Abstract
Polyploids generally show strong environmental adaptation and diverse morphological variations. Therefore, polyploid induction is an important protocol for plant breeding. Lilium rosthornii is a wild lily species with high horticultural value and excellent disease resistance. In this study, seeds of L. rosthornii were subjected to polyploidy induction treatments to obtain multiple genotypes of tetraploids. Germinated seeds were immersed in two antimitotic agents at different concentrations for various times. In total, 199 tetraploid genotypes were obtained. The most efficient treatments of each agent were immersed in 0.05% colchicine for 36 h and in 0.01% oryzalin for 24 h; the induction rate of the former (27.78%) was significantly higher than that of the latter (22.22%). The swollen hypocotyl phenotype after colchicine and oryzalin treatments was strongly correlated with tetraploidy (0.989** and 0.975**, respectively), suggesting that this phenotype could serve as an early ploidy selection trait. The correlations were weaker between stomata length/density and tetraploidy (0.773** and 0.695**, respectively), implying that stomatal characters are affected by both the ploidy level and genotype. After several rounds subculture in vitro, the morphology and growth traits were not significantly different between diploids and tetraploids, but there were wider variations in these parameters in tetraploids than in diploids. After transplanting, the bulblet germination rate was higher in tetraploids than in diploids. The leaf phenotype did not differ between tetraploids and diploids initially, but the leaves of tetraploids became larger than those of diploids over time. Together, these suggest that tetraploids, may contribute diverse characteristics to lily breeding.
Key message
Multiple genotypes of tetraploid Lilium rosthornii were induced by colchicine and oryzalin. Swollen hypocotyl was associated with polyploidy. Compared with diploids, tetraploids showed wider variations in morphological parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyploid induction is an important protocol for plant breeding. According to the chromosome origin, polyploids can be classified as allopolyploids and autopolyploids. Compared with diploids, most allopolyploids show superior growth and stress resistance because of heterosis and heterozygosity, whereas autopolyploids may show either enhanced or reduced these traits due to differences in heterozygosity among different genotypes (Podwyszyńska et al. 2014, 2018; Pozo and Elena 2015; Zhang et al. 2017). Wild plants have many genes and diverse phenotypes that may have been lost from cultivated varieties, so they are important germplasm resources (Kasteele and Stoop 1974). To acclimatize wild plants, seeds with multiple genotypes may provide wider range of morphological variations and stress resistance (Acosta-Gallegos et al. 2007; Tian et al. 2013; Mei et al. 2009). Chromosome duplication using seeds as the starting material can yield multi-genotypic autopolyploids with diverse heterozygosity (Bingham et al. 1994; Eliášová et al. 2014; Pozo and Ramirez-Parra 2014).
The efficiency of polyploidy induction depends on many variables, including explant type, the type and concentration of the antimitotic agent, and the exposure time. The most commonly used antimitotic agent is colchicine, which has a high affinity for microtubules in animal cells but a much lower affinity for plant tubulins (Dhooghe et al. 2010; Morejohn et al. 1984). Alternative antimitotic agents, such as oryzalin, trifluralin, surflan, and amiprohpos methyl (APM), have been used in previous studies (Khosravi et al. 2008; Nimura et al. 2006). Their efficiency depends on their concentration, as well as exposure time and solvent (Petersen et al. 2003, 2010; Zhang et al. 2017; Zhou et al. 2016). Leaves, stem apex, and seeds are the most widely used plant materials in induction treatments (Tu et al. 2018; Yu et al. 2015). The pre-culture period of plant materials also affect the polyploid induction efficiency (Kafawin and Chen 1991). For the induction of polyploids in Lilium, scales and shoots are the most widely used materials to date (Emsweller and Brierley 1940; Zhang et al. 2017). These studies for polyploidy induction using scales or shoots have used only a few individuals, so that the characteristics of polyploidization may have reflected only a few genotypes. Seeds, produced by fertile lily, may have multiple genotypes (Lim et al. 2008). However, seeds have rarely been used for polyploid induction in lily. Frist, hybrid seeds may lose the target characteristics, because character segregation may occur during seed formation (Lim et al. 2008). Second, many important variates are interspecific hybridization, such as OA hybrids, which are infertility and cannot produce seeds (Barba-Gonzalez et al. 2006; Tuyl and Arens 2010). Unlike varieties, seeds are an important propagative organ for majority of wild Lilium species (Liang and Tamura 2000).
Lilium rosthornii (2n = 2x) is a wild lily species with high horticultural value and excellent disease resistance (Du 2014). To make full use of this excellent material, we devised a method to induce polyploids of L. rosthornii. To establish an efficient method for inducing multiple genotypes of tetraploids, seeds were treated with two antimitotic agents at various concentrations for a range of exposure times. Moreover, to select abundant variations for acclimation and further breeding, we compared the morphological characters and growth rate between the genotypes of diploids and tetraploids in tissue culture and after transplanting.
Materials and methods
Seed pre-culture
Mature seeds of L. rosthornii were collected from Hunan Province. After 2 months of stratification in cold sand, the disinfected seeds were cultured on germination medium (Murashige–Skoog (MS) medium containing 0.02 mg L−1 naphthylacetic acid (NAA), 30 g L−1 sugars, and 6 g L−1 agar, pH 5.8). The plant materials were incubated at 23 ± 2 °C, under a 16/8 h (light/dark) photoperiod with light (40 μm m−2 s−1) supplied by cool-white fluorescent lamps.
Polyploidy induction and morphology investigation during seeds germination
Germinated seeds with hypocotyl 0.2–0.5 cm in length, were selected as the materials for polyploidy induction. Oryzalin was dissolved in small amount of 1 M NaOH then diluted with distilled water to final concentrations of 0.005%, 0.01%, and 0.02% (w/v). Colchicine was directly dissolved in distilled water and diluted to final concentrations of 0.025%, 0.05%, and 0.1% (w/v). The solutions were sterilized by autoclaving and then used to treat the germinated seeds for different periods (12 h, 24 h, and 36 h). The control groups were treated with sterile distilled water. Each treatment contained 30 seeds and had three repeats. After induction, seeds were cultured on germination medium and the hypocotyl length, euphylla number, and other phenotypic traits were observed every 7 days. The survival rate of seeds was determined in the 3rd and 6th month after treatments. Each surviving seed and seeding propagated from it was marked as a clone. Newly generated shoots were transplanted onto proliferation medium (MS medium containing 0.1 mg L−1 NAA, 2 mg L−1 6-Benzylaminopurine (6-BA), 30 g L−1 sugars, and 6 g L−1 agar, pH 5.8) and subcultured every 60 days. After three to four rounds of subculturing, some shoots were transplanted onto rooting medium (MS medium containing 0.02 mg L−1 NAA, 60 g L−1 sucrose, and 6 g L−1 agar, pH 5.8) to promote bulblet growth and obtain roots for chromosomal counting, and some shoots were further cultured on proliferation medium to obtain more microshoots.
Polyploidy level detection
Flow cytometry (FCM) identification
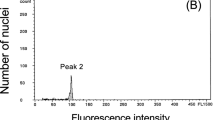
Young leaf tissue (0.5 × 0.5 cm) was selected for analysis by flow cytometry (FCM). The FCM identification was conducted according to a previously reported protocol, with minor modifications (Zhang et al. 2017). Histograms were generated after analyzing ≥ 5000 nuclei using FSC 3.0. Flow-Cytometry Express software.
Chromosomal counting
After FCM identification, tetraploids were randomly reconfirmed by chromosomal counting using a method of Zhou (2007) with a minor modifications.
Stomatal characteristics and comparison of diploid and tetraploid growth in tissue culture
After 60 days of cultivation on MS medium (containing 0.5 g L−1 active carbon), uniformly sized leaves were selected from 30 diploid and 30 tetraploid genotypes to investigate the stomatal characteristics of each genotype (with three replications). The nail polish impression method was used to analyze stomatal characteristics (Ozturk et al. 2014). The stomata size and density were observed under 10 × lens of a light microscope (Lecia DM500, Heerhrugg, Switzerland). Stomata density was observed under three different horizons. The length and width of 10 stomata were measured for each genotype. The stoma index was calculated by dividing stoma length by stoma width. To calculate the scale differentiation rate for each ploidy level, 90 outer-layer scales were selected from similarly sized bulblets of 15 genotypes and then cultured on proliferation medium. The degree of differentiation was observed every 5 days and the number of newly generated microshoots was recorded after 60 days. We selected 32 diploid and 32 tetraploid shoots with a uniform size from eight genotypes of each ploidy level and cultured them on MS medium (containing 0.5 g L−1 activated carbon) to compare shoot growth characteristics. Roots length was recorded every 7 days to calculate the root generation rate. After 3 months of culture, phenotype parameters including the increase of shoot weight, root number and length, and leaf number and size were determined.
Comparison of growth of diploids and tetraploids in the greenhouse
Tissue-cultured bulblets (diameter, 0.8–1.0 cm) of 30 diploid genotypes (N = 2863) and 30 tetraploid genotypes (N = 2818) were transplanted into pots (5 × 5 cm, 2:1 mixture of grass peat and sands) after 2 months of dormancy breaking. After 3 months of growth, the seedlings were transplanted into large pots. The germination rate of bulblets was investigated after 1 month. For each genotype, the number of leaves, and the length and width of new fully extended leaf blades were recorded as morphological characters. The plants were cultivated in the greenhouse from 12 November 2018 to 31 June 2019 (13 to 27 °C in winter and 20 to 33 °C in spring during day, 5 to 15 °C in winter and 10–20 °C in spring during night).
Statistical analyses
Data were processed using Microsoft Excel 2010 software (Microsoft Corp, Richmond, CA, USA) and IBM SPSS Statistics 22 (IBM, Chicago, IL, USA). The significance of differences among treatments was evaluated using independent-samples T test or one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests. The significance of differences in each parameter was evaluated using multivariate analysis of variance (in a general linear model) followed by Duncan’s multiple range tests. The linear relationship between two variables was evaluated by Pearson’s correlation analysis. Differences at P < 0.05 were considered significant. Graphs and figures were generated using GraphPad Prism 7.00 and Adobe Photoshop CS3 software.
Results
Changes in seeds morphology and growth after polyploidy induction
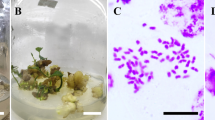
As illustrated in Fig. 1, the survival rate, morphology, and development rate of seeds differed significantly between those treated with antimitotic agents and the control groups. In the control groups, 100% of seeds survived from 0 to 36 h after water treatment. The hypocotyl rapidly extended to 4–5 cm in length over 2 weeks, then stopped elongating. The hypocotyl was thin, yellowish green, and had a smooth surface with abundant root hairs (Fig. 1b). Seeds in the control groups generated 1–2 euphylla in 2–4 weeks (Fig. 1d). In the treated groups, most generating seeds showed abnormal hypocotyl phenotype, apart from a few that were not significantly different from the control groups. As shown in Fig. 1c, the abnormal hypocotyl was swollen, had a rough surface, a yellowish-green color, and few root hairs. The swollen hypocotyl extended to 0.5–2 cm in length in 2 weeks, and then stopped elongating. No euphylla were generated in 2 months. After 2–3 months of cultivation, the color of the swollen hypocotyl became brown. One part of the hypocotyl gradually produced new shoots (Fig. 1e). Other parts of the hypocotyl did not generate new shoots in 3 months and died, but one seed generated callus in 6 months (Fig. 1f). Finally, 137 oryzalin-treated and 239 colchicine-treated seeds were survival, of which 87 oryzalin-treated and 125 colchicine-treated seeds formed abnormal hypocotyl (Table 1).
Phenotype of L. rosthornii seeds after treatment with anti-mitosis agents. Germinated seeds immersed in anti-mitosis agent (a); phenotype of seeds in control group after 14 days (b); variant hypocotyl phenotype of seeds at 14 days after anti-mitosis treatments (c); phenotype of seeds in control group at 30 days (d); shoots directly variant generated from swollen hypocotyl (e); shoots indirectly generated from callus (f) in 6th month of culture; bars = 1 cm
Ploidy level after induction by oryzalin and colchicine
The ploidy levels of seeds in each treatment were identified by FCM (Table 1). There were 81 tetraploids in the oryzalin-treated group, 118 in the colchicine-treated group, and none in the control group. As shown in Fig. 2, selected tetraploids were randomly checked by chromosomal counting, and all re-identified plants had 48 chromosomes, double the number of chromosomes in diploids (2n = 2x = 24). The induction efficiency of treatments was compared by ANOVA. We compared 18 combinations of the two antimitotic agents, and found that the most efficient induction treatment was 0.05% colchicine for 36 h (27.78%), followed by 0.1% colchicine for 24 h (23.33%), 0.01% oryzalin for 24 h (22.22%) and 0.05% colchicine for 24 h (21.11%). The former treatment was significantly more efficient than the other treatments, and the latter three treatments were not significantly different from each other in terms of efficiency.
To determine the influence of each parameter, between-subjects effects were tested in multivariate analyses (Tables 2 and 3). The survival rate, frequency of swollen hypocotyl, and induction frequency of tetraploids were significantly affected by the concentration of each antimitotic agent, treatment time, and the interaction between concentration and treatment time (P < 0.01). An ANOVA multiple comparison analysis was conducted to analyze the effects of agent concentration and treatment time (Table 4). The numbers of swollen hypocotyl and tetraploids were markedly lower in the 0.02% oryzalin treatment than in the 0.005% and 0.01% oryzalin treatments, and not significantly different between the latter two treatments. The numbers of swollen hypocotyl and tetraploids were significantly higher after the 24-h oryzalin induction treatment than after the 12-h and 36-h oryzalin induction treatments. The numbers of swollen hypocotyl and tetraploids were not significantly different between the 0.05% and 0.1% colchicine treatments, but were significantly higher in those treatments than in the 0.025% colchicine treatment. The numbers of swollen hypocotyl and tetraploids were almost same after the 24-h and 36-h colchicine induction treatments, and were significantly higher after those treatments than after 12-h colchicine induction treatment. In conclusion, the highest relative efficiency of tetraploid induction was achieved with 0.005–0.01% oryzalin treatments for 24 h, and 0.05–0.1% colchicine treatments for 24 to 36 h.
Correlation between swollen hypocotyl phenotype and ploidy level
The results of FCM analyses showed that the plantlets developing from hypocotyl with a normal phenotype were diploids, while the majority of shoots developing from swollen hypocotyl had altered ploidy levels. As shown in Table 1, the 87 plants that developed from swollen hypocotyl after oryzalin treatment consisted of 81 tetraploids, one mixploid and five diploids; and the 125 plants that developed from swollen hypocotyl after colchicine treatment consisted of 118 tetraploids, four mixploids, and three diploids. In correlation tests between the swollen hypocotyl phenotype and the frequency of tetraploids, the Pearson’s coefficient values were 0.975** and 0.989** (P < 0.01) for the oryzalin and colchicine treatments, respectively (Table 5).
Stomatal characteristics of tissue cultured plantlets in diploid and tetraploid populations
As shown in Table 6, the stomatal length, width, and stomatal index of tetraploids was 61.17%, 22.33%, and 32.47% higher than those of tetraploids, respectively, while stomata density was 78.33% lower in tetraploids than in diploids. Except for stomata density, all other stomatal parameters showed larger coefficients of variation in tetraploids than in diploids. The results of correlation analyses between stomata characteristics and ploidy levels for tissue-cultured plantlets are summarized in Table 7. The Pearson’s coefficient for stomatal traits of tetraploids (length, width, and density) were 0.773**, 0.504**, and 0.695** (P < 0.01), respectively.
Scale differentiation and adventitious shoot growth of plantlets with different ploidy levels in tissue culture
Scale differentiation and adventitious shoot growth were monitored in plantlets (Table 8). Scale differentiation did not differ significantly between diploids and tetraploids. The scales were swelled by 10 days of culture and generated new microshoots in 25 days. After 60 days of culture, diploids and tetraplolids had generated an average of 2.40 and 2.61 adventitious shoots, respectively. The rooting percentage from diploid and tetraploid adventitious shoots was 39.59% and 40.12%, respectively, at 14 days and 100% at 30 days (on MS medium containing 0.5 g L−1 activated carbon). Finally, diploids and tetraploids formed, on average, 9.38 and 10.88 roots, respectively, with an average length of 2.48 cm and 2.08 cm, respectively. The average increased weight of diploid and tetraploid shoots was 0.47 g and 0.48 g, respectively. The values of four leaf parameters (leaf number, length, width, and area) were all slightly higher for tetraploids than for diploids, but the differences were not significant. There were no significant differences between the two ploidy levels in terms of differentiation, growth, and morphology, but for most parameters, the coefficient of variations was higher for tetraploids than for diploids.
Germination rate of bulbltes and leaf growth rates of plantlets after transplanting
Average bulblet germination rates of diploids and tetraploids after transplanting were 55.08% and 65.31%, respectively (Table 9). The germination rate of diploid genotypes ranged from 40.58 to 70.43% (coefficient of variation, 16.63%), while that of tetraploid genotypes ranged from 48.39 to 80.30% (coefficient of variation, 12.87%). The bulblet germination rate of diploid and tetraploid genotype is shown in Fig. 3. The germination rate of seven tetraploid genotypes was greater than the maximum germination rate of diploids.
Table 10 summarizes morphological characteristics after transplanting. Leaf number did not differ significantly between diploids and tetraploids at each time point. Leaf length was similar between diploids and tetraploids initially, but leaves of tetraploids became significantly larger than those of diploids over time. For example, after 7 months of cultivation, the average leaf length was significantly greater in tetraploids (14.11 cm) than in diploids (11.35 cm). The leaf width of tetraploids was greater than that of diploids at all time points, except in the 3rd month of culture. Leaf area did not differ significantly between the two ploidy levels initially, but was greater for tetraploids than for diploids in the 5th to the 7th month of culture. After 7 months of culture, the coefficient of variation in leaf area was greater in tetraploids (23.06%) than in diploids (14.86%).
Discussion
An efficient method for tetraploid induction is crucial for breeding. To eliminate the effects of poor seed germination, we selected germinated seeds as the materials for induction in this study. The use of germinated seeds to improve the induction rate has been reported in many studies (Ascough and Staden 2008; Dhamayanthi and Gotmare 2010; Feng et al. 2016; Schnell 2015). However, the appropriate period of pre-culture was different among different species (Aqafarini et al. 2019; Feng et al. 2016; Omidbaigi et al. 2010). In this study, we selected seeds with hypocotyl about 0.2–0.5 cm in length because longer hypocotyl was easily detached from the endosperm, and was prone to rotting during subsequent steps. Among the induction treatments, the most efficient one was 36-h immersion in 0.05% colchicine (induction rate, 27.78%). Our results indicated that colchicine is more efficient than oryzalin, as it resulted in a higher tetraploid induction rate (Tables 1 and 4). However, the results of other studies suggested that polyploidy induction is more efficient with oryzalin treatment than with colchicine treatment (Bouvier et al. 2010; Lehrer et al. 2008; Sakhanokho et al. 2009; Tuyl et al. 1990). Oryzalin, a kind of herbicide, is characterized by more affinity to microtubules of plant cells than animal cells and it always resulted in a higher mortality rate compared with colchicine (Bajer and Mole-Bajer 1986; Morejohn et al. 1987; Morejohn and Fosket 1991). Moreover, oryzalin cannot directly dissolve in water, so solvents may affect the survival and induction rates as reported in previous studies (Petersen et al. 2003). Inclusion, the higher fatality rate of oryzalin than that of colchicine may directly responsible for less efficiency of polyploidy induction. Similar results have been reported in previous studies (Chung et al. 2014; Petersen et al. 2003, 2010).
The phenomenon that morphological variations in the hypocotyl after the induction treatments was widely reported in previous studies, but the correlation between the altered hypocotyl phenotype and the ploidy level was rarely reported (Ascough and Staden 2008; Dhamayanthi and Gotmare 2010; Schnell 2015). For both of the antimitotic agents, high Pearson’s coefficient values were obtained for the relationship between hypocotyl changes and tetraploidy. These findings suggested that morphological variations in the hypocotyl may serve as an early ploidy selection character. Stomatal characters are also widely used as ploidy selection traits. For the majority of plants, the stomata size was positively correlated with ploidy levels, but the stomata density was negative correlated with ploidy levels (Kaensaksiri et al. 2011; Podwyszyńska et al. 2014). In addition, stomatal characters remain stable during cultivation and through generations (Husband et al. 2016; Pavlíková et al. 2017). In this study, stomata length and density were correlated with ploidy levels, as indicated by the Pearson’s coefficient values (Table 7). However, some tetraploids had similar stomata length and density to those of diploids (Table 6 and Fig. 3). Similar stomatal characters between diploids and tetraploids have also been reported for some genotypes of apple (Hias et al. 2017), potato (Aversano et al. 2013), Mentha canadensis (Yu et al. 2015), and Rhynchostylis gigantean var. rubrum (Kerdsuwan and Te-chato 2012). These findings suggested that stomatal characters are affected by both the ploidy level and genotype.
Previous studies have been reported that the size of the nucleus and cells increases with whole chromosome duplication, and this affects mitosis and the cell cycle (Pozo and Elena 2015). Slow growth of tetraploids is a typical characteristic of neopolyploids (Allario et al. 2011; Podwyszyńska et al. 2018). In this study, delayed development of seeds and slow differentiation of bulblets only occurred initially. After several rounds of subcultural, there were no differences in scale differentiation, shoot growth, and leaf morphology between tetraploids and diploids (Table 8). There are several possible explanations for the transient growth retardation observed in this study. Firstly, the toxic effect of the antimitotic agent may be decreased or disappeared during long-term cultivation (Drunen and Husband 2018; Munzbergova 2017). Secondly, DNA elimination and/or chromosome rearrangements may be have occurred during genome stabilization (Dar et al. 2013). Thirdly, there may have been changes in DNA methylation patterns (Podwyszyńska et al. 2014; Xu et al. 2017).
Most losses occur when tissue-cultured plants are transplanted. In this study, the average germination rate was higher for tetraploids than diploids, suggesting that tetraploids had better environmental fitness than diploids in these conditions. Several diploid and tetraploid genotypes shown similar germination rates, suggesting that environmental fitness is not only affected by ploidy level but also by genotype. As previous studies reported that the degree of genomic changes after polyploidization is highly variable within a species (Spoelhof et al. 2017) and tetraploids may not vigorous than diploids for some genotypes (Podwyszyńska et al. 2018). Growth characters were also compared after transplanting. Initially, the leaf size was not significantly different between diploids and tetraploids, but the leaf area of tetraploid plants was significantly greater than that of diploid plants after 5 months of cultivation. Previous studies suggested that photosynthetic capability of tetraploid always significantly higher than that of diploid (Cao et al. 2018; Zhou et al. 2016). However, tetraploid primary characteristics by increased in nuclear and cell volume which need speed longer time and greater bulk of materials in building up larger cells (Levan 1943; Knight and Beaulieu 2008). We hypothesis that, the photosynthesis area was increase with increased leaf number and size which may attributed more accumulation of photosynthetic products for tetraploid to building up larger leaves compared with diploid in later stage of growth. This assumption needs further comparison the photosynthesis capability between the two ploidy levels.
Most studies on polyploids have concentrated on one or a few genotypes, and therefore, their results may only reflect characteristics of given genotypes rather than the actual characteristics of polyploidization. Multiple genotypes may provide more opportunities for morphological variation in other characters (Chen et al. 2016; Emsweller and Brielery 1949). In this study, many characters had larger coefficients of variation in tetraploids than in diploids, both in tissue culture and after transplanting. Therefore, compared with diploids, tetraploids may provide a wider range of variation for lily breeding.
Conclusion
In the present study, we have developed an effective protocol for polyploidy induction with germinated seeds treated by oryzalin and colchicine. Both mitotic inhibitors were efficient in polyploidy induction but the treatment with colchicine presented a higher survival rate and higher induction rate than those treatment with oryzalin. After treated with both mitotic inhibitors, the swollen hypocotyl phenotype was prevalence observed and it was strongly correlated with polyploidy. After transplanting, tetraploids were more vigorous than diploids for higher germination rate of bulblets and larger size of leaves.
Abbreviations
- ANOVA:
-
One-way analysis of variance
- BA:
-
Benzylaminopurine
- FCM:
-
Flow cytometry
- MS:
-
Murashige–Skoog
- NAA:
-
Naphthylacetic
References
Acosta-Gallegos JA, Kelly JD, Gepts P (2007) Prebreeding in common bean and use of genetic diversity from wild germplasm. Crop Sci 47(Supplement 3):44–59
Allario T, Brumos J, Colmenero-Flores JM et al (2011) Large changes in anatomy and physiology between diploid Rangpur lime (Citrus limonia) and its autotetraploid are not associated with large changes in leaf gene expression. J Exp Bot 62:2507–2519
Aqafarini A, Lotfi M, Norouzi M et al (2019) Induction of tetraploidy in garden cress: morphological and cytological changes. Plant Cell Tissue Organ Cult 37:627–635
Ascough GD, Staden JV (2008) Effectiveness of colchicine and oryzalin at inducing polyploidy in Watsonia lepida N.E Brown. HortScience 43:2248–2251
Aversano R, Caruso I, Aronne G et al (2013) Stochastic changes affect Solanum wild species following autopolyploidization. J Exp Bot 64:625–635
Bajer A, Mole-Bajer J (1986) Drugs with colchicine-like effects that specifically disassemble plant but not animal microtubules. Ann N Y Acad Sci 466:767–784
Barba-Gonzalez R, Miller C, Ramanna M et al (2006) Induction of 2n gametes for overcoming F1-sterility in lily and tulip. In: XXII international Eucarpia symposium, section ornamentals, breeding for beauty 714, pp 99–106
Bingham E, Groose R, Woodfield D et al (1994) Complementary gene interactions in alfalfa are greater in autotetraploids than diploids. Crop Sci 34:823–829
Bouvier L, Fillon FR, Lespinasse Y (2010) Oryzalin as an efficient agent for chromosome doubling of haploid apple shoots in vitro. Plant Breed 113:343–346
Cao Q, Zhang X, Gao X et al (2018) Effects of ploidy level on the cellular, photochemical and photosynthetic characteristics in Lilium FO hybrids. Plant Physiol Biochem 133:50–56
Chen R, Jiang WZ, Li QL et al (2016) Comparison of seven colchicine-induced tetraploid clones with their original diploid clones in purple coneflower (Echinacea purpurea L.). Euphytica 207:387–399
Chung MY, King CY, Min JS et al (2014) In vitro induction of tetraploids in an interspecific hybrid of Calanthe (Calanthe discolor x Calanthe sieboldii) through colchicine and oryzalin treatments. Plant Biotechnol Rep 8:251–257
Dar TH, Raina SN, Goel S (2013) Molecular analysis of genomic changes in synthetic autotetraploid Phlox drummondii Hook. Biol J Linn Soc 110:591–605
Dhamayanthi KPM, Gotmare V (2010) Induction of polyploidy in two diploid wild cotton (G. armourianum and G. aridum) species by colchicine treatment. Electron J Plant Breed 1:966–972
Dhooghe E, Van LK, Eeckhaunt T et al (2010) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult 104:359–373
Drunen WEV, Husband BC (2018) Immediate vs. evolutionary consequences of polyploidy on clonal reproduction in an autopolyploid plant. Ann Bot 122:195–205
Du Y P (2014) Collection and evaluation of Lilium spp. in China (Liliaceae) and the Resarch on Resistance Gene Anslog (RGA). Doctor, Beijing Forestry University (in Chinese)
Eliášová A, Trávníček P, Mandák B et al (2014) Autotetraploids of Vicia cracca show a higher allelic richness in natural populations and a higher seed set after artificial selfing than diploids. Ann Bot 113:159–170
Emsweller SL, Brierley P (1940) Colchicine-induced tetraploidin Lilium. J Hered 5:223–230
Feng H, Wang M, Cong R et al (2016) Colchicine- and trifluralin-mediated polyploidization of Rosa multiflora Thunb. var. inermis and Rosa roxburghii f. normalis. J Hortic Sci Biotechnol 92:279–287
Hias N, Leus L, Davey MW et al (2017) Effect of polyploidization on morphology in two apple (Malus x domestica) genotypes. Horticult Sci 44:55–63
Husband BC, Baldwin SJ, Sabara HA (2016) Direct vs. indirect effects of whole-genome duplication on prezygotic isolation in Chamerion angustifolium: Implications for rapid speciation. Am J Bot 103:1259–1271
Kaensaksiri T, Soontornchainaksaeng P, Soonthornchareonnon N et al (2011) In vitro induction of polyploidy in Centella asiatica (L.) Urban. Plant Cell Tissue Organ Cult 107:187–194
Kafawin OM, Chen CH (1991) Induction of tetraploid Lilium longiflorum Thunb plants by colchicine treatment of cultured bulbscale discs. Proc Natl Acad Sci 70:31–37
Kasteele VD, Stoop FSCS (1974) Conservation of wild Lilium species. Biol Cons 6:26–31
Kerdsuwan N, Te-chato S (2012) Effects of colchicine on survival rate, morphological, physiological and cytological characters of chang daeng orchid (Rhynchostylis gigantean var. rubrum Sagatik) in vitro. J Agric Technol 8:1451–1460
Khosravi P, Kermani MJ, Nematzadeh GA et al (2008) Role of mitotic inhibitors and genotype on chromosome doubling of Rosa. Euphytica 160:267–275
Knight CA, Beaulieu JM (2008) Genome size scaling through phenotype space. Ann Bot 101:759–766
Lehrer JM, Brand MH, Lubell JD (2008) Induction of tetraploidy in meristematically active seeds of Japanese barberry (Berberis thunbergii var. atropurpurea) through exposure to colchicine and oryzalin. Sci Hortic 119:67–71
Levan A (1943) The pigment content of polyploid plants. Hereditas 29:255–268
Liang SY, Tamura M (2000) Lilium. In: Chen (ed) Flora of China, vol 24. Science Press, St. Louis, pp 118–152
Lim KB, Barba-Gonzalez R, Zhou S et al (2008) Interspecific hybridization in lily (Lilium): taxonomic and commercial aspects of using species hybrids in breeding. Floricult Ornam Plant Biotechnol 5:138–145
Mei L, Chen XZ, Wang SH et al (2009) Relationship between bruchid resistance and seed mass in mungbean based on QTL analysis. Genome 52:589–596
Morejohn L, Bureau T, Mole-Bajer J et al (1987) Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro. Planta 172:252–264
Morejohn LC, Bureau TE, Tocchi LP et al (1984) Tubulins from different higher plant species are immunologically nonidentical and bind colchicine differentially. Proc Natl Acad Sci 81:1440–1444
Morejohn LC, Fosket DE (1991) The biochemistry of compounds with anti-microtubule activity in plant cells. Pharmacol Ther 51:217–230
Munzbergova Z (2017) Colchicine application significantly affects plant performance in the second generation of synthetic polyploids and its effects vary between populations. Ann Bot 120:329–339
Nimura M, Kato J, Horaguchi H et al (2006) Induction of fertile amphidiploids by artificial chromosome-doubling in interspecific hybrid between Dianthus caryophyllus L. and D. japonicus Thunb. Breed Sci 56:303–310
Omidbaigi R, Mirzaee M, Hassani M et al (2010) Induction and identification of polyploidy in basil (Ocimum basilicum L.) medicinal plant by colchicine treatment. Int J Plant Prod 4:87–98
Ozturk A, Umit S, Gürgör PN et al (2014) The effect of different nursery conditions on some of the leaf and stomata characteristics in Chestnuts (Castanea sativa Mill.). J Appl Bot Food Qual 87:190–195
Pavlíková Z, Paštová L, Münzbergová Z (2017) Synthetic polyploids in Vicia cracca: methodology, effects on plant performance and aneuploidy. Plant Syst Evol 303:827–839
Petersen KK, Hagberg P, Kristiansen K (2003) Colchicine and oryzalin mediated chromosome doubling in different genotypes of Miscanthus sinensis. Plant Cell Tissue Organ Cult 73:137–146
Petersen KK, Hagberg P, Kristiansen K et al (2010) In vitro chromosome doubling of Miscanthus sinesis. Plant Breed 121:445–450
Podwyszyńska M, Gabryszewska E, Dyki B et al (2014) Phenotypic and genome size changes (variation) in synthetic tetraploids of daylily (Hemerocallis) in relation to their diploid counterparts. Euphytica 203:1–16
Podwyszyńska M, Trzewik A, Marasek-Ciolakowska A (2018) In vitro polyploidisation of tulips (Tulipa gesneriana L.)—Phenotype assessment of tetraploids. Sci Hortic 242:155–163
Pozo dJC, Ramirez-Parra E (2014) Deciphering the molecular bases for drought tolerance in Arabidopsis autotetraploids. Plant Cell Environ 37:2722–2737
Pozo JCD, Elena R (2015) Deciphering the molecular bases for drought tolerance in Arabidopsis autotetraploids. Plant Cell Environ 37:2722–2737
Sakhanokho HF, Rajasekaran K, Kelley RY et al (2009) Induced polyploidy in diploid ornamental ginger (Hedychium muluense R. M. Smith) using colchicine and oryzalin. HortScience 44:1809–1814
Schnell L (2015) The Induction of Polyploidy in Vinca Rosea L. Proc Okla Acad Sci 21:67
Spoelhof JP, Soltis PS, Soltis DE (2017) Pure polyploidy: closing the gaps in autopolyploid research. J Syst Evol 55:340–352
Tian J, Isemura T, Kaga A et al (2013) Genetic diversity of the rice bean (Vigna umbellata) genepool as assessed by SSR markers. Genome 56:717–727
Tu H-Y, Zhang AL, Xiao W et al (2018) Induction and identification of tetraploid Hedychium coronarium through thin cell layer culture. Plant Cell Tiss Organ Cult 135:395–406
Tuyl JMV, Arens P (2010) Lilium: breeding history of the modern cultivar assortment. In: II international symposium on the genus Lilium 900, pp 223–230
Tuyl JMV, Meijer B, Diën MPV (1990) The use of oryzalin as an alternative for colchicine in in-vitro chromosome doubling of Lilium. Lily Yearb N Am Lily Soc 43:19–22
Xu C, Zhang Z, Huang Z et al (2017) Impact of the leaf cut callus development stages of Populus on the tetraploid production rate by colchicine treatment. J Plant Growth Regul 37:635–644
Yu X, Wang HT, Liu Y et al (2015) In vitro induction of chromosome-doubling in cultured shoots of three cultivars of mint (Mentha canadensis L.) treated with colchicine. J Hortic Sci Biotechnol 88:306–312
Zhang XQ, Cao QZ, Jia GX (2017) A protocol for fertility restoration of F1 hybrid derived from Lilium x formolongi 'Raizan 3' x Oriental hybrid 'Sorbonne'. Plant Cell Tissue Organ Cult 129:375–386
Zhou HW, Zeng WD, Yan HB (2016) In vitro induction of tetraploids in cassava variety 'Xinxuan 048' using colchicine. Plant Cell Tissue Organ Cult 128:1–7
Zhou SJ (2007) Intergenomic recombination and introgression breeding in Longiflorum x Asiatic lilies. Wagenigen Universit, Doctor
Acknowledgements
This work was supported by Grants from the National Key R & D Program of China (2019YFD1001002) and Funds for the National Natural Science Foundation of China (Grant No. 31772348).
Author information
Authors and Affiliations
Contributions
G-XJ and L-JW conceived and designed the experiments; L-JW, QZ and XG performed the experiments; L-JW analyzed the data and wrote the manuscript; G-XJ, L-JW and Q-ZC revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by M. I. Beruto.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, LJ., Zhang, Q., Cao, QZ. et al. An efficient method for inducing multiple genotypes of tetraploids Lilium rosthornii Diels. Plant Cell Tiss Organ Cult 141, 499–510 (2020). https://doi.org/10.1007/s11240-020-01807-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01807-4