Abstract
Plumbago auriculata Lam. (Plumbaginaceae) is an ornamental/medicinal flowering shrub. However, it could be stop growing under 5 °C resulting in its poor germplasm resources. Increasing the tolerance of polyploids to environmental stress will enrich its germplasm resources. In this study, we thus employed the stem segments and buds exposed to pendimethalin and trifluralin in vitro to conduct an effective polyploid protocol, in which buds were cultured for 7 days in MS medium with 800 μM. In this study, we have developed a novel polyploid identification method based on accuracy and efficiency. Early screening was performed based on the morphological characteristics of this plants, and then polyploidy was determined by flow cytometry. The number of chromosomes was determined by the chromosomal accounting. At the anatomical level, in comparison with diploids, the root parenchyma, stem collenchyma and tetraploid spongy tissues were thickened, the guard cell size and vascular bundles number were increased, and the stomata were widened; but the stomatal density was decreased. After 24 h of cold stress, the maximal quantum yield of photosystem II (Fv/Fm) and maximum primary yield of photochemistry of PSII (Fv/Fo) of diploids decreased significantly. Conversely, the minimal value of chlorophyll a fluorescence (Fo), relative electrical conductivity and malondialdehyde increased markedly. Accordingly, the cold tolerance of the tetraploids presented better than these of the diploids. Taken together, our study has first developed an effective protocol to obtain and identify the tetraploid of P. auriculate plants. Furthermore, it was proved that this polyploid has presented improving cold tolerance and enriching phenotypic properties. These findings could be useful for improving cold-tolerance breeding and enriching genetic diversity of P. auriculate plants.
Key message
We used stem segments and buds exposed to pendimethalin and trifluralin in vitro to conduct polyploids of Plumbago auriculata and compared characterizations including cold tolerance between diploids and polyploids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plumbago auriculata Lam. (Plumbaginaceae) is an ornamental and medical flowering evergreen shrub worldwide that is native to South Africa. The chromosome number of P. auriculata is 12, i.e., 2n = 2x = 12 (Lei et al. 2016), and has a long flowering period with richness in flowers. Moreover, the species have many medicinal and phytochemical properties, e.g., anti-cancer (Gou et al. 2015; Bao et al. 2017; Liu et al. 2017), anti-obesity (Jaradat et al. 2016), antidepressant (Dhingra and Bansal 2015) and antimicrobial properties (Gupta et al. 2017). However, previous studies have verified that this species will stop growing at 1–5 °C and begin to wither at below 0 °C, which therefore limit the productivity and distribution of this polyploids species. Notwithstanding, polyploids have great breeding values and characteristics (Otto 2007), such as enhancing plant biomass (Zhou et al. 2017), increasing species diversity (Soltis et al. 2009; Schneider et al. 2017), regulating potential metabolic (Soltis and Soltis 2009; Luo et al. 2018), and improving tolerance/genetic adaptability resistance to heterogeneous environment (Hollister 2014; Zhang et al. 2015; Oustric et al. 2017). Further, compared with other variety methods, such as genome editing (Nadakuduti et al. 2018), polyploid breeding has a minimal environmental impact. Accordingly, we hypothesized that polyploids may contribute to improve cold-tolerance ability and species diversity in P. auriculate plants.
Colchicine is a commonly used anti-mitotic agent that induces polyploidy in plants. However, this compound has high-cost and strong-toxicity to humans (Kong et al. 2016). Recent decades, alternative colchicine has been gradually discovered. For example, herbicides such as pendimethalin and trifluralin have a high affinity for plant tubulin, which are less harmful to plants and humans with lower price (Liu et al. 2014). In recent years, the flow cytometry (FCM) has been a reliable technique for evaluating DNA ploidy levels in plants due to its greater ease of use, faster speed and higher accuracy (Tavan et al. 2015; Saghahazrati et al. 2020). However, it is difficult to measure polyploids employing this protocol alone at a large scale because of its high price in related apparatus (Schurer et al. 2019). Since polyploids have obvious morphological features, such as large leaves, the induced polyploids can be first filtered by morphological variation and then identified by the FCM. The selected putative polyploid is then directly and accurately verified by chromosome counting (Eng and Ho 2019). Therefore, these combinations by different methods can increase the efficiency and cost savings of polyploid level identification.
Besides, the chlorophyll fluorescence, such as the minimal value of chlorophyll a fluorescence (Fo), maximal quantum yield of photosystem II (Fv/Fm) and maximum primary yield of photochemistry of PSII (Fv/Fo), are used to reflect the photosynthetic performance of plants under abiotic stress especially under temperature stress, which is a lossless, fast and easy way to get information in situ (Baker 2004; Banks 2017; Zhou et al. 2018). Previous publications have showed that the identification of cold-tolerance using chlorophyll fluorescence has been successful in many plants, such as watermelons (Hou et al. 2016) and walnuts plants (Dong et al. 2018). Additionally, different severities of cold-stress lead to physiological and biochemical changes in the plants (Karimi and Ershadi 2015). For example, under cold stress, the leaf membrane permeability increases, and membrane integrity is disrupted, resulting in membrane damage in plants. Based on the above backgrounds, the objectives of this study were to (1) investigate an efficient methodology for obtaining and identifying tetraploids of P. auriculata plants, and (2) examine the impacts of chromosome doubling in these studied plants.
Materials and methods
Plant materials and polyploidization
The plant materials were collected from the same batch of P. auriculata sterile buds (introduced by axillary buds) and stem segments, which were cultured in the laboratory of Landscape Architecture Institute at Sichuan Agricultural University, Chengdu, China. The culture-media conditions for this plant include pH of 5.8–6.0 in MS-media, room temperature of 25 ± 1 °C, photoperiod of 12 h/day, and light intensity of 1500–2000 Lx. During the cultivation process, the Pendimethalin (Jiangsu Longlight Chemical Co., Ltd.) and Trifluralin (Jiangsu Longlight Chemical Co., Ltd.) were added into MS medium. The concentrations of these mutagens (i.e., Pendimethalin & Trifluralin) were arranged as 400, 600, and 800 μM for the treatments of 7-, 14-, and 21-day, respectively. In this study, each treatment contained 90 collected samples with three replications. Under strict aseptic conditions, we decomposed into the treated sterile buds into a single bud and then inserted them into the medium. The control group was blank MS-medium. After treatment, single buds were washed four to 5 five times with sterile water and then cultured in blank MS-medium. Each sterile stem was cut into segments of more than three leaves, and the rest for stems were the same as those for sterile buds. The survival rate and morphological-variation rate in the blank medium were observed and calculated after 30 days culture. Then, the top buds newly grown from the material were cultured in new MS-medium per 2-week, which was repeated six times to acquire purified tetraploids.
Tetraploid identification
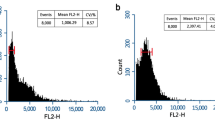
For flow cytometry analysis, we employed the FCM (BD FACSVerse flow cytometry, American) technology to identify the ploidy levels of the newly grown from sterile buds and stem segments. The light source was a 15 mw-488 nm argon ion laser with the channel of FL-2. The mature leaves of P. auriculata seedlings (2n = 2x = 12) with morphological variation (~ 0.1 g) were selected as an external standard. In briefly, 1.5 mL of Weibel–Palade-Body (WPB) cell lysate from precooled lysate was added to a Petri dish (Tian et al. 2011). Then, a blade was used to quickly cut the leaf material into the fine particles, and the material was submerged in the dissociation solution. The mixture was filtered twice with a 300-mesh nylon mesh, and the filtrate was placed in a 1.5 mL centrifuge tube. The supernatant was discarded by centrifugation at 1000×g for 5 min. Subsequently, 500 μL of propidium iodide (PI) solution was added to a final concentration of 5 μg/mL. After dyeing, samples were measured using the FCM. Data were acquired using the sample acquisition software BD CSampler (BD FACSVerse flow cytometry, American), and at least 10,000 nuclei were collected from each sample with three replicates.
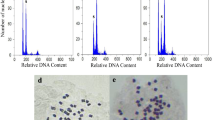
For chromosome counting, plants with vigorous growth underwent the FCM testing after growing new roots. The plants initially identified as a polyploids were selected for chromosome counting. The plant root tips (~ 2 mm) were cut, and then were treated with 0.05% colchicine at 10–20 °C. After 2 h, samples were fixed at 4 °C with Carnot's fixing solution (anhydrous ethanol: glacial acetic acid = 3:1) for 24 h. Immediately, the root tips were washed three times and placed in a water bath at 60 °C for 8 min to preheat the 1 M hydrochloric acid solution for good dyeing. Each plant root tip was stained in a cardo-red-dye for 8 min and then compressed. Finally, the ploidy level (including chromosome number) was identified by a microscope (Olympus BX53, Tokyo, Japan) with a 100-x objective lens.
Morphological and anatomical structure
Ten tetraploids and ten diploid plants with uniformly grown were selected for morphological trait analysis. In the present study, the basic morphological features such as stem length, flower diameter, stem diameter, leaf width and leaf length were measured. To avoid errors, all of these measurements were repeated three times. To further understand the effects of polyploidy on plant stomata, in our study, we thus measured the length and density of the stomata by the Olympus-BX53 microscope (Olympus BX53, Tokyo, Japan).
For the anatomy traits, we also measured the leaves, stems and root tips of collected tetraploid and diploid plants following the previous protocol (Liu et al. 2014) employing the Olympus-BX53 microscope. In detail, the thickness of leaf, leaf-sponge tissue, root epidermal cells, stem epidermal cells, stem stratum corneum, root parenchyma; and diameter of roots and stems were investigated.
Tetraploid cold resistance testing
Forty tetraploids and diploids plants with similar size were cultured in the cultivation substrate with nutrient soil, vermiculite and perlite (v:v:v = 1:1:1). The plants were placed in the intelligent biochemical incubator (Ningbo Jiangnan Instrument Factory, China). The culture conditions were 25/20 °C (day/night), a photoperiod of 12 h/day, a light intensity of 6600 Lx and a relative humidity of 70%. To avoid damage to, the experiment adopts a step-by-step cooling method that is, cooling down to 5 °C to ensure the adaptability of plants to low temperature. When the temperature dropped to 10/5 °C, the incubators were set to a constant temperature for 24 h at − 5 °C, 0 °C, 5 °C, and 25 °C, where − 5 °C, 0 °C, and 5 °C were low-temperature treatments, and 25 °C was a normal temperature control. The other incubation conditions were the same.
The mid-upper and healthy leaf of plants were dark adapted for 30 min using a leaf clip (Hansatech Instrument, King’s Lynn, England). When measuring, the blade was evenly clamped in the leaf clip, and the direction and angle of the probe were consistent. Then, a Handy Plant-Efficiency-Analyzer (high speed continuous excitation fluorometer; Hansatch Instrument, King’s Lynn, England) was used to measure the Fo, Fm, Fv/Fm and Fv/Fo (Zhou et al. 2018). Besides, the damage level of the membrane can be measured by the relative electrical conductivity (REC; Zhou and Guo 2009). Malondialdehyde (MDA) is a marker of excessive production of peroxidic products. Lipid peroxidation are positively correlated with the extent of damage to plants exposed to low temperatures (Liu et al. 2011). The temperature gradient in which the chlorophyll fluorescence parameters were significantly different was selected, and leaves were therefore collected to measure these cold-resistance physiological indicators according to the protocol of Li (2003).
Statistical analysis
Forms were produced in Microsoft Excel 2010 (Microsoft Corporation, Richmond, WA USA). Data were analyzed using SPSS 22.0 (SPSS Inc. Chicago, IL, USA). The results were subjected to one-way ANOVA, with an HSD-Tukey test in the post hoc analysis for comparisons among groups.
Results
Analysis of the tetraploid induction method
In this study, the main peak of diploid was situated at a value of 6000, the main peak of tetraploid was situated at a value of 12,000 in the FCM histogram (Fig. 1). The chromosome counting analysis have further verified this finding (Fig. 2). As showed in Table 1, the survival rate for pendimethalin was higher than that for trifluralin at the same concentration and treatment time, while the survival rate of stem segments was generally higher than that of sterile buds under the same treatment. Moreover, the polyploidization rate of pendimethalin was also higher than that of trifluralin, while the polyploidization rate of the sterile buds was higher than that of the stem. Therefore, we preliminarily speculated that the treatment of sterile buds with pendimethalin for 7 days was identified as the optimal treatment, with a survival rate of 60%, morphological variation rate of 79.63%, and polyploidization rate of 23.26%.
Characterization of tetraploid P. auriculata
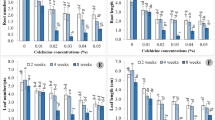
In tetraploid plants, the leaf width, leaf length, stem diameter and corolla diameter increased by 34.51%, 17.84%, 22.99%, and 29.38%, respectively. However, no significant difference in stem length and leaf shape index were observed (Fig. 3; Table 2). The stomata size of diploid plants was 10.0 × 3.51 μm, while the stomata size of tetraploid plants was 10.29 × 4.18 μm. The width of stomatal and guard-cell, and the length of guard-cell increased by 19.09%, 9.71% and 10.66, respectively. The stomatal density decreased by 16.67% compared to the control (Fig. 4; Table 3). The leaves of the tetraploid were thicker than those of the diploid, while the sponge tissue of the tetraploid leaves was thickened by 68.80%. The vascular bundle diameter of the tetraploid leaves was slightly reduced, but the number was increased. The diameter of the tetraploid roots increased by 26.78%, while the root parenchyma thickness of the tetraploid increased by 22.36%. Interestingly, no significant difference between the thickness of stem epidermal cell and root epidermal cell in tetraploid and diploid plants was recorded (Table 4).
Comparison of tetraploid and diploid anatomical structures. a Tetraploid stomata; b Diploid stomata; c, e Tetraploid leaf cross section; d, f Diploid leaf cross section; g Tetraploid root cross-section; h Diploid root cross-section; i tetraploid stem cross-section; j diploid stem cross-section, Plumbago auriculata plants cultured in MS medium for 45 days
Analysis of cold resistance of tetraploid and diploid plants
At 5 °C, the upper leaves of the tetraploid were slightly wilted, while the leaves and the upper stems of the diploid were wilting (Fig. 5). However, there was no significant difference in Fo, Fv/Fm and Fv/Fo between the tetraploid and diploid plants (Fig. 6). At 0 °C, the tetraploid stem tip was wilting, and the main stem of the diploid was wilting. The Fo of the diploid was higher than that of the tetraploid, but the Fv/Fm and Fv/Fo of the diploid was lower than that of the tetraploid. At − 5 °C, some leaves of the tetraploid were wilted, but the main stem of the tetraploid stood upright. All the leaves of the diploid and most stems of the diploid were wilted. The Fo of the diploid was higher (by 16.76%) than that of the tetraploid, but the Fv/Fm and Fv/Fo of the diploid was lower (by ca. 50%) than that of the tetraploid. Compared with the control, the REC of diploid and tetraploid was increased by 109.62%, 66.51%, while the MDA content of diploid and tetraploid was increased by 181.84%, 80.42% (Table 5).
Discussion
Method for polyploidization of P. auriculata and identification of tetraploids
In polyploidization, as colchicine is toxic and expensive, colchicine is often replaced by less toxic and cheaper herbicides, such as pendimethalin, oryzalin or trifluralin (Dimitrov 2000; Denaeghel et al. 2018; Podwyszyńska et al. 2018). For example, colchicine and oryzalin can induce polyploid plants Hebe ‘Oratia Beauty’ (Gallone et al. 2014). Due to the low survival rate and polyploidization rate of trifluralin, in our study, pendimethalin is more suitable than trifluralin for the polyploidization of P. auriculate plants. However, few reports regarding pendimethalin were found in recent decades. One explanation is that mixoploid plants may result in the current appearance. Due to different growth speeds, the original cells eliminate polyploid cells, which makes mixoploids unstable (Hussy and Falavigna 1980; Regalado et al. 2015). Further, using multicellular organs as initial explants may increase the mixoploid rates (Cai et al. 2015; Zhou et al. 2017). Besides, leaves and flowers are larger and stems are thicker in tetraploids than in diploids of our studied plants, which is consistent with the conclusions of Gallone et al. (2014). However, previous studied have shown that the tetraploid structure was not tighter than the diploid (Tavan et al. 2015; Regalado et al. 2017). Therefore, we speculate that the amount of morphological differences of polyploids is dependent on the plant species and the ploidy level (Leitch and Bennett 1997; Denaeghel et al. 2018).
To avoid mixoploid plants, a recent effective solution is the use of calluses as the initial explants. For example, previous studies have shown that a method of obtaining polyploids without mixing multiples by callus and in vivo shoot regeneration can speed up the process of multiple sports (Shi et al. 2015). However, it takes at least three years to purify the plants and flower in the fields. According to our results, interestingly, the mixoploidy problem can also be effectively solved by inoculating newly grown apical buds into the new medium, although the initial explants were not callusing. This is consistent with the findings of Zhou et al. (2017). Calluses could decrease the occurrence of mixoploid plants, but it is time-consuming to regenerate calluses to a complete plant from buds (Regalado et al. 2015; Podwyszyńska et al. 2018). The speed of regeneration is based on the plant species. For P. auriculata, the speed of regeneration of sterile buds and stem segments is fast, while sterile buds were better than stem segments at increasing the polyploidization rate. Our finding will help save the time spent by the introduction of calluses in vivo.
Chromosome accounting is usually the most straightforward way to determine ploidy. However, the numbers of variables and treatment gradients involved in the exploration of polyploid induction methods are large, resulting in a large sample size for identification (Lei et al. 2016). Therefore, the use of chromosome counting methods is time consuming, laborious and difficult. In recent years, the combination of indirect recognition methods such as morphology and FCM has shown significant advantages, although they each have their own shortcomings (Regalado et al. 2015, 2017; Eng and Ho 2019). Morphological identification can greatly reduce the workload, but its accuracy is insufficient. The disadvantage of FCM is that the instrument is expensive (Saghahazrati et al. 2020), making it unpopular when many samples must be identified, and information on aspects such as chromosome morphology and karyotype cannot be provided by the FCM (Schurer et al. 2019). In this study, our morphologically mutated plants were first screened using the FCM, and the tetraploids identified by the FCM were identified again by chromosome counting.
Anatomical structure changes of tetraploids contribute to cold tolerance
Previous studies have confirmed that a low resource requirement leads to the poly-tolerance of polyploids, which may be caused by the overexpression of certain genes under different abiotic stresses (Deng et al. 2012; Yeap et al. 2018). Studies showed that significant changes in leaf anatomical structure are caused by polyploidization, which also has an important regulatory effect on the photosystems of polyploid plants because of its higher light energy requirements (Cao et al. 2018). In the present study, we thus investigated the anatomical changes in polyploid P. auriculata under cold stresses. Our study showed that the P. auriculata tetraploid had thickened sponge tissue, widened leaf stomata, and an increased guard cell size, which may increase the chloroplast content, thereby enhancing the photosynthesis of tetraploids (Cao et al. 2018; Porcel et al. 2018). In other studies, it has also been shown that tetraploid thickened root parenchyma provides more support for plants under adverse conditions. (Qiang 2006; Mao et al. 2018).
Chlorophyll fluorescence parameters are sensitive to various stress and are ideal indicators for identifying plant stress resistance (Baker 2004). Among them, Fo reflects the degree of permanent damage to plant leaf PSII, and the degree of environmental stress is often indicated by Fv/Fm. When plants are subjected to extreme natural conditions, such as extreme temperature (Du et al. 2012), the Fv/Fm values of the plant leaves decreased (less than normal value of 0.8). Moreover, Fv/Fo reflects the potential activity of PSII (Hou et al. 2016; Dong et al. 2018). In our study, compared to the tetraploid, the Fv/Fm and Fv/Fo of the diploid decreased but the Fo of the diploid increased, revealing that the cold resistance of tetraploid plants is better than that of diploid plants. This result is consistent with the results of Liu et al. (2011). One of the main explanations is that the light energy capture efficiency of the diploid PSII antenna pigment is low, which leads to the blade not fully absorbing light energy for photosynthesis, thus reducing the maximum photochemical efficiency of PSII (Baker 2004; Banks 2017; Zhou et al. 2018). Thus, the better PSII activity of P auriculata tetraploids compared to diploids may be caused by the significant changes in the leaf, stem and root anatomical structures.
Additionally, the stability of plant membrane system is an important indicator for evaluating the strength of plant resistance. Among them, MDA is the final product of membrane lipid peroxidation, indicating cell membrane damage in plants under adverse conditions (Wang et al. 2017). In this study, under low-temperature stress, the increase of the diploid REC and MDA is twice than that of the tetraploid. In detail, 5 °C had little effect on the tetraploid, and made the diploid wilt slightly. However, 0 °C and − 5 °C greatly weakened the photosynthetic capacity of the diploids, while the tetraploids had better cold tolerance. The results indicated that the stability of the tetraploid plant membrane system is better than that of the diploid. This finding is consistent with the chlorophyll fluorescence parameter results (Oustric et al. 2017).
Conclusions
To our knowledge, we have successfully developed a fast, easy and feasible method for the polyploidization of P. auriculata plants firstly. Our study has demonstrated that for this polyploidization, pendimethalin is superior to trifluralin, while in vitro induction is superior to in vivo induction. Besides, the tetraploid of P. auriculata had larger leaves, thicker stems, and larger flowers than the diploid, proving that the cold resistance of the tetraploid was better. The feasibility of using morphological characteristics and the FCM as an indirect identification method was verified. Further, the rapid and accurate polyploid identification technology is summarized in the current study, which can improve the identification efficiency of polyploids and greatly reduce the associated workload. Taken together, our findings provide a technical and theoretical support for the cold-tolerance breeding and have important practical significance for enriching the germplasm resources of new varieties of P. auriculate plants.
Abbreviations
- FCM:
-
Flow cytometry
- Fm:
-
Maximum fluorescence
- Fo:
-
Minimal value of chlorophyll a fluorescence
- Fv:
-
Variable fluorescence of photosystem II
- Fv/Fm:
-
The maximal quantum yield of photosystem II
- Fv/Fo:
-
Maximum primary yield of photochemistry of PSII
- MDA:
-
Malondialdehyde
- PSII:
-
Photosystem II
- REC:
-
Relative electrical conductivity
References
Baker NR (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621
Banks JM (2017) Continuous excitation chlorophyll fluorescence parameters: a review for practitioners. Tree Physiol 37:1128–1136
Bao N, Ou J, Xu M, Guan F, Shi W, Sun J, Chen L (2017) Novel NO-releasing plumbagin derivatives: design, synthesis and evaluation of antiproliferative activity. Eur J Med Chem 137:88–95
Cai X, Cao Z, Xu S, Deng Z (2015) Induction, regeneration and characterization of tetraploids and variants in ‘Tapestry’ caladium. Plant Cell Tissue Organ Cult 120:689–700
Cao Q, Zhang X, Gao X, Wang L, Jia G (2018) Effects of ploidy level on the cellular, photochemical and photosynthetic characteristics in Lilium FO hybrids. Plant Physiol Biochem 133:50–56
Denaeghel HER, Van Laere K, Leus L, Lootens P, Van Huylenbroeck J, Van Labeke M (2018) The variable effect of polyploidization on the phenotype in escallonia. Front Plant Sci 9:12321–12334
Deng B, Du W, Liu C, Sun W, Tian S, Dong H (2012) Antioxidant response to drought, cold and nutrient stress in two ploidy levels of tobacco plants: low resource requirement confers polytolerance in polyploids? Plant Growth Regul 66:37–47
Dhingra D, Bansal S (2015) Antidepressant-like activity of plumbagin in unstressed and stressed mice. Pharmacol Rep 67:1024–1032
Dimitrov ADAB (2000) Influence of the herbicide stomp 330 on morphogenetic response of triticale callus cultures. Cytologica evidences for its mutagenic action. Cytologia 23:123–125
Dong N, Li Y, Qi J, Chen Y, Hao Y (2018) Nitric oxide synthase-dependent nitric oxide production enhances chilling tolerance of walnut shoots in vitro via involvement chlorophyll fluorescence and other physiological parameter levels. Sci Hortic 230:68–77
Du Y, Li J, Wang H, Tang X, Hu F (2012) Effects of high temperature stress on photosynthesis and Chlorophyll fluorescence of flag leaves of rice. J Ecol 31:2541–2548
Eng W, Ho W (2019) Polyploidization using colchicine in horticultural plants: a review. Sci Hortic 246:604–617
Gallone A, Hunter A, Douglas GC (2014) Polyploid induction in vitro using colchicine and oryzalin on Hebe ‘Oratia Beauty’: production and characterization of the vegetative traits. Sci Hortic 179:59–66
Gou Y, Zhang Y, Qi J, Kong L, Zhou Z, Liang S, Yang F, Liang H (2015) Binding and anticancer properties of plumbagin with human serum albumin. Chem Biol Drug Des 86:362–369
Gupta P, Sarkar A, Sandhu P, Daware A, Das MC, Akhter Y, Bhattacharjee S (2017) Potentiation of antibiotic against Pseudomonas aeruginosa biofilm: a study with plumbagin and gentamicin. J Appl Microbiol 123:246–261
Hollister JD (2014) Polyploidy: adaptation to the genomic environment. New Phytol 270:488
Hou W, Sun AH, Chen HL, Yang FS, Pan JL, Guan MY (2016) Effects of chilling and high temperatures on photosynthesis and chlorophyll fluorescence in leaves of watermelon seedlings. Biol Plant 60:148–154
Hussey G, Falavigna A (1980) Origin and production of in vitro adventitious shoots in the onion, Allium cepa L. J Exp Bot 125:1675–1686
Jaradat NA, Zaid AN, Hussein F (2016) Investigation of the antiobesity and antioxidant properties of wild Plumbago europaea and Plumbago auriculata from North Palestine. Chem Biol Technol Agric 3:156–158
Karimi R, Ershadi A (2015) Role of exogenous abscisic acid in adapting of ‘Sultana’ grapevine to low-temperature stress. Acta Physiol Plant 37:1–11
Kong S, Duan N, Liu B, Gao L, Liu B, Xu K (2016) Effects of different mutagens on the induction of tetraploid garlic. J Nucl Agric Sci 30:1067–1073
Lei T, Zhao C, Li, F, Gao S (2016) Optimization of Plumbago auriculata chromosome production and karyotype analysis. In: 2016 China ornamental horticulture symposium. Changsha, Hunan, p 5
Leitch IJ, Bennett MD (1997) Polyploidy in angiosperms. Trends Plant Sci 1:470–476
Li H (2003) Principles and techniques of plant physiological and biochemical experiments. Higher Education Press, Beijing, pp 37–281
Liu S, Chen S, Chen Y, Guan Z, Yin D, Chen F (2011) In vitro induced tetraploid of Dendranthema nankingense (Nakai) Tzvel. shows an improved level of abiotic stress tolerance. Sci Hortic 127:411–419
Liu H, Gao S, Jiang F, Lei X, Deng M, Wang C (2014) Comparative study on the occurrence of polyploidy in Dioscorea opposita Thunb. in vitro induced by pendimethalin and colchicine. J Nucl Agric Sci 28:1985–1992
Liu Y, Cai Y, He C, Chen M, Li H (2017) Anticancer properties and pharmaceutical applications of plumbagin: a review. Am J Chin Med 45:423–441
Luo Z, Iaffaldano BJ, Cornish K (2018) Colchicine-induced polyploidy has the potential to improve rubber yield in Taraxacum koksaghyz. Ind Crop Prod 112:75–81
Mao H, Chen M, Su Y, Wu N, Yuan M, Yuan S, Brestic M, Zivcak M, Zhang H, Chen Y (2018) Comparison on photosynthesis and antioxidant defense systems in wheat with different ploidy levels and octoploid triticale. IJMS 19:3006
Nadakuduti SS, Buell CR, Voytas DF, Starker CG, Douches DS (2018) Genome editing for crop improvement-applications in clonally propagated polyploids with a focus on potato (Solanum tuberosum L.). Front Plant Sci 132:1356–1476
Otto SP (2007) The evolutionary consequences of polyploidy. Cell 131:452–462
Oustric J, Morillon R, Luro F, Herbette S, Lourkisti R, Giannettini J, Berti L, Santini J (2017) Tetraploid Carrizo citrange rootstock (Citrus sinensis Osb. ×Poncirus trifoliata L. Raf.) enhances natural chilling stress tolerance of common clementine (Citrus clementina Hort. ex Tan). J Plant Physiol 214:108–115
Podwyszyńska M, Trzewik A, Marasek-Ciolakowska A (2018) In vitro polyploidisation of tulips (Tulipa gesneriana L.) Phenotype assessment of tetraploids. Sci Hortic 242:155–163
Porcel R, Bustamante A, Ros R, Serrano R, Mulet Salort JM (2018) BvCOLD1: a novel aquaporin from sugar beet (Beta vulgaris L.) involved in boron homeostasis and abiotic stress. Plant Cell Environ 41:2844–2857
Qiang S (2006) Botany. Higher Education Press, Beijing, p 134
Regalado JJ, Carmona Martín E, Castro P, Moreno R, Gil J, Encina CL (2015) Study of the somaclonal variation produced by different methods of polyploidization in Asparagus officinalis L. Plant Cell Tissue Organ Cult 122:31–44
Regalado JJ, Carmona-Martín E, Querol V, Veléz CG, Encina CL, Pitta-Alvarez SI (2017) Production of compact petunias through polyploidization. Plant Cell Tissue Organ Cult 129:61–71
Saghahazrati S, Ayatollahi SA, Kobarfard F, Zang BM (2020) The synergistic effect of glucagon-like peptide-1 and chamomile oil on differentiation of mesenchymal stem cells into insulin-producing cells. Cell J 21:371–378.
Schneider H, Liu H, Chang Y, Ohlsen D, Perrie LR, Shepherd L, Kessler M, Karger DN, Hennequin S, Marquardt J, Russell S, Ansell S, Lu NT, Kamau P, Lóriga J, Regalado L, Heinrichs J, Ebihara A, Smith AR, Gibby M (2017) Neo- and paleopolyploidy contribute to the species diversity of Asplenium -the most species-rich genus of ferns. J Syst Evol 55:353–364
Schurer R, Schippers JC, Kennedy MD, Cornelissen ER, Salinas-Rodriguez SG, Hijnen WAM, van der Wal A (2019) Enhancing biological stability of disinfectant-free drinking water by reducing high molecular weight organic compounds with ultrafiltration posttreatment. Water Res 164:114927. https://doi.org/10.1016/j.watres.2019.114927
Shi Q, Liu P, Wang J, Xu J, Ning Q, Liu M (2015) A novel in vivo shoot regeneration system via callus in woody fruit tree Chinese jujube (Ziziphus jujuba Mill.). Sci Hortic 188:30–35
Soltis PS, Soltis DE (2009) The role of hybridization in plant speciation. Annu Rev Plant Biol 60:561–588
Soltis DE, Albert VA, Leebens-Mack J, Bell CD, Paterson AH, Zheng C, Sankoff D, de Pamphilis CW, Wall PK, Soltis PS (2009) Polyploidy and angiosperm diversification. Am J Bot 96:336–348
Tavan M, Mirjalili MH, Karimzadeh G (2015) In vitro polyploidy induction: changes in morphological, anatomical and phytochemical characteristics of Thymus persicus (Lamiaceae). Plant Cell Tissue Organ Cult 122:573–583
Tian X, Zhou X, Gong N (2011) Application of flow cytometry in botanical research——detecting nuclear DNA content and ploidy level in plants. Chin Agric Sci Bull 9:21–27
Wang T, Pei Y, Guo X, Li J, Song X (2017) Identification of cold tolerance of three maize varieties at seedling stage. Chin J Nucl Agric 31:803–808
Yeap WC, Namasivayam P, Ooi TEK, Appleton DR, Kulaveerasingam H, Ho CL (2018) EgRBP42 from oil palm enhances adaptation to stress in Arabidopsis through regulation of nucleocytoplasmic transport of stress-responsive mRNAs. Plant Cell Environ 12:1456–1459
Zhang F, Xue H, Lu X, Zhang B, Wang F, Ma Y, Zhang Z (2015) Autotetraploidization enhances drought stress tolerance in two apple cultivars. Trees 29:1773–1780
Zhou B, Guo Z (2009) Calcium is involved in the abscisic acid-induced ascorbate peroxidase, superoxide dismutase and chilling resistance in Stylosanthes guianensis. Biol Plantarum 53:63–68
Zhou H, Zeng W, Yan H (2017) In vitro induction of tetraploids in cassava variety ‘Xinxuan 048’ using colchicine. Plant Cell Tissue Organ Cult 128:723–729
Zhou R, Wu Z, Wang X, Rosenqvist E, Wang Y, Zhao T, Ottosen C (2018) Evaluation of temperature stress tolerance in cultivated and wild tomatoes using photosynthesis and chlorophyll fluorescence. Hortic Environ Biotechnol 59:499–509
Acknowledgements
This work was partial supported by the Breeding Project of the Sichuan Province 13th Five-Year Plan to Tackle Key Problems, China (No. 2016NYZ0038).
Author information
Authors and Affiliations
Contributions
YJ wrote the manuscript. YJ, GH and WL performed the experiments. SG and TL assisted with the experimental design. SL, SG, JH, and YL helped to improve the manuscript. XC, QL, LY, DH and JL assisted with analyzing the experimental results. SL, YJ and SG revised the original manuscript based on the comments of reviewers and editors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Alison M.R. Ferrie.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, Y., Liu, S., Hu, J. et al. Polyploidization of Plumbago auriculata Lam. in vitro and its characterization including cold tolerance. Plant Cell Tiss Organ Cult 140, 315–325 (2020). https://doi.org/10.1007/s11240-019-01729-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01729-w