscyamus reticulatus L.) using shoot tip explants cultured on Murashige and Skoog (MS) medium supplemented with N6-benzylaminopurine (BAP) and indolyl-3-acetic acid (IAA). In vitro-induced autotetraploid plants of H. reticulatus L. exhibiting high yield of scopolamine were successfully induced by different concentrations of colchicine solutions (0.00, 0.05, 0.1, 0.2 and 0.5 %) which were applied for 24, 48 and 72 h. Treated shoot tips were regenerated on MS medium supplemented with 8.8 mM BAP and 2.2 mM IAA and rooted on ½MS medium containing 2.2 mM indolyl-3-butyric acid, then were acclimatized and transferred to soil. According to the results, 0.1 % (w/v) of colchicine for 48 h can be effective for induction of polyploidy in H. reticulatus L. The induced tetraploid plants represented a different structural, physiological and biochemical characteristics. Autotetraploid plants of H. reticulatus also showed a higher conversion of hyoscyamine to scopolamine as scopolamine content was increased from 0.23 in diploids to 8.66 % in the induced tetraploid plants. Regarding the higher commercial value of scopolamine than the other tropane alkaloids, tetraploidy can efficiently be used to improve scopolamine production of H. reticulatus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lattice henbane (Hyoscyamus reticulatus L.) is one of the most important medicinal plants in solanaceae family (Bahmanzadegan et al. 2009). This species is native to arid and semi-arid regions of south-west Asia and Iran (Deivis 1978). The plant consists of an erect, simple or branched stem, covered with basal and cauline leaves petiolate. The corolla is pale yellowish, soon becoming purplish-violet. It is found on cereal fields and waste places from 10 to 1,700 m (Deivis 1978). Hyoscyamine, scopolamine and atropine are of the most important tropane alkaloid compounds which were found in lattice henbane (Manske and Holmes 1950). Hyoscyamine and scopolamine are inhibitor drugs of parasympathetic nervous system, also are competitive antagonist of muscarinic acetylcholine receptors (Kutchan 1995). Scopolamine is also applied to suppress the central nervous system, whereas hyoscyamine excites it (Ionkova 2010). Considering world demand for scopolamine which is about 10 times higher than the hyoscyamine, many attempts have been conducted to optimize production of scopolamine in plants and hairy root cultures (Ghafarzadegan et al. 2010). Because many tropane alkaloid production species accumulate hyoscyamine as the major alkaloid and scopolamine in minor quantities, it is of commercial importance to increase scopolamine content in this species (Hashimoto and Yamada 1994).
Polyploidy is a widespread phenomenon in the evolution of flowering plants and is considered as a key element in plant speciation and diversification (Wendel and Doyle 2005). In plants, it was estimated that polyploidy has an occurrence between 30 and 70 % (Wolfe 2001). Polyploid plants may have a wide variety of uses including overcoming hybridization barriers, improved stress tolerance, improved pest resistance, and restoring fertility in wide hybrids (Levin 1983). Polyploid plants can have larger flowers and large thick leaves with shorter internodes (Pryor and Frazier 1968) and increased concentration and/or qualitative changes in active plant bio-compounds (Lavania 2005). Induction of polyploidy in plants has been of considerable interest for plant breeders and has been used for obtaining new plant characteristics (Cheng and Korban 2011).
With the development of tissue culture techniques, in vitro polyploidization has become the main method for induction of polyploid plants (Zhang et al. 2008). Because of high effectiveness and convenience, tissue culture-mediated induction of tetraploidy is more advantageous than traditional methods (Gao et al. 1996). The in vitro induction of polyploidy using colchicine has been reported in several plants, such as Centella asiatica (Kaensaksiri et al. 2011), Artemisia annua (Lin et al. 2011), Dendranthema nankingense (Liu et al. 2011), Lagerstroemia indica (Zhang et al. 2010) and Dioscorea zingiberensis (Heping et al. 2008). Artificial polyploidy was applied successfully for increasing scopolamine accumulation in plants and hairy root cultures of H. muticus (Dehghan et al. 2012). We do not know how tetraploidy changes biomass production and profile of tropane alkaloid accumulation in H. reticulatuce plant. So our aim was to develop an efficient protocol to regenerate and induce tetraploidy in vitro in H. reticulatus L. for the first time. We also want to investigate whether the ploidy manipulation in this plant is able to change profile of tropane alkaloid accumulation toward scopolamine or not.
Materials and methods
Plant materials and in vitro regeneration
The seeds of lattice henbane were collected from the foothills surrounding areas of Naqhadeh. The seeds were surface sterilized in 70 % (v/v) ethanol for one min, then sterilized 10 min in 12 % (v/v) NaOCl, and finally washed three times by sterile distilled water, then were cultured in MS medium (Murashige and Skoog 1962). Approximately 2 weeks after seed germination, the apical parts of the seedlings were used as explants for plant proliferation on MS medium supplemented with 3 % sucrose, 7.2 gl−1 Agar (Duchefa, Netherlands) and 0.1 gl−1 myo-inositol (Duchefa, Netherlands). To optimize shoot proliferation, three levels of BAP (0, 4.4 and 8.8 mM) and three levels of IAA (0, 1.1 and 2.2 mM) were supplemented to culture medium. The pH of medium was adjusted to 5.8 before autoclaving. Five explants per jar were grown on MS media and provided with 16 h artificial light (fluorescent tube giving light intensity 3,500 Lux) followed by 8 h dark period, at 25 °C and the explants were allowed to grow for a period of 3 weeks.
Root formation was induced in ½MS medium supplemented with 2.2 mM IBA. The cultures were incubated in a growth chamber at 25 °C under a 16/8 h (light/dark) photoperiod. The regenerated plants were acclimatized in the plastic pots containing sterilized soil and sand mixture in the growth chamber at 25 °C under a 16/8 h (light/dark) photoperiod. Thereafter, they were transferred to the greenhouse and maintained in the similar conditions.
Induction and establishment of tetraploid plants
Proliferated shoot apicals with 1 cm length were used as a source of explants for colchicine treatment. In vitro tetraploid was induced in solid MS medium supplemented with 3 % sucrose, 7.2 gl−1 agar (Duchefa, Netherlands), 2 % dimethyl sulfoxide (DMSO) and filter-sterilized colchicine (0.0, 0.05, 0.1, 0.2 and 0.5 %) for 24, 48 and 72 h at 25 °C under dark condition. Each treatment contained 75 explants in 3 replicates (25 explants per replicate). The treated shoot apicals were washed three times (2–3 min) with sterilized distilled water and transferred to MS medium (pH 5.7) containing 3 % sucrose, 7.2 gl−1 agar and 8.8 mM BAP and 2.2 mM IAA at 25 °C under a 16/8 h (light/dark) photoperiod at a photon flux rate of 60 μmol m−2 s−1, provided by cool-white fluorescent tubes. Explants were subcultured on to the similar fresh media every 3 weeks and after 2 months all of the regenerated shoots placed in the rooting medium, ½MS containing 3 % sucrose, 7 gl−1 agar and 2.2 mM IBA. Rooted plants were acclimatized and transferred to the greenhouse in conditions as mentioned above.
Flow cytometry experiments
The ploidy level of putative tetraploid and control plants was measured by flow cytometry (partec PA, Germany). Fresh leaves were first harvested and nuclei suspensions were prepared by chopping the leaf tissue (50 mm2) of colchicine’s treated and diploid (control) plants, with a fresh razor blade in 400 µl of nuclei extraction buffer (Kit A, Partec PA, Germany) for 30–60 s. After filtrations through a 30-µm Partec Cell-Trice disposable filter, 1,600 µl of 4-6 diamidino 2-2 phenylindole (DAPI, provided by Partec PA, Germany) was added. A minimum of 5,000 nuclei were measured per sample and histograms of DNA content were generated using Mode Fit software (Dehghan et al. 2012).
Chromosome counting
Root tips were pretreated with 8-hydroxyquinoline (C9H7NO) for 4 h and fixed in Carnoy’s solution (glacial acetic acid: 95 % ethanol, 1:3) for 1 day then were kept in 70 % ethanol, for 24 h. After the fixative was washed out with water, the fixed roots were hydrolyzed in 1 N HCl at 60 °C for 15 min. The samples were prepared by soaking the tissues in hematoxylin and eosin stains for 50 min at 60 °C and squashing the samples with 45 % glacial acetic acid on the microscopic slide. The chromosome numbers were observed under a light Olympus microscope with 100× magnification.
Measurement of several structural and physiological characteristics
Selected morphological and physiological characteristics of tetraploid plants (confirmed by flow cytometry and chromosome karyotyping) were compared with diploid control plants. Leaf characteristics of diploid and putative tetraploids were measured from in vitro material and fully established glasshouse plants (6 months old). Size and density of stomata on the abaxial surface of leaves were measured. For leaf sampling, three branches were randomly selected from the tetraploid and diploid plants, and from each branch, the fully developed basal leaves were observed under 100× magnifications using a fluorescence microscope (Olympus CX21, Olympus America Inc.). Stomata in 25 microscopic fields were counted for each leaf. Counts were taken twice per leaf at random locations across the surface in the unit of 0.15 mm2 (Lin et al. 2011). Other morphological characteristics such as length and width of leaf, length of petiole and thickness of petiole and leaves were also measured.
The content of a and b chlorophylls and total leaf chlorophyll was estimated following Arnon’s (1949) equations 1, 2 and 3. The absorbance of the solutions was read at 645 and 663 nm for chlorophyll a and chlorophyll b, respectively.
Extraction and analysis of tropane alkaloids
Tropane alkaloid of solid tetraploids and diploid plants was extracted by the method of Kamada et al. (1986). Four grams of powdered plant material from leafs were homogenized in chloroform, methanol and 25 % ammonia (75:25:5). After 130 min at room temperature in dark condition, the homogenate was filtered through paper filter and was washed 2 times with 1 ml chloroform. Then solvent was evaporated and dried with rotary evaporator. 50 ml chloroform and 20 ml 1 N H2SO4 were added and mixed completely. Chloroform fraction was removed and the solvent was adjusted to pH 10 by 25 % ammonia. After addition of anhydrous Na2SO4, the solvent was filtered through filter paper then the residue was washed with 1 ml chloroform. At the final stage, after evaporation, the powder was dissolved in 1–2 ml MeOH and the obtained extract was injected to Gas chromatography–mass spectrometry. GC–MS analyses were performed with a Shimadzu GCMS-QP5050A spectrometer with DB-1 columns (60 m length, internal diameter 0.25 mm and layer thickness 0.25 µm). Injector temperature was set at 290 °C. Helium was the carrier gas, at a flow rate of 0.8 ml/min. 1.0 µl of diluted samples (1/10 in methanol v/v) was injected in the split/splitless (10:1 split) mode. The identification of alkaloids was based on the comparison of their GC retention time and mass spectra (MS) data with their standard substances (HYO. HCl and SCO. HBr, Merck).
Statistical analysis
All experiments were done as a factorial based on randomized complete design with three replications. Analysis of variance (ANOVA) of the results was performed using one-way ANOVA. Means were compared using Duncan’s multiple range test (DMRT) at the 95 % confidence level (P ≤ 0.05) using SAS 9.1 software.
Results and discussion
In vitro regeneration of lattice henbane
In vitro shoot multiplication and root induction were successfully performed in lattice henbane. Shoot bud development and shoot regeneration were significantly (P < 0.05) affected by media containing various levels of BAP in combination with IAA. Among different treatments, the highest number of shoots (about 13 shoots per explant) was obtained when 8.8 mM BAP and 2.2 mM IAA were supplemented to MS medium (Figs. 1, 3).
Effects of different BAP and IAA concentrations on the average proliferated shoots of Hyoscyamus reticulatus L. Means within each column with different letters are significantly different at P = 0.05 (Duncan). I1 = 0 µM IAA, I2 = 1.1 µM IAA, I3 = 2.2 µM IAA, B1 = 0 µM BAP, B2 = 4.4 µM BAP, B3 = 8.8 µM BAP
Effects of basal MS media and different concentrations of IBA and IAA on the average number of roots in Hyoscyamus reticulatus L. Means within each column with different letters are significantly different at P = 0.05 (Duncan). S1 = MS, S2 = ½MS, I1 = 1.1 µM IAA, I2 = 2.2 µM IAA, I3 = 1.1 µM IBA, I4 = 2.2 µM IBA, I5 = Control
The shoot apical explants responded very well to the applied plant growth regulators, producing developed shoots after 3 weeks of culture (Fig. 3). In this study, IBA treatment was very effective for inducing roots in proliferated shoots. The roots were induced 3 weeks after transferring of shoots on solid ½MS medium containing 3 % sucrose and 2.2 mM IBA (Figs. 2, 3). Most probably, high concentration of cytokinin stimulates development of meristems and at its optimal concentration promotes shoot proliferations, and the inclusion of low concentration of auxin(s) along with cytokinin(s) triggers the rate of shoot proliferations (Maharana et al. 2012). The results of present study indicated the effects of plant growth regulators on micropropagation of lattice henbane. Kang et al. (2004) reported that supplementation of 3 % sucrose was the optimum level for shoot and root formation from rhizomes of Scopolia parviflora, a tropane alkaloid producing species. Their study also indicated the effectiveness of IBA for induction of roots. In the Artemisia absinthium plants, the highest regeneration rate belonged to the treatments containing 2.2 mM BAP and 0.5 mM NAA (Zia et al. 2007). It has been reported that MS medium containing 2.2 mM BAP and 1.4 mM IAA has been a suitable medium for direct regeneration of Basil plants (Gopi et al. 2006). Similar results were also reported for several medicinal plants such as Atropa acuminate (Ahuja et al. 2002), Papaver somniferum (Ovečka et al. 1997), Agastachi anisata (Kayani et al. 2013), Atropa belladonna (Asha Rani and Prasad 2013), Echinacea purpurea (Koul et al. 2010), Solanum nigrum (Sundari et al. 2010) and Phyllanthus amarus (Bhattacharyya and Bhattacharya 2001).
Induction and confirmation of tetraploidy
The effect of colchicine on survival rate of explants was assessed 15 days after treatment. The entire colchicine-treated explants demonstrated lower survival rate than those of the control ones (Table 1). The observed inverse relationship between the concentration of colchicine and survival rate of explants was consistent with the other studies on different species, such as Cichorium intybus (Ghotbi Ravandi et al. 2013) Gerbera jamesonii (Gantait et al. 2011), Smallanthus sonchifolius (Viehmannová et al. 2009), Phlox subulata (Zhang et al. 2008), Astragalus memberanaceus (Chen and Gao 2007), Chaenomeles japonica (Stanys et al. 2006), Zizyphus jujuba (Gu et al. 2005) and Humulus lupulus L. (Roy et al. 2001). Lower survival rates may be due to a physiological disturbance caused by spindle inhibitor activity of colchicine, resulting in a reduced rate of cell division (Swanson 1957). In addition, there is an inverse relation between DNA content and development rate of the cells (Bennett 1972).
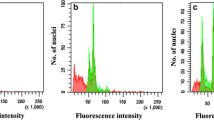
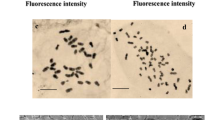
In vitro plantlets with different ploidy levels were regenerated using appropriate concentration of plant growth regulators in the culture media. The ploidy level of the surviving plants was first determined by morphological and structural characteristics and then by flow cytometry, followed by chromosome counting. As it is represented in Fig. 4, flow cytometry experiments confirmed the colchicine-induced autotetraploidy of lattice henbane. The G1 peak of diploid plants (2x) was at channel 36 while it was at channel 72 for induced tetraploids (4x) (Fig. 4). Various mixoploid plants were also observed as two fluorescence peaks appeared, one at channel 36 and the other at channel 72, which indicated that they possessed both diploid and tetraploid nuclei. In consent with the other results, chromosome counting also confirmed tetraploidy of the plants (Table 2; Fig. 5). The chromosome number of diploid plants was 2n = 2x = 34 and that of the tetraploid plants was 2n = 4x = 68.
The highest number of tetraploid (12 %) and mixoploid (60 %) plants was obtained when the apical meristems were cultured in the MS medium supplemented with 3 % sucrose, 2 % dimethyl sulfoxide (DMSO) and 0.1 % colchicine for 48 h (Table 1). Similar to our results, the highest number of tetraploid plants of Z. jujuba was obtained by treatment of shoot tip explants with 0.1 % colchicine for 48 h (Gu et al. 2005).
Because of the difference in division rate between central zone and peripheral zone, the cells in the apical meristem not equally affecting by colchicine treatment, so arising chimeric plants (mixoploids) with diploid and tetraploid cells are a usual effect of polyploidization by application of colchicine (Ghotbi Ravandi et al. 2013).
Comparison of structural and physiological characteristics
Several morphological and physiological characteristics were compared between the tetraploid and control diploid plants (Table 2; Figs. 6, 7). As shown in Fig. 6, stomatal characteristics of H. reticulatus L. were affected by the increase in ploidy level. Significant difference in stomatal size and frequency was also observed between diploid and induced tetraploid plants (Table 2; Fig. 6). While the stomatal density was significantly decreased by induction of tetraploidy, the average lengths and widths of the stomata were increased in tetraploid plants in comparison to that of the diploid counterparts. Roots of tetraploids were shorter, thicker and darker in color and more branched than those of the diploids.
Similar results were also reported for several tetraploid plants such as C. intybus (Ghotbi Ravandi et al. 2013), D. nankingense (Liu et al. 2011), C. asiatica L. (Kaensaksiri et al. 2011), L. indica (Zhang et al. 2010), Hyoscyamus muticus (Shahriari-Ahmadi et al. 2009) and Chrysanthemum cineraiifolium (Liu and Gao 2007). As it is shown in Table 2, several selected characteristics of lattice henbane were changed by manipulation of ploidy level. While the length of leaves and petioles was decreased by induction of tetraploidy, the thickness and width of leaves and petioles, leaf area and contents of chlorophyll a, b and total chlorophyll were increased in tetraploid plants in comparison to the diploid ones. The increase of chlorophyll content as a result of induction of tetraploidy has also been reported in Lavandula angustifolia (Urwin et al. 2007) and Acacia mearnsii (Mathura et al. 2006) and Salvia leriffolia (unpublished data).
Tropane alkaloids production in diploid and tetraploid H. reticulatus L.
The results showed the severe effect of ploidy level on hyoscyamine and scopolamine profiles of H. reticulatus plants. The hyoscyamine and scopolamine content of diploid plants was 0.26 and 0.23 % (DW), respectively. While hyoscyamine was not detected in the induced tetraploid plants, the scopolamine content was sharply increased (8.66 % DW) as a result of the ploidy level manipulation (Table 3). As it was already reported by Dehghan et al. (2012), it seems that the conversion of hyoscyamine to scopolamine was accelerated by increasing of the ploidy level. Therefore, the ratio of scopolamine/hyoscyamine was higher in tetraploid plants. Philipov and Berkov (2002) reported 2.63-(0.15–0.40 % DW) and 1.41-fold (0.05–0.08 % DW) increase of scopolamine and hyoscyamine content in the leaves of D. stramonium L., after induction of tetraploidy by colchicine treatment. In our study, the increase in scopolamine content and decrease in hyoscyamine content may be due to the higher activity of hyoscyamine-6-beta-hydroxylase (H6H) that catalyzes conversion of hyoscyamine to scopolamine (Dehghan et al. 2012). Our recent experiments indicated higher expression of h6h gene and sco/hyo ratio in tetraploid plants and genetically engineered tetraploid hairy root cultures of H. muticus than their diploid counterparts (unpublished data). Polyploidy often increases the production of secondary metabolites in medicinal plants (Dhawan and Lavania 1996). Polyploidy can also alter the relative amounts of different combinations of secondary metabolites (Chavadej and Becker 1984). As it is reported that main alkaloid contents of Hyoscyamus niger, H. muticus, H. albus and Datura innoxia were increased by manipulation of ploidy level (Lavania 2005). Recently Ghotbi Ravandi et al. (2013) reported the tetraploid plants of C. intybus with a significant increase in total phenolic compounds and chlorogenic acid content of leaves. In another study, Estaji et al. (unpublished data) observed qualitative and quantitative changes of metabolite production in induced tetraploid plants of S. leriffolia.
In autopolyploid, the basic genetic material remains the same, but gene dosage is multiplied and there is potential to modulate gene expression and hence enzyme activity per unit protein (Lavania 2005). Due to manipulation of ploidy, significant upregulation of several key enzymes related to artemisinin biosynthetic pathway was observed in autotetraploid plants of A. annua (Lin et al. 2011). In this study, tropane alkaloids were extracted from the leaves during flowering time. The highest amount of alkaloids was reported in the leaves at flowering time of H. reticulatus and other Hyoscyamus species such as H. muticus (Bahmanzadegan et al. 2009). Plant leaves were the most valuable organs for alkaloid accumulation followed by stems, roots, and crowns of D. innoxia Mill, plants (Al-Humaid 2003).
In conclusion, an efficient and rapid in vitro propagation method was developed through shoot tip cultures of H. reticulatus. In vitro-induced tetraploid plants of H. reticulatus L. with positive attitudes in morphological traits and scopolamine production were reported for the first time. In this research, the increase of scopolamine content was important due to its high pharmaceutical value.
Author contribution statement
Hadi Madani and Bahman Hosseini are responsible for tissue culture experiments, sampling, and chromosome counting and statistical analyses presented in this paper. Esmail Rezaei-chiyaneh is responsible for extraction of alkaloids and GC/MS analysis. Esmail Dehghan is contributed to flow cytometry analysis. All the authors read and approved the final manuscript.
References
Ahuja A, Sambyal M, Koul S (2002) In vitro propagation and conservation of Atropa acuminata Royle ex Lindl-An indigenous threatened medicinal plant. J Plant Biochem Biotechnol 11(2):121–124
Al-Humaid AI (2003) Effects of compound fertilization on growth and alkaloids of Datura innoxia Mill. plants. J Agr Rural Dev Trop. 104:151–165
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. J Plant Physiol 24(1):1
Asha Rani NS, Prasad MP (2013) Studies on the organogenesis of Atropa Belladonna in in-vitro conditions. J Biotechnol Bioeng 4:457–464
Bahmanzadegan JA, Sefidkon F, Sanboli A (2009) Determination of hyoscyamine and scopolamine in Four Hyoscyamus species from Iran. Iranian J Pharm Res 8(1):65–70
Bennett M (1972) Nuclear DNA content and minimum generation time in herbaceous plants. Proc Roy Soc Lon Ser B Biol Sci 181(1063):109–135
Bhattacharyya R, Bhattacharya S (2001) High frequency in vitro propagation of Phyllanthus amarus Schum. & Thom. by shoot tip culture. Indian J Exp Biol 39(11):1184–1187
Chavadej S, Becker H (1984) Influence of Colchicine treatment on chromosome number and growth rate of tissue Culture of Valeriana wallichii DC. Plant Cell Tiss Organ Cult. 3:265–272
Chen LL, Gao SL (2007) In vitro tetraploid induction and generation of tetraploids from mixoploids in Astragalus membranaceus. Sci Hort 112(3):339–344
Cheng ZM, Korban SS (2011) In vitro ploidy manipulation in the genomics era. Plant Cell Tiss Organ Cult 104:281–282
Dehghan E, Häkkinen ST, Oksman-Caldentey KM, Ahmadi FS (2012) Production of tropane alkaloids in diploid and tetraploid plants and in vitro hairy root cultures of Egyptian henbane (Hyoscyamus muticus L.). Plant Cell Tissue Organ Cult 110(1):35–44
Deivis TH (1978) Flora of Turkey, vol 6. University Press, Edinburg, pp 454–455
Dhawan OP, Lavania UC (1996) Enhancing the productivity of secondary metabolites via induced polyploidy: a review. Euphytica 87:81–89
Gantait S, Mandal N, Bhattacharyya S, Das PK (2011) Induction and identification of tetraploids using in vitro colchicine treatment of Gerbera jamesonii Bolus cv Sciella. Plant Cell Tissue Organ Cult 106(3):485–493
Gao S, Zhu D, Cai Z, Xu D (1996) Autotetraploid plants from colchicine-treated bud culture of Salvia miltiorrhiza Bge. Plant Cell Tissue Organ Cult 47(1):73–77
Ghafarzadegan R, Khadiv PP, Khalighi Sigaroudi F, Pirali Hamedani M, Kadkhoda Z, Rezazadeh SA (2010) Optimization of extraction method of hyoscine from hyoscyamus niger L. J Med Plants 9(36):87–95
Ghotbi Ravandi E, Rezanejad F, Zolala J, Dehghan E (2013) The effects of chromosome-doubling on selected morphological and phytochemical characteristics of Cichorium intybus L. J Hortic Sci Biotech 88(8):701–709
Gopi C, Sekhar YN, Ponmurugan P (2006) In vitro multiplication of Ocimum gratissimum L. through direct regeneration. Afr J Biotechnol 5:9
Gu X, Yang A, Meng H, Zhang J (2005) In vitro induction of tetraploid plants from diploid Zizyphus jujuba Mill. cv. Zhanhua. Plant Cell Rep 24(11):671–676
Hashimoto T, Yamada Y (1994) Alkaloid biogenesis: molecular aspects. Annu Rev Plant Phys 45:257–285
Heping H, Shanlin G, Lanlan C, Xiaoke J (2008) In vitro induction and identification of autotetraploids of Dioscorea zingiberensis. In Vitro Cell Dev-Pl 44(5):448–455
Ionkova I (2010) Tropane alkaloids in Hyoscyamus reticulatus L. In: Awaad AS, Govil JN, Singh VK Drug plants I. pp 373–389
Kaensaksiri T, Soontornchainaksaeng P, Soonthornchareonnon N, Prathanturarug S (2011) In vitro induction of polyploidy in Centella asiatica (L.) Urban. Plant Cell Tissue Organ Cult 107(2):187–194
Kamada H, Okamura N, Satake M, Harada H, Shimomura K (1986) Alkaloid production by hairy root cultures in Atropa belladonna. Plant Cell Rep 5(4):239–242
Kang Y, Min J, Moon H, Karigar C, Prasad D, Lee C, Choi M (2004) Rapid in vitro adventitious shoot propagation of Scopolia parviflora through rhizome cultures for enhanced production of tropane alkaloids. Plant Cell Rep 23(3):128–133
Kayani HA, Khan S, Naz S, Chaudhary MI (2013) Micropropagation of Agastache anisata using nodal segments as explants and cytotoxic activity of its methanolic extracts. Pak J Bot 45(6):2105–2109
Koul S, Sambyal M, Kitchlu S, Bakshi S, Kaul M (2010) Development, micropropagation and characterization of colchiploid of Echinacea purpurea (L.) Moench. Indian J Biotech 9:221–224
Kutchan TM (1995) Alkaloid biosynthesis [mdash] the basis for metabolic engineering of medicinal plants. Plant Cell 7(7):1059
Lavania U (2005) Genomic and ploidy manipulation for enhanced production of phyto-pharmaceuticals. Acta Hortic 3(2):170–177
Levin DA (1983) Polyploidy and novelty in flowering plants. Am Nat 122(1):1–25
Lin X, Zhou Y, Zhang J, Lu X, Zhang F, Shen Q, Wu S, Chen Y, Wang T, Tang K (2011) Enhancement of artemisinin content in tetraploid Artemisia annua plants by modulating the expression of genes in artemisinin biosynthetic pathway. Biotechnol Appl Bioc 58(1):50–57
Liu Z, Gao S (2007) Micropropagation and induction of autotetraploid plants of Chrysanthemum cinerariifolium (Trev.) Vis. In vitro Cell Dev-Pl 43(5):404–408
Liu S, Chen S, Chen Y, Guan Z, Yin D, Chen F (2011) In vitro induced tetraploid of Dendranthema nankingense (Nakai) Tzvel. shows an improved level of abiotic stress tolerance. Sci Hort 127(3):411–419
Maharana SB, Mahato V, Behera M, Mishra RR (2012) In vitro regeneration from node and leaf explants of Jatropha curcas L. and evaluation of genetic fidelity through RAPD markers. Indian J Biotechnol 11:280–287
Manske RRHF, Holmes HHL (1950) The alkaloids: chemistry and physiology V1. J Chem Physiol 1:1–525
Mathura S, Fossey A, Beck SL (2006) Comparative study of chlorophyll content in diploid and tetraploid black wattle (Acacia mearnsii). Forestry 79(4):381–388
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Omidbaigi R (1997) Approaches to production and processing of medicinal plants, vol 2. Tarrahane Nashr Public Tehran 14:70–78
Ovečka M, Bobák M, Šamaj J (1997) Development of shoot primordia in tissue culture of Papaver somniferum L. Biol Plant 39(4):499–506
Philipov S, Berkov S (2002) GC-MS investigation of tropane alkaloids in Datura stramonium. Z Naturforsch C 57(5/6):559–561
Pryor R, Frazier L (1968) Colchicine-induced tetraploid azaleas. Sci Hort 3(4):283–286
Roy A, Leggett G, Koutoulis A (2001) In vitro tetraploid induction and generation of tetraploids from mixoploids in hop (Humulus lupulus L.). Plant Cell Rep 20(6):489–495
Shahriari-Ahmadi F, Dehghan E, Farsi M, Azizi M (2009) Tetraploid induction of Hyoscyamus muticus L. using colchicine treatment. Pak J Biol Sci 11(24):2653–2659
Stanys V, Weckman A, Staniene G, Duchovskis P (2006) In vitro induction of polyploidy in Japanese quince (Chaenomeles japonica). Plant Cell Tissue Organ Cult 84(3):263–268
Sundari M, Benniamin A, Manickam V (2010) Micropropagation and in vitro flowering in Solanum nigrum Linn. a medicinal plant. Int J Biotechnol 1(1):29–32
Swanson CP (1957) Cytology and cytogenetics. Carl P, Swanson
Urwin NA, Horsnell J, Moon T (2007) Generation and characterisation of colchicine-induced autotetraploid Lavandula angustifolia. Euphytica 156(1–2):257–266
Viehmannová I, Cusimamani EF, Bechyne M, Vyvadilová M, Greplová M (2009) In vitro induction of polyploidy in yacon (Smallanthus sonchifolius). Plant Cell Tissue Organ Cult 97(1):21–25
Wendel J, Doyle J (2005) Polyploidy and evolution in plants. Plant diversity and evolution: genotypic and phenotypic variation in higher plants, p 97
Wolfe KH (2001) Yesterday’s polyploids and the mystery of diploidization. Nat Rev Genet 2(5):333–341
Zhang Z, Dai H, Xiao M, Liu X (2008) In vitro induction of tetraploids in Phlox subulata L. Euphytica 159(1–2):59–65
Zhang Q, Luo F, Liu L, Guo F (2010) In vitro induction of tetraploids in crape myrtle (Lagerstroemia indica L.). Plant Cell Tissue Organ Cult 101(1):41–47
Zia M, Rehman R, Chaudhary MF (2007) Hormonal regulation for callogenesis and organogenesis of Artemisia absinthium L. Afr J Biotechnol 6:16
Acknowledgments
This work was supported by Urmia University, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Y. Paek.
Rights and permissions
About this article
Cite this article
Madani, H., Hosseini, B., Dehghan, E. et al. Enhanced production of scopolamine in induced autotetraploid plants of Hyoscyamus reticulatus L.. Acta Physiol Plant 37, 55 (2015). https://doi.org/10.1007/s11738-015-1795-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1795-x