Abstract
Mutation induction is a feasible and established breeding method for crop improvement and genetic diversity creation to introduce new plant cultivars. The present study was aimed at mutagenesis of four chrysanthemum cultivars (‘Homa’, ‘Fariba2’, ‘Arina’, and ‘Delkash’) using ethyl methanesulfonate (EMS) (0, 0.125, 0.25, and 0.5%) as mutagen and leaf disks as explants to obtain novel variants. In addition, genetic polymorphism among mutants and their parents was detected using inter simple sequence repeat (ISSR) and inter-retrotransposon amplified polymorphism (IRAP) molecular markers. A total of 2082 plantlets were produced through EMS induced mutagenesis under in vitro conditions and at the end 58 mutants including 28 leaf and 32 flower mutants were analyzed for phenotypic and molecular variation. The explant survival rate decreased by increasing EMS concentration. A wide range of phenotypic leaf and inflorescence variability was obtained in four studied chrysanthemum cultivars confirming the efficiency of EMS to create genetic variation and desired mutants. All generated variants with different inflorescence and leaf shape and color were maintained through cuttings and they expressed same traits in the next generation. The mutants were different in leaf size and shape, plant height, day to flowering, inflorescence head size, ray floret color and ray floret size. The used ISSR and IRAP primers could classify chrysanthemum mutants based on cultivar and somewhat based on used EMS concentration confirming their effectiveness for the discrimination of real variants that allow their earlier selection and reduction of the mutant population size. The in vitro EMS-induced mutation can be a promising tool to assist breeding programs for the generation of new chrysanthemum cultivars.
Key message
New chrysanthemum mutants with distinct colors and inflorescence shape were obtained in four chrysanthemum cultivars using ethyl methanesulfonate (EMS). IRAP and ISSR could successfully classify the EMS-induced mutants and their relationship with mother plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetic diversity is a key source for breeding and selection of superior genotypes to access innovative genetic make-up and to develop unique and superior cultivars (Clegg 1990). Ornamental chrysanthemum (Chrysanthemum morifolium Ramat.) is a member of the Asteraceae family and is one of the most important commercial cut and pot flowers cultivated worldwide. In the chrysanthemum market, there is a continuous demand and necessity for novel traits, and modern growers are always the applicants for newly introduced cultivars (Su et al. 2019). Traditional reproduction and thus creation of diversity by conventional hybridization methods is not versatile in chrysanthemum because of high degree of heterozygosity and self-incompatibility, as well as parental ploidy differences (Teixeira da Silva et al. 2013; Teixeira da Silva and Kulus 2014; Zalewska et al. 2007).
The available gene pools for many ornamental crops are limited and getting eroded continuously which limits the generation of desirable recombinants; therefore other sources of diversity need to be exploited (Shirasawa et al. 2016; Ibrahim et al. 2018). In vitro tissue culture is a relevant and valuable tool for mutagenesis studies to create novel variants as plant cells are totipotent and can regenerate to calli, tissues, organs, and complete plants on a large scale (Misra and Saema 2016; Dhaliwal et al. 2015; Wannajindaporn et al. 2016). This strategy can speed up the breeding programs as the resulted genetic variation can directly lead to the development of new varieties (Ahloowalia and Maluszynski 2001). Ornamental plants are ideal candidates for mutation breeding programs as their various commercial attributes, including flower traits (color, size, phenotype, odor, longevity), leaves traits (shape, dimensions, pigment formation, chimera), growth pattern (dwarf or trailing), and physiological characteristics (photoperiod response, flowering time, tolerance to biotic and abiotic stresses) can be improved (Datta 2020). In this connection, the creation of novel flower colors has a great significance in the floriculture market as it is the primary feature with the highest appeal for consumers, even higher than flower scent (Ibrahim et al. 2018; Datta and Chakrabarty 2009; Datta 2020). Furthermore, many commercially important traits can be quickly screened, assorted, and maintained in mutated lines (Ahloowalia and Maluszynski 2001; Datta 2020).
Mutational agents including physical (gamma and X rays, and fast neutrons) and chemical (colchicine, DMS dimethyl sulfate, DES diethyl sulfate, EMS Ethyl methanesulfonate, MNU 1-methyl-1-nitrosourea, and SA Sodium azide) mutagens are extensively employed for in vitro mutagenesis purposes. In this regard, chemical mutagens have successfully been examined to generate new flower varieties in a short period of time and with higher effciency, in particular when rays are not readily available (Shirasawa et al. 2016; Ibrahim et al. 2018; Begum and Dasgupta 2010). Among the chemical mutagens used to develop new cultivars in ornamental plants, EMS has shown to be very effective as it induces many point mutations in the plant genomes. Besides high levels of gene mutations, EMS causes low rates of chromosomal aberrations during mutagenesis (Jankowicz-Cieslak et al. 2012; Luan et al. 2006).
Currently several in vitro studies have recently been performed in chrysanthemum to develop novel variants and to release new varieties. For example, Latado et al. (2004) induced mutation in Ingrid cultivar using EMS on immature floral pedicel explants and obtained 910 putative variant lines which 48 lines were real mutations (5.2% successful mutation rate) deviating in petal color. Purente et al. (2020) used different EMS concentrations to induce phenotypic variation in C. indicum var. aromaticum and obtained leaf and stem mutants with different leaf size and plant height as well as lignin and cellulose content. Stable NaCl-tolerant chrysanthemum mutant lines were successfully generated through in vitro mutagenesis using EMS and the novel variants represented higher antioxidant enzymatic activity than mother plants under saline condition (Hossain et al. 2006). Despite successful generation of chrysanthemum mutants, the cultivars with yellow original flower color have been considered as stable to mutation by both radio- and chemo-mutagens and there just few reported color mutants with different from mother plant (Schum 2003; Miler et al. 2020). Therefore, efficient mutation of yellow color chrysanthemum varieties may lead to introduction of mutants with distinct novel inflorescence shape and color.
Traditionally, chemo- and radio-mutants are screened based on the phenotypic characteristics; however this method of selection is tedious and laborious, and the traits of interest may be affected by environmental conditions (Xi et al. 2012). Moreover, mutagenesis studies require large plant populations as mutation is a low-frequency event in plant cells, and most regenerants are not affected by mutagenic treatment. This highlight need to maintain the candidate regenerated lines until the final selection step of desired mutants that increase the costs of breeding programs (Akhar et al. 2016). Today, molecular markers have been effectively used in mutagenesis breeding for accurate detection of real mutants and for elimination of non-mutated plants in the early steps of the experiment (Xi et al. 2012). Moreover, molecular markers can reveal the genetic relationship between the mutants and original mother plants (Kang et al. 2013).
Inter-retrotransposon amplified polymorphism (IRAP) is a transposon-based marker with widespread distribution in plants genome (Kaki et al. 2020; Kalendar et al. 1999). IRAP markers has been successfully used for fingerprinting of plant germplasm, discrimination of genetic variation, and also for detection of radio-mutants in mutagenesis studies (Kalendar et al. 1999). Despite the reproducibility and power of IRAP markers, they have not yet been used to elucidate genetic relationship of chemo-mutant lines, as mutation mechanism imposed by radio- and chemo-mutagens (in particular EMS) is basically different. Inter-simple sequence repeats (ISSR) markers are 100–3000 bp genomic fragments located between adjacent, oppositely oriented microsatellite regions with more advantages over other marker systems like random amplified polymorphic DNA (RAPD), amplified fragment length polymorphism (AFLP), and short sequence repeat (SSR). This is because ISSRs do not require previous information of the genome, variable primer length, motif, and anchor are available, and they are highly polymorphic and informative (Reddy et al. 2002; Gholami et al. 2021b).
Yellow chrysanthemums are claimed to be the most stable to mutagens treatments and, there are few mutants inflorescence color derived from yellow mother plant. On the other hand, there is no report on the generation of new mutant variants with novel important traits in chrysanthemum cultivars cultivated in Iran. Therefore, the present study was framed for mutagenesis of four chrysanthemum cultivars (‘Homa’, ‘Fariba2’, ‘Arina’, and ‘Delkash’) using ethyl methanesulfonate (EMS) (0, 0.125, 0.25, and 0.5%) as mutagen and leaf disk as explant to obtain novel variants. Furthermore, we tried to examine applicability of IRAP markers to detect polymorphism of EMS-induced mutants and their genetic relationships for the first time. This would be more worthwhile when this marker is used in combination with ISSR markers as both markers cover different parts of genome.
Materials and methods
Plant material
Terminal cuttings (8–10 cm long) of four well-known Iranian chrysanthemum cultivars (‘Homa’, ‘Fariba2’, ‘Arina’, and ‘Delkash’) were obtained from the National Institute of Ornamental Plants (NIOP), Mahallat, Iran. The cultivars were the mid- to late- season garden cultivars with medium and bushy plant size. The inflorescence color of mother plants was yellow, red, yellow and yellowish-orange for ‘Homa’, ‘Fariba2’, ‘Arina’, and ‘Delkash’, respectively. The shape of inflorescence for Homa’, ‘Fariba2’, ‘Arina’, and ‘Delkash’ was double-type, anemone-type, mono-type and double type, respectively. The cuttings were then transferred to the research greenhouse at the Department of Horticultural Sciences and Engineering, University of Kurdistan (35°16′ 51.4′′ N 46°59′46.5′′ E) for further experimentation.
Explant preparation and sterilization
The rooted cuttings grown under greenhouse conditions (temperature 20–23 °C, RH 65–75% and PAR 700–1200 mol m−2 s−1) were used. Young and healthy leaves of chrysanthemum cultivars were then collected and immediately transferred to the lab. For surface sterilization, leaves were first washed in tap water for 15 min, followed by dipping in 70% ethanol/water (v/v) for 1 min and twice rinsing in double-distilled water. The samples were then dipped in sodium hypochlorite solution (1% v/v) containing 2–3 droplets of Tween 20 for 12 min and finally rinsed in sterile distilled water 3–4 times.
Mutagenesis experiment
For the mutation treatment, 1% EMS stock solution)v/v( was used to prepare 0.125%, 0.25%, and 0.5% (v/v) solutions using 0.1 M phosphate buffer (pH 7.2) which were then filter-sterilized with a SFCA-PF 0.2 μm filter (Corning, NY, USA) under aseptic conditions. The leaves were cut into 1 cm pieces and were wounded using a sterile surgical blade, and were then immersed in the tested EMS solutions and sterile distilled water (as control) on a rotary shaker at 60–90 rpm for 60 min. After the mutagenic treatment, all explants were washed with sterile distilled water 4–5 times.
Embryogenic callus induction, embryo conversion and shoot development
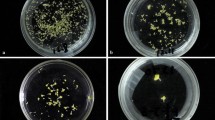
To induce embryogenesis, the EMS-treated leaf disks were cultured abaxially on MS medium (Murashige and Skoog 1962) containing 2 mg L−1 2,4-D and 2 mg L−1 BAP based on our previous optimized protocol (Nasri et al. 2018). All media were prepared using 30 g L−1 sucrose, and the media were solidified with 0.6% agar at pH 5.8 and were then incubated at 25 ± 2 °C in the dark for 30 days, and the explants were then weekly sub-cultured on fresh media with the same PGR combinations and concentrations in a Conviron growth chamber (Conviron Ltd., Winnipeg, Canada) with a 16/8-h light–dark photoperiod at photosynthetically active radiation (PAR) of 160 µmol m−2 s−1 and 60% RH. After 4–5 weeks of incubation, the frequency of explant survival and callus formation (%) from the leaf explants were recorded. In the next step, the calli with somatic embryos (yellowish and compact calli differentiated into various stages of embryogenesis) (Fig. 1B) were cut into 0.5–0.75 cm3 clumps and cultured on media supplemented with 2 mg L−1 BAP and 0.05 mg L−1 NAA for embryo conversion and shoot growth as recommended by Nasri et al. (2018). Finally, 8–9 weeks after EMS treatment, regeneration percentage, the number of shoot (developed from embryos) per explant (clump with 1 cm length and higher Fig. 1E) and days to the development of shoots were recorded.
Callus induction, embryogenesis, and shoot developed from embryos under EMS treatment in C. morifolium. A Yellowish and compact embryogenic callus on EMS-treated leaf explants on MS medium. B Callus differentiated into various stages of embryos on MS medium supplemented with 2 mg L−1 2–4 D and 2 mg L−1 BA, C Green shoot primordia from conversed embryos on MS medium supplemented with 2 mg L−1 BAP and 0.05 mg L−1 NAA, D Shoot clump developed from embryos, E Shoot multiplication and elongation after 4–6 weeks, F In vitro raised mutants plantlets, G Rooting of mutant plantlets on hormone-free half strength MS medium, H, I Acclimizated in vitro raised chrysanthemum plantlets. (Color figure online)
Root growth and development and plantlet acclimization
All plantlets developed from embryogenic calli were transferred to hormone-free half strength MS medium for root growth and development (Fig. 1F). For acclimization, the rooted individual plantlets (3 cm length or higher) (Fig. 1G) were excised from the media and, after washing agar debris, they were directly planted in the small pots containing perlite and coco peat (1:1 v/v) covered with transparent poly bags to inhibit dehydration and desiccation (Fig. 1H). Acclimization process performed in the research greenhouse (24/18 °C day/night temperature, 16/8 h light/dark photoperiod, 60–70% RH and 650–1200 mol m−2 s−1 PAR). The containers were gradually uncovered during the next two weeks when plants were well-established in the soil mixture (Fig. 1I). During the next three weeks, well-rooted, irrigated, and acclimizated chrysanthemum plants were transferred to the greenhouse and kept under nursery conditions for further growth and flowering. During growing and flowering in the greenhouse, characteristics including plant height, inflorescence head type, inflorescence head diameter, length and width of leaf, days to flowering, number of leaf mutants, number of flower mutants, and flower mutation rate (%) were evaluated. The color of the inflorescences were determined based on the Royal Horticultural Society Color Chart (RHS 2015).
DNA extraction and molecular marker analyses
All flower mutants (26 lines) with different inflorescence shapes and ray floret colors along with the mother plant of each cultivar (4 plants) were selected for molecular analysis. Total genomic DNA was purified from leaf tissue using the cetyltrimethylammonium bromide (CTAB) method developed by Doyle and Doyle (1987) with minor modifications. The quantity and quality of purified DNA samples were analyzed by both spectrophotometry at 260 nm and 280 nm wavelengths and agarose gel electrophoresis. Five ISSR (Meyer et al. 1993) and five IRAP primers (Kalendar et al. 1999) were employed (Table 1) for molecular analysis. PCR reactions carried out in a final volume of 10 µL, comprising 20 ng template DNA, 200 µM dNTPs, 0.4 U Taq DNA polymerase (SinaClone, Tehran, Iran), 1 µL 10X PCR buffer, 2 mM MgCl2, and 10 pmol/μl primer. PCR reactions performed in a BioRad thermocycler (Bio-Rad, Hercules, CA, USA) based on the following program: 5 min initial denaturation at 94 °C followed by 28 cycles of 30 s denaturation at 94 °C, 30 s primer annealing at 50–57 °C (depend on used primer) and 45 s extension at 72 °C, with a final extension of 7 min at 72 °C. The amplicons were separated in 1.5% agarose gels and subsequently stained with ethidium bromide. The scoring and size estimation of the amplified bands was done as described by Gholami et al. (2021a). The similarity matrix was obtained based on the Jaccard’s coefficient using the SIMQUAL implemented in the NTSYS software version 2.20 (Rohlf 2000), and the dendrogram was constructed using the unweighted pair group method with arithmetic means (UPGMA). To evaluate the efficiency of primers, the total number of polymorphic bands, polymorphism information content (PIC), and marker index (MI), were estimated according to Powell et al. (1996).
Statistical analyses
The experiment was arranged as factorial based on the completely randomized design (CRD) with 10 replication (each replication defined as petri dish or test container containing ten explants). Cultivars (‘Homa’, ‘Fariba2’, ‘Delkash’, and ‘Arina’) and EMS (0, 0.125%, 0.25%, and 0.5%) were considered as the first and second factors, respectively. The resulted data were subjected to analysis of variance (ANOVA). Mean comparisons were done based on Duncan’s multiple range test at a probability level of 5% (P < 0.05). Data analyses were performed using SAS version 9.1.
Results and Discussion
in vitro regeneration and plantlet development
The explants of studied chrysanthemum cultivars responded differently to the applied EMS concentrations with the highest explant survival rate being 88.48% in ‘Homa’ at 0.125% EMS treatment (Table 1). In contrast, the 0.25% EMS concentration reduced the explant survival rate by 76.91% in ‘Homa’, 71.16% in ‘Arina’, 64.22% in ‘Delkash’, and 54.96% in ‘Fariba2’ compared with the control explants (Table 1). Similarly, the 0.5% EMS concentration reduced the explant survival rates in all studied cultivars. Overall, the ‘Homa’ cultivar was considered to be more tolerant to EMS treatment than the other evaluated cultivars. High explant survival rate is essential to establish an optimized mutagenesis platform using EMS. Survival rate under chemical mutagens varies depend on species and even cultivar type (Wani 2009; Arisha et al. 2014). In total, the survival rate expectedly shown an inverse response with increasing EMS concentrations.
Reduction in the survival of explants as a result of mutagenic treatments has also been reported in other herbaceous species such as Dianthus carophyllus L. (Roychowdhury et al. 2012), Cajanus cajan (Sangle et al. 2011), Sorghum bicolor (Ramulu 1970), and Leucaena leucocephala (ZakyZayed et al. 2014). Reduced survival rate can be attributed an increase in the frequency of chromosomal damage induced by high concentrations of mutagens (Kumar and Rai 2007; Bashir et al. 2013). It may also be due to specific toxic effects imposed by certain biochemical substances in plant cells which finally result in tissue dead (Gocke et al. 2009).
The highest callus induction rates were obtained in non-treated chrysanthemum explants in which they represented the earliest signs of callus formation after 12–24 days of culture establishment (Table 2). Among studied cultivars, ‘Homa’ represented the highest rate of callus induction (80.16%) with 0.125% EMS treatment, followed by ‘Arina’ (71.57%), ‘Delkash’ (63.47%), and ‘Fariba2’ (59.64%) under the same EMS concentration. These results indicate that callus induction rate under EMS pressure was lower than the control samples as it expected. After 30 days, the control and leaf explants treated with 0.125 and 0.25% EMS, produced yellowish and compact embryogenic calli (Fig. 1A), while most of calli obtained under 0.5% EMS treatment, particularly in ‘Fariba2’, were brown and soft, which eventually died after two subcultures.
Plant cultivars represent different callogenesis responses under in vitro condition most probably because of differences in the genetic background that is reflected as different endogenous hormones content and sensitivity to exogenous PGRs (Sarker and Biswas 2002). Decrease in callogenesis under the pressure of high EMS concentrations has also been reported by Purnamaningsih and Hutami (2016) and Koch et al. (2012). This effect may be also due to lethal mutations in the form of chromosomal aberrations induced by EMS (Bashir et al. 2013).
Regeneration rate, the number of shoot developed from embryos indicating shoot multiplication, and days to shoot development were significantly affected by increasing concentrations of EMS (Table 2). Calli derived from leaf explants were started to develop shoots from embryos 3–7 weeks after culture establishment. The lowest (28.43) and highest (53.46) mean number of days to shoot development were recorded in ‘Homa’ (0.125% EMS) and ‘Fariba2’ (0.5% EMS) cultivars, respectively. Increase in EMS concentration imposed an evident toxicity on the regeneration percentage and the number of shoot developed from embryos per explant. The maximum regeneration rate and the highest the number of shoot developed from embryos per explant were detected in control plants, followed by 0.125% and 0.25% EMS treatments, respectively. Several abnormal shoots were observed under high EMS doses, which may probably indicate chromosomal damages and abnormalities induced by EMS. Reduction of regeneration percentage and delaying regeneration time under EMS treatment was also reported in chrysanthemum (Latado et al. 2004), banana (Jankowicz-Cieslak et al. 2012), petunia (Berenschot et al. 2008), and soybean (Li et al. 2017). This effect which has been also reported by using other chemical mutagens can be associated with the production of toxic substances. The alkylating agents induce mutations by adding a methyl or ethyl group to one of the four DNA nucleotides and eventually lead to a modified base form. The presence of such modified bases in the template DNA can block DNA replication (Britt 1996) and thus suppress cell division and differentiation.
Greenhouse observations
A total of 2082 plantlets were produced through the EMS-induced mutagenesis under in vitro conditions, and at the end, 58 mutants (with distinct leaf and inflorescence morphology from mother plants), including 32 leaf and 26 flower mutants (Figs. 2 and 3), were analyzed at the morphological level. The phenotypic analysis represented a significant variations between EMS-treated and control plants (Table 2). All developed flower and leaf variants were maintained through cuttings in the next generation, where they expressed the same traits.
Inflorescence morphology in EMS-induced mutants, A cv. ‘Homa’, Hom-C Control (RHSCC code: 10C); Hom-E1-1 (RHSCC code: 155B), Hom-E1-2 (RHSCC code: 10B), mutant obtained with 0.125% EMS; Hom-E2-1 (RHSCC code: 10B), Hom-E2-2 (RHSCC code: 8B), Hom-E2-3 (RHSCC code: 10C), Hom-E2-4 (RHSCC code: 9C) mutants obtained with 0.25% EMS; Hom-E3-1 (RHSCC code: 12C) mutant obtained with 0.5% EMS, B cv. ‘Arina’, Ari-C Control (RHSCC code: 43B), Ari-E1-1 (RHSCC code: 43A), Ari-E1-2 (RHSCC code: 43A), Ari-E1-3 (RHSCC code: 44C) mutants obtained with 0.125% EMS; Ari-E2-1 (RHSCC code: 32C), Ari-E2-2 (RHSCC code: 22A), Ari-E2-3 (RHSCC code: 14C) mutants obtained with 0.25% EMS; Ari-E3-1 (RHSCC code: 46A) mutant obtained with 0.5% EMS. The bar under each inflorescence represent 1 cm
Inflorescence morphology of EMS-induced mutants in four C. morifolium cultivars, A cv. ‘Fariba2’, Far-C Control (RHSCC code: 8C), Far-E1-1 (RHSCC code: 10C) mutant obtained with EMS 0.125%; Far-E2-1 (RHSCC code: 25B) mutant obtained with 0.25% EMS; Far-E3-1 (RHSCC code: 69C), Far-E3-2 (RHSCC code: 47C), Far-E3-3 (RHSCC code: 49C) mutants obtained with 0.5% EMS B cv. ‘Delkash’, Del-C Control (RHSCC code: 23B), Del-E1-1 (RHSCC code: 22B), Del-E1-2 (RHSCC code: 21C) mutant obtained with EMS 0.125%; Far-E2-1 (RHSCC code: 23C), Far-E2-2 (RHSCC code: 25A), Far-E2-3 (RHSCC code: 15C), Far-E2-4 (RHSCC code: 15C) mutants obtained with 0.25% EMS; Del-E3-1 (RHSCC code: 25A) mutants obtained with 0.5% EMS. The bar under each inflorescence represent 1 cm
Compared to control plants, a significant reduction in height was observed in mutants generated with 0.25 and 0.5% EMS, while 0.125% EMS treatment increased plant height. In this regard, the highest (55. 95 ± 1.56 cm) and the lowest (19.16 ± 2.21 cm) mean plant height was recorded in ‘Homa’ treated with 125% EMS and ‘Delkash’ treated with 0.5% EMS, respectively. Moreover, among all 2082 putative mutant plants, six dwarf variants were identified. The plants propagated from cuttings of these mutants represented similar range of plant height. This stability was also shown by Miler et al. (2021) on chrysanthemum radio-mutants.
Higher mutagenic efficiency on plant height using the lower EMS concentrations has earlier been shown in chrysanthemum (Datta et al. 2005), calendula (El-Nashar and Asrar 2016), tomato (Laskar et al. 2018), and lentil (Amin et al., 2015). The increase in plant height could be due to higher rates of cell division and expansion in internode cells (Biro et al. 1980). Seedling and plant length has been generally utilized as an index to evaluate the overall biological impact of different physical and chemical mutagens (Bhat et al., 2007). The control of plant height in chrysanthemum is one of the main breeding goals for the generation of small, compact, and dwarf pot cultivars that are used in edging borders and rock gardens (Anderson 2006). The present results are in line with the findings obtained in chrysanthemum (Purente et al. 2020), Withania somnifera (Das et al. 2010), and pepper (Arisha et al. 2015), in which plant height decreased in response to high EMS concentrations. This effect may be attributed to the inhibition of cell elongation in epidermis, defect in GA biosynthesis, cell cycle suspension during somatic cell division, meristematic cells injuries, and delay in mitosis (Arisha et al. 2015; Shinoyama et al. 2006; Gocke et al. 2009).
In cut and pot flowers, early flowering or predictable flowering time, are important in timely sales during the holiday periods. In the present study, compared to control plants, the number of days to flowering significantly decreased in mutant lines generated with 0.125% EMS, while late flowering was observed under higher EMS levels (0.25 and 0.5%). Under 0.125% EMS treatment, ‘Homa’ had the minimum time for flowering (69 ± 2.1 days), followed by Ariana and ‘Fariba2’ (73 ± 1.76) and ‘Delkash’ (74 ± 1.11 days), while the maximum time to flowering (84 ± 2.14 days) was recorded in ‘Delkash’ variants obtained with 0.5% EMS. Mutagenesis could provide a sufficient variability of flowering time in variant populations with the potential of exploitation in the breeding of early or late flowering cultivars (El-Nashar and Asrar 2016). Change in flowering time and duration induced by mutagenic treatments could be due to the alteration of molecular pathways, which are directly and/or indirectly associated with the flowering physiology (Mahure et al. 2010). Inhibition of cell growth and decreasing growth rate could be the main reason of late flowering under high EMS concentrations (El-Nashar and Asrar 2016).
A significant increase in inflorescence head diameter was observed in mutants obtained with 0.125 and 0.25% EMS concentrations, while this parameter was decreased under 0.5% EMS treatment. The inflorescence head diameter in plants treated with 0.125% EMS was 5.91 ± 0.81, 5.08 ± 0.11, 6.42 ± 0.33 and 6.89 ± 0.27 cm in ‘Homa’, Ariana, ‘Fariba2’, and ‘Delkash’ cultivars, respectively (Table 3), showing the highest inflorescence diameter values. The reducing effect of high concentration of chemical mutagens (SA and DES) on inflorescence size was also observed in Calendula officinalis L. (El-Nashar and Asrar 2016). Kapadiya et al. (2014) recorded similar observations in chrysanthemum cv. Maghi and they concluded that higher concentrations of mutagen could impose deleterious physiological and molecular effects on overall plant growth and development, resulting in smaller plants and in turn smaller leaf and inflorescence size probably due to both chromosomal damages and extrachromosomal origins.
As expected, no visible change in inflorescence color was observed in the control population, but the mutant lines treated with EMS (in particular 0.25%) produced solid and chimeric variants with high rate of variation in inflorescence color. Solid variants were the mutated lines with only one color different from the mother plant, while the chimeric mutants had ray florets with more than one color sectors. Compared to control plants, the highest flower mutation rates were observed in ‘Delkash’ (2.81%), ‘Fariba2’ (2.08%), ‘Homa’ (1.87%), and ‘Arina’ (1.55%) cultivars, respectively, all treated with 0.25% EMS (Table 3). The original ray floret color of cv. ‘Homa’ was yellow (Fig. 2 Hom-C,), while one of mutants generated with 0.125% and 0.5% EMS had white (Fig. 2 Hom-E1-1) and light yellow (Fig. 2 Hom-E2-4) ray floret colors, respectively. On the other hand, the original shape of ray floret in this cultivar was ligulate with shallow keels, while in one of the mutants generated by 0.125% EMS (Fig. 2 Hom-E2-4), the floret rays represented deep keels. Furthermore, this mutant represented an anemone-like disk type but the non-mutated plants had a daisy-like disk type. More interestingly, in one of the ‘Homa’ mutants obtained with 0.125% EMS, there were morphologically different ray florets with darker yellow color and spatulate shape (Fig. 2 Hom-E2-2). Similar to ‘Homa’, EMS treatments resulted in mutants with new inflorescence attributes in ‘Arina’ cultivar. However, there was no distinct color change in ‘Arina’ mutants generated with 125% EMS, a semi-full inflorescence with was observed in these mutants, in which disk florets transformed to ray florets (Fig. 2 Ari-E1-1, Ari-E1-2 and Ari-E1-3). In contrast, three ‘Arina’ mutants generated with 0.25% EMS had chimeric petals with a mix of red and predominant yellow colors (Fig. 2 Ari-E2-1, Ari-E2-2 and Ari-E2-3), where one of these mutants developed tabular ray florets (Fig. 2 Ari-E2-2). Change in both color and shape of ray florets was observed in mutants of ‘Fariba2’ cultivar, in which their color varied from light yellow (Fig. 3 Far-E1-1) to orange (Fig. 3 Far-E2-1), pink (Fig. 3 Far-E3-1), and red (Fig. 3 Far-E3-2 and Far-E3-3), whereas the control plants had yellow color ray florets (Fig. 1 Far-C). Interestingly, the shape of ray floret in ‘Fariba2’ variant generated with 0.125% EMS changed to quilled form (Fig. 3 Far-E2-1), while the original mother plant had ligulate ray florets. Unlike ‘Fariba2’, ‘Homa’ and ‘Arina’ cultivars, there were fewer changes in inflorescence color and shape in mutants of the ‘Delkash’ cultivar. The original color of ray floret in ‘Delkash’ was yellowish-orange (Fig. 3 Del-C), while in mutants obtained with 0.125 and 0.25% EMS, ray floret tended to be more yellowish (Fig. 3 Del-E1-1, Del-E1-2, Del-E2-2, Del-E2-3 and Del-E2-4). Furthermore, in one of the ‘Delkash’ mutants obtained with 0.25 EMS (Fig. 3 Del-E2-2), ray florets were narrow and quilled-shape, while the non-mutated mother plant had ligulate ray florets.
Chemo- and radio-mutants with modified ray floret colors have also been reported in other chrysanthemum cultivars, including Ingrid (Latado et al. 2004), Flirt, Sunil, Puja, and Maghi (Datta et al. 2005), Snow Ball (Kaul et al. 2011), Bindiya (Mahure et al. 2010), H13 and Shiroyamate (Matsumura et al. 2010), Lalima (Misra et al. 2003), Youka (Soliman et al. 2014), and Albugo, Alchimist, and Satinbleu (Zalewska et al. 2010). One of the most important finding of the present study was the creation of mutation in cultivars with original yellow inflorescence color in particular Fariba2 for which five mutant with inflorescence color completely different from mother plants were obtained. This is because yellow chrysanthemum cultivars are claimed to be the most stable under mutagens treatment (Schum 2003; Miler et al. 2020). Langton (1980) attributed this difficulty to the direct inheritance of carotenoid genes which are present at L1 layer of corolla cells. However, now it is well-known that the color of chrysanthemum inflorescence is mediated by plant pigments that their nature and level are affected by various genetic factors, including structural genes, transcriptional factors, and genes controlling the complete metabolic pathways. Anthocyanins, carotenoids, and flavones are among the main pigment groups determining ray floret color in chrysanthemum (Malaure et al. 1991; Lema-Rumińska and Mellem 2017). The change in the color of ray florets may be most probably due to mutations in one or few genes involved in biosynthesis of anthocyanins or carotenoids (Yoosumran et al. 2018; Latado et al. 2004; Liu et al. 2021). In chrysanthemum, there are some key functional genes such as violaxanthin de epoxidase (VDE) and lycopene e-cyclase (LCYE) that control carotenoid biosynthesis, and their mutation by chemical or radiant mutagens can induce change in the nature and content of carotenoids in mutated variants (Zalewska et al. 2011; Lema‐Rumiñska et al. 2004; Soliman et al. 2014). On the other hand, chemical mutagens like EMS could lead to partial or complete inactivation of the genes encoding the functional enzymes in the anthocyanin biosynthesis pathway (Shi et al. 2021). This probably leads to the accumulation of intermediate compounds and change in the combination of anthocyanins and in turn modification of petal color. Mutations may also take place in the genes encoding proteins responsible for the transport of anthocyanidins over membranes to vacuoles (GS-X), where they are deposited (Lema‐Rumiñska et al. 2004; Shi et al. 2021). Chimerism is phenomenon which is usually take place in chrysanthemum mutagenesis studies and it hinders the selection of real mutants (Miler and Zalewska 2014). In fact the observed change in leaves or inflorescences could be the results of epigenetic changes due to transient gene expression change which can not be inherited to the next generation (Frank and Chitwood 2016). However, as we could observe same characteristics in the next generation (plant obtained from the cuttings of in vitro raised and acclimatized mutants), where they expressed the same traits, the resultant phenotypically plants can be real mutants.
We obtained morphologically different inflorescence types (in whole inflorescence and/or in the shape and size of ray and disk floret) of the mutated lines. It has been stated that change in inflorescence structure induced by mutagens may be due to the mutations in transcription factors and MADS-box genes responsible for initiation and development of flower parts (Lee et al. 2008; Benlloch et al. 2009; Liu et al. 2021). Some of these mutations, particularly those affecting floret shape, can be pivotal for the development of new fascinating chrysanthemum cultivars. For example, the tubular shape of ray florets has been known as an attractive novel character, and there is high demand in the flower industry for cultivars with tabular ray florets. Different mutagens have been employed to develop novel cultivars with tubular florets and in this connection, several EMS-induced variants with tubular florets and other phenotypically important traits have already been developed and commercialized (Padmadevi and Jawaharlal 2011; Datta et al. 2005). However, the mutants obtained in the present study are different from variants reported in previous studies as they have new combination of ray floret colors and shape. This was more obvious in case of ‘Homa’ and ‘Arina’ cultivars where such unique and novel mutants have not already produced and they have potential to be introduced as novel cultivars in chrysanthemum market.
During the growth under greenhouse conditions, there was a clear leaf morphology difference between the mutant and control populations. In this regard, the leaf size was increased in mutants obtained with 0.125% EMS, while it decreased in plants treated with 0.25 and 0.5% EMS (Table 2). Leaf size is the most significant vegetative character that could indirectly affect plant performance and even flower size and quality. The variation in leaf size induced by different mutagens has been reported in chrysanthemum (Kapadiya et al. 2014) and other plant species (Behera et al. 2012; Chen et al. 2018; El-Nashar and Asrar 2016). It has been stated that increase in leaf size under mutagenic chemical application could be result of chromosomal aberrations and disturbance in DNA replication favoring enlargement of palisade and spongy mesophyll cells (Shah et al. 2015). In contrast, suppressed cell division and inhibited auxin biosynthesis could be the main reasons of growth retardant observed under high levels of mutagens (Mohd-Yusoff et al. 2015). Among leaf mutants, there was a visible mutant in cv. ‘Homa’ with small leaves (Fig. 4 Home-E3) and also a chimeric leaf variant in cv. ‘Arina’ (Fig. 4 Ari-E3), both obtained with 5% EMS. This type of mutants can be used for breeding of novel candidate cultivars as the foliage provides ornamental interest when plants are not at flowering stage. Furthermore, these mutations are important to identify the function of genes responsible for leaf morphogenesis and development and also to elucidate chlorophyll metabolism and its regulation (Arisha et al. 2015).
Leaf morphology of EMS-induced mutants in four C. morifolium cultivars, A cv. ‘Homa’, Hom-C Control; Hom-E1 mutant obtained with 0.125% EMS; Hom-E2 mutant obtained with 0.25% EMS; Hom-E3 mutant obtained with 0.5% EMS, B cv. ‘Arina’, Ari-C Control, Ari-E1 mutant obtained with 0.125% EMS; Ari-E2 mutants obtained with 0.25% EMS; Ari-E3 mutant obtained with 0.5% EMS. C cv. ‘Fariba2’, Far-C Control, Far-E1 mutant obtained with EMS 0.125%; Far-E2 mutant obtained with 0.25% EMS; Far-E3 mutants obtained with 0.5% EMS D cv. ‘Delkash’, Del-C Control, Del-E1 mutant obtained with EMS 0.125%; Far-E2 mutant obtained with 0.25% EMS; Del-E3 mutants obtained with 0.5% EMS. The bar under each leaf represent 1 cm
Molecular marker analyses
Study of relationship and genetic variation of in vitro-raised mutants via more than one marker system has been recommended as each marker system can cover and target different regions of plants genome (Rahmani et al. 2015). In the present study, all 10 studied IRAP and ISSR primers produced sharp, re-amplifiable, and scorable bands. In total, 62 (ranged from 150 to 3000 bp) and 59 (ranged from 100 to 3000 bp) fragments were amplified for IRAP and ISSR markers, respectively (Table 3). The highest (0.3) and the lowest (0.15) PIC values were obtained for LTR6149 and URP1F primers, respectively. However, ISSR primers represented higher averages of polymorphic bands, IRAP primers provided more PIC values. The PIC is an indicator of marker informativeness to estimate the discriminating power of loci (Ebadi et al. 2019) and higher PIC values of IRAP marker may show they were generally better in distinguishing and discriminating EMS-induced mutants. This was further supported by higher MI values of the IRAP primers. As pivotal criteria, PIC and MI give an important benchmark helping to assess the efficiency of the primers used in genetic diversity and mutagenesis studies (Hossain et al. 2006).
The resulted UPGMA dendrogram classified the EMS-induced mutants based on cultivar and in some cases, based on the applied EMS concentrations (Fig. 5A and B). In ISSR dendrogram, all mutants of cv. ‘Fariba2’ were grouped under a separate cluster excluding Far-E1-1. The same pattern was observed for ‘Homa’’s variants. The mutants of ‘Arina’ and ‘Delkash’ were classified as subclusters in a common group (Fig. 5A). In IRAP dendrogram, mutants of Ariana and ‘Homa’ cultivars were separated in individual clusters, while ‘Delkash’ and ‘Fariba2’ variants were grouped under the same cluster (Fig. 5B). In subclusters of both dendrograms, some of the variants generated with the same EMS concentration were closed together, probably showing similar frequency of mutation. ISSR markers involve the amplification of DNA segments present at an amplifiable distance between two identical microsatellite repeat regions oriented in the opposite direction (Reddy et al. 2002), while the IRAP marker is based on transposable elements that can replicate in the genome (Kalendar et al. 1999). Despite the suitability of ISSR and IRAP markers in the classification of EMS-induced mutations, employment of other molecular markers such as start codon targeted (SCoT) and also the sequencing of amplified and polymorph fragments can aid the accurate identification of mutated genes responsible for observed phenotypic traits. The ISSR and IRAP primers used in the present study could also be used in marker assisted-selection (MAS) programs for chrysanthemum breeding. The application of ISSR for detection of genetic relationship between Chemo- or radio-mutants, has been reported in chrysanthemum (Wang et al. 2020), Lilium (Xi et al. 2012) and Leucaena leucocephala, Miscanthus × giganteus, and Helainthus tuberosus (Altindal 2019), while IRAP marker has not yet been used for this purpose.
Conclusion
Traits such as flower color, shape, size, and scent, are important factors of consumer preference and the global floriculture market thrives on the new introduced cultivars with novel floral characteristics. In the present research, a wide range of phenotypic variability in four well-known Iranian chrysanthemum cultivars was obtained with EMS application confirming its efficiency in creation of genetic variation and desired mutants. The mutants obtained in the present study possess new combination of ray floret colors and shape. These unique and novel mutants have great commercial potential for introduction as novel cultivars in chrysanthemum market. Furthermore, our results proved the effectiveness of ISSR and IRAP markers for the discrimination of EMS-induced chrysanthemum mutants allowing their earlier selection and reduction of the mutant population size.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxy acetic acid
- BAP:
-
N6-benzylaminopurine
- IRAP:
-
Inter retrotransposon amplified polymorphism
- ISSR:
-
Inter simple sequence repeat
- MS:
-
Murashige and Skoog
- NAA:
-
a-Naphthalene acetic acid
- PGRs:
-
Plant growth regulators
- SCoT:
-
Start codon targeted polymorphism
References
Ahloowalia BS, Maluszynski M (2001) Induced mutations: a new paradigm in plant breeding. Euphytica 118(2):167–173. https://doi.org/10.1023/A:1004162323428
Akhar FK, Khadem A, Sharifi A, Nemati Z, Yazdi M, Bagheri A (2016) In vitro mutation induction on TCL explants of Lilium (Lilium spp.) with Ethyl Methane Sulfunate (EMS). J Biol Today’s World 5(10):177–185
Altindal N (2019) Molecular characterization of Helianthus tuberosus L. treated with ethyl methanesulfonate based on inter-simple sequence repeat markers. Int J Environ Sci Technol 16(9):5311–5318. https://doi.org/10.1007/s13762-019-02486-1
Anderson NO (2006) Dendranthema x grandiflora Tzvelv. In: Flower breeding and genetics: issues, challenges and opportunities for the 21st Century. p 389
Arisha M, Liang B, Shah SM, Gong Z, Li D (2014) Kill curve analysis and response of first generation Capsicum annuum L. B12 cultivar to ethyl methane sulfonate. Genet Mol Res 13:10049–10061
Arisha MH, Shah SNM, Gong Z-H, Jing H, Li C, Zhang H-X (2015) Ethyl methane sulfonate induced mutations in M2 generation and physiological variations in M1 generation of peppers (Capsicum annuum L.). Front Plant Sci. https://doi.org/10.3389/fpls.2015.00399
Bashir S, Wani AA, Nawchoo IA (2013) Chromosomal damage induced by gamma rays, ethyl methyl sulphonate and sodium azide in Trigonella foenum-graecum L. Chromosome Bot 8(1):1–6
Begum T, Dasgupta T (2010) A comparison of the effects of physical and chemical mutagens in sesame (Sesamum indicum L.). Genet Mol Biol 33(4):761–766
Behera M, Panigrahi J, Mishra RR, Rath SP (2012) Analysis of EMS induced in vitro mutants of Asteracantha longifolia (L.) Nees using RAPD markers. Indian J Biotech 11:39–47
Benlloch R, Roque E, Ferrandiz C, Cosson V, Caballero T, Penmetsa RV, Beltran JP, Canas LA, Ratet P, Madueno F (2009) Analysis of B function in legumes: PISTILLATA proteins do not require the PI motif for floral organ development in Medicago truncatula. Plant J 60(1):102–111. https://doi.org/10.1111/j.1365-313X.2009.03939.x
Berenschot AS, Zucchi MI, Tulmann-Neto A, Quecini V (2008) Mutagenesis in Petunia x hybrida Vilm and isolation of a novel morphological mutant. Braz J Plant Physiol 20(2):95–103
Biro RL, Hunt ER Jr, Erner Y, Jaffe MJ (1980) Thigmomorphogenesis: changes in cell division and elongation in the internodes of mechanically-perturbed or ethrel-treated bean plants. Ann Bot 45:655–664
Britt AB (1996) DNA damage and repair in plants. Annu Rev Plant Biol 47(1):75–100
Chen C, Cui Q-z, Huang S-w, Wang S-h, Liu X-h, Lu X-y, Chen H-m, Tian Y (2018) An EMS mutant library for cucumber. J Integr Agric 17(7):1612–1619. https://doi.org/10.1016/s2095-3119(17)61765-9
Clegg MT (1990) Molecular diversity in plant populations. Plant Popul Genet Breed Genet Resour 48:98–115
Das A, Datta AK, Bhattacharya A, Bhattacharyya A, Ghose S (2010) EMS induced mutagenesis in Poshita and Jawahar 22 of Withania somnifera (L.) Dunal (Solanaceae). Cytologia 75(3):305–311
Datta S, Misra P, Mandal A (2005) In vitro mutagenesis–a quick method for establishment of solid mutant in chrysanthemum. Curr Sci 28:155–158
Datta SK (2020) Induced mutations: technological advancement for development of new ornamental varieties. Nucleus 63(2):119–129. https://doi.org/10.1007/s13237-020-00310-7
Datta SK, Chakrabarty D (2009) Management of chimera and in vitro mutagenesis for development of new flower color/shape and chlorophyll variegated mutants in Chrysanthemum. Induced plant mutations in the genomics era FAO/IAEA, Rome: pp 303–305
Dhaliwal AK, Mohan A, Sidhu G, Maqbool R, Gill KS (2015) An Ethylmethane sulfonate mutant resource in pre-green revolution hexaploid wheat. PLoS ONE 10(12):e0145227. https://doi.org/10.1371/journal.pone.0145227
Doyle JJ, Doyle JL (1987) A rapid isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Ebadi A, Ghaderi N, Vafaee Y (2019) Genetic diversity of Iranian and some European grapes as revealed by nuclear and chloroplast microsatellite and SNP molecular markers. J Hortic Sci Biotechnol 94(5):599–610. https://doi.org/10.1080/14620316.2019.1585210
El-Nashar YI, Asrar AA (2016) Phenotypic and biochemical profile changes in calendula (Calendula officinalis L.) plants treated with two chemical mutagenesis. Genet Mol Res. https://doi.org/10.4238/gmr.15028071
Frank MH, Chitwood DH (2016) Plant chimeras: the good, the bad, and the ‘Bizzaria.’ Dev Biol 419(1):41–53. https://doi.org/10.1016/j.ydbio.2016.07.003
Gholami S, Vafaee Y, Nazari F, Ghorbani A (2021a) Exploring genetic variations in threatened medicinal orchids using start codon targeted (SCoT) polymorphism and marker-association with seed morphometric traits. Physiol Mol Biol Plants. https://doi.org/10.1007/s12298-021-00978-4
Gholami S, Vafaee Y, Nazari F, Ghorbani A (2021b) Molecular characterization of endangered Iranian terrestrial orchids using ISSR markers and association with floral and tuber-related phenotypic traits. Physiol Mol Biol Plants 27(1):53–68. https://doi.org/10.1007/s12298-020-00920-0
Gocke E, Bürgin H, Müller L, Pfister T (2009) Literature review on the genotoxicity, reproductive toxicity, and carcinogenicity of ethyl methanesulfonate. Toxicol Lett 190(3):254–265
Hossain Z, Mandal AK, Datta SK, Biswas AK (2006) Development of NaCl-tolerant strain in Chrysanthemum morifolium Ramat through in vitro mutagenesis. Plant Biol (stuttg) 8(4):450–461. https://doi.org/10.1055/s-2006-923951
Ibrahim R, Ahmad Z, Salleh S, Hassan AA, Ariffin S (2018) Mutation breeding in ornamentals. In: Ornamental Crops. Springer, pp 175–211
Jankowicz-Cieslak J, Huynh OA, Brozynska M, Nakitandwe J, Till BJ (2012) Induction, rapid fixation and retention of mutations in vegetatively propagated banana. Plant Biotechnol J 10(9):1056–1066. https://doi.org/10.1111/j.1467-7652.2012.00733.x
Kaki A, Vafaee Y, Khadivi A (2020) Genetic variation of Anacamptis coriophora, Dactylorhiza umbrosa, Himantoglossum affine, Orchis mascula, and Ophrys schulzei in the western parts of Iran. Ind Crops Prod 156:112854. https://doi.org/10.1016/j.indcrop.2020.112854
Kalendar R, Grob T, Regina M, Suoniemi A, Schulman A (1999) IRAP and REMAP: two new retrotransposon-based DNA fingerprinting techniques. Theor Appl Genet 98(5):704–711. https://doi.org/10.1007/s001220051124
Kang E-J, Lee Y-M, Sung SY, Ha B-K, Kim SH, Kim DS, Kim J-B, Kang S-Y (2013) Analysis of the genetic relationship of gamma-irradiated in vitro mutants derived from standard-type chrysanthemum cv Migok. Horticult Environ Biotechnol 54(1):76–81. https://doi.org/10.1007/s13580-013-0124-9
Kapadiya D, Chawla S, Patel A, Ahlawat T (2014) Exploitation of variability through mutagenesis in Chrysanthemum (Chrysanthemum morifolium Ramat) var Maghi. Bioscan 94:1799–1804
Kaul A, Kumar S, Ghani M (2011) In vitro mutagenesis and detection of variability among radiomutants of chrysanthemum using RAPD. Adv Horticult Sci 24:106–111
Koch AC, Ramgareeb S, Rutherford RS, Snyman SJ, Watt MP (2012) An in vitro mutagenesis protocol for the production of sugarcane tolerant to the herbicide imazapyr. Vitro Cell Dev Biol Plant 48(4):417–427. https://doi.org/10.1007/s11627-012-9448-x
Kumar G, Rai PK (2007) EMS induced karyomorphological variations in maize (Zea mays L.) inbreds. Turk J Biol 31(4):187–195
Langton FA (1980) Chimerical structure and carotenoid inheritance in Chrysanthemum morifolium (Ramat). Euphytica 29(3):807–812. https://doi.org/10.1007/BF00023228
Laskar RA, Chaudhary C, Khan S, Chandra A (2018) Induction of mutagenized tomato populations for investigation on agronomic traits and mutant phenotyping. J Saudi Soc Agric Sci 17(1):51–60. https://doi.org/10.1016/j.jssas.2016.01.002
Latado RR, Adames AH, Neto AT (2004) In vitro mutation of chrysanthemum (Dendranthema grandiflora Tzvelev) with ethylmethanesulphonate (EMS) in immature floral pedicels. Plant Cell Tissue Organ Cult 77(1):103–106
Lee J, Oh M, Park H, Lee I (2008) SOC1 translocated to the nucleus by interaction with AGL24 directly regulates leafy. Plant J 55(5):832–843. https://doi.org/10.1111/j.1365-313X.2008.03552.x
Lema-Rumińska J, Mellem A (2017) Genetic diversity of chrysanthemum plants derived via somatic embryogenesis using rapd markers. Acta Scientiarum Polonorum Hortorum Cultus 16(6):149–156. https://doi.org/10.24326/asphc.2017.6.13
Lema-Rumiñska J, Zalewska M, Sadoch Z (2004) Radiomutants of chrysanthemum (Dendranthema grandiflora Tzvelev) of the Lady group: RAPD analysis of the genetic diversity. Plant Breed 123(3):290–293
Li Z, Jiang L, Ma Y, Wei Z, Hong H, Liu Z, Lei J, Liu Y, Guan R, Guo Y (2017) Development and utilization of a new chemically-induced soybean library with a high mutation density. J Integr Plant Biol 59(1):60–74
Liu H, Luo C, Chen D, Wang Y, Guo S, Chen X, Bai J, Li M, Huang X, Cheng X, Huang C (2021) Whole-transcriptome analysis of differentially expressed genes in the mutant and normal capitula of Chrysanthemum morifolium. BMC Genom Data 22(1):2. https://doi.org/10.1186/s12863-021-00959-2
Luan Y-S, Zhang J, Gao X-R, An L-J (2006) Mutation induced by ethylmethanesulphonate (EMS), in vitro screening for salt tolerance and plant regeneration of sweet potato (Ipomoea batatas L.). Plant Cell Tissue Organ Cult 88(1):77–81. https://doi.org/10.1007/s11240-006-9183-2
Mahure H, Choudhary M, Prasad K, Singh S (2010) Mutation in chrysanthemum through gamma irradiation. Indian J Hort 67:356–358
Malaure RS, Barclay G, Power JB, Davey MR (1991) The production of novel plants from florets of chrysanthemum morifolium using tissue culture 1: shoot regeneration from ray florets and somaclonal variation exhibited by the regenerated plants. J Plant Physiol 139(1):8–13. https://doi.org/10.1016/s0176-1617(11)80156-2
Matsumura A, Nomizu T, Furutani N, Hayashi K, Minamiyama Y, Hase Y (2010) Ray florets color and shape mutants induced by 12C5+ ion beam irradiation in chrysanthemum. Sci Hortic 123(4):558–561. https://doi.org/10.1016/j.scienta.2009.11.004
Meyer W, Mitchell TG, Freedman E, Vilgalys R (1993) Hybridization probes for conventional DNA fingerprinting used as single primers in the polymerase chain reaction to distinguish strains of Cryptococcus neoformans. J Clin Microbiol 31(9):2274–2280
Miler N, Jedrzejczyk I, Jakubowski S, Winiecki J (2021) Ovaries of Chrysanthemum Irradiated with High-Energy Photons and High-Energy Electrons Can Regenerate Plants with Novel Traits. Agronomy. https://doi.org/10.3390/agronomy11061111
Miler N, Kulus D, Sliwinska E (2020) Nuclear DNA content as an indicator of inflorescence colour stability of in vitro propagated solid and chimera mutants of chrysanthemum. Plant Cell Tissue Organ Cult (PCTOC). 143(2):421–430. https://doi.org/10.1007/s11240-020-01929-9
Miler N, Zalewska M (2014) Somaclonal variation of chrysanthemum propagated in vitro from different explants types. Acta Sci Pol Hortorum Cultus 13(2):69–82
Misra P, Datta S, Chakrabarty D (2003) Mutation in flower colour and shape of Chrysanthemum morifolium induced by γ-radiation. Biol Plant 47(1):153–156
Misra P, Saema S (2016) Plant tissue culture for in vitro mutagenesis, large-scale propagation, and genetic transformation. In: Anis M, Ahmad N (eds) Plant tissue culture: propagation, conservation and crop improvement. Springer Singapore, Singapore, pp 309–342
Mohd-Yusoff NF, Ruperao P, Tomoyoshi NE, Edwards D, Gresshoff PM, Biswas B, Batley J (2015) Scanning the effects of ethyl methanesulfonate on the whole genome of Lotus japonicus using second-generation sequencing analysis. G3 Bethesda 5(4):559–567. https://doi.org/10.1534/g3.114.014571
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nasri F, Zakizadeh H, Vafaee Y, Mozafari AA (2018) Callus induction and plant regeneration of Chrysanthemum morifolium and C. coccineum via direct and indirect organogenesis and genetic fidelity analysis using IRAP, ISSR and SCoT molecular markers. J Ornament Plants 8(4):265–284
Padmadevi K, Jawaharlal M (2011) Induction of in vitro mutation in chrysanthemum (Dendranthema grandiflora Tzvelev) ray florets (var. Ravi Kiran) using gamma rays and EMS. Floricult Ornament Biotechnol 5(1):74–77
Powell W, Morgante M, Andre C, Hanafey M, Vogel J, Tingey S, Rafalski A (1996) The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed 2:225–238
Purente N, Chen B, Liu X, Zhou Y, He M (2020) Effect of ethyl methanesulfonate on induced morphological variation in M3 generation of chrysanthemum indicum var. aromaticum. HortScience 55(7):1099–1104. https://doi.org/10.21273/hortsci15068-20
Purnamaningsih R, Hutami S (2016) Increasing Al-tolerance of sugarcane using ethyl methane sulphonate and in vitro selection in the low pH media. Hayati J Biosci 23(1):1–6
Rahmani M-S, Pijut PM, Shabanian N, Nasri M (2015) Genetic fidelity assessment of in vitro-regenerated plants of Albizia julibrissin using SCoT and IRAP fingerprinting. Vitro Cell Dev Biol Plant 51(4):407–419. https://doi.org/10.1007/s11627-015-9692-y
Ramulu KS (1970) Sensitivity and induction of mutations in sorghum. Mutat Res 10(3):197–206
Reddy MP, Sarla N, Siddiq EA (2002) Inter simple sequence repeat (ISSR) polymorphism and its application in plant breeding. Euphytica 128(1):9–17
RHS (2015) RHS large colour chart, 6th edn. Royal Horticultural Society, London
Rohlf FJ (2000) NTSYS-pc numerical taxonomy and multivariate analysis system. Version 21 Exeter Software, Setauket
Roychowdhury R, Mamgain A, Ray S, Tah J (2012) Effect of gibberellic acid, kinetin and indole 3-acetic acid on seed germination performance of Dianthus caryophyllus (Carnation). Agric Conspec Sci 77(3):157–160
Sangle SM, Mahamune SE, Kharat S, Kothekar V (2011) Effect of mutagenisis on germination and pollen sterility in pigeonpea. Biosci Discov J 2(1):127–130
Sarker R, Biswas A (2002) In vitro plantlet regeneration and Agrobacterium-mediated genetic transformation of wheat (Triticum aestivum L). Plant Tissue Cult 12(2):155–165
Schum A (2003) Mutation breeding in ornamentals: an efficient breeding method? Acta Hort 612:47–60. https://doi.org/10.17660/ActaHortic.2003.612.6
Shah SN, Gong ZH, Arisha MH, Khan A, Tian SL (2015) Effect of ethyl methyl sulfonate concentration and different treatment conditions on germination and seedling growth of the cucumber cultivar Chinese long (9930). Genet Mol Res 14(1):2440–2449. https://doi.org/10.4238/2015.March.30.2
Shi H, Geng B, Zhao Y, Liu Y, Huang R, Zhao P, Guo Z (2021) EMS-induced mutations in common vetch (Vicia sativa L.) and two mutants without anthocyanin accumulation showing increased cold tolerance. Grassland Sci 67(2):148–155. https://doi.org/10.1111/grs.12301
Shinoyama H, Anderson N, Furuta H, Mochizuki A, Nomura Y, Singh R, Datta SK, Wang B, Teixeira da Silva J (2006) Chrysanthemum biotechnology. Floricultu Ornamental Plant Biotechnol 2:140–163
Shirasawa K, Hirakawa H, Nunome T, Tabata S, Isobe S (2016) Genome-wide survey of artificial mutations induced by ethyl methanesulfonate and gamma rays in tomato. Plant Biotechnol J 14(1):51–60. https://doi.org/10.1111/pbi.12348
Soliman TMA, Lv S, Yang H, Hong B, Ma N, Zhao L (2014) Isolation of flower color and shape mutations by gamma radiation of Chrysanthemum morifolium Ramat cv. Youka. Euphytica 199(3):317–324. https://doi.org/10.1007/s10681-014-1127-z
Su J, Jiang J, Zhang F, Liu Y, Ding L, Chen S, Chen F (2019) Current achievements and future prospects in the genetic breeding of chrysanthemum: a review. Horticult Res 6(1):1–19
Teixeira da Silva JA, Kulus D (2014) Chrysanthemum biotechnology: discoveries from the recent literature. Folia Horticult 26(2):67–77
Teixeira da Silva JA, Shinoyama H, Aida R, Matsushita Y, Raj SK, Chen F (2013) Chrysanthemum biotechnology: quo vadis? Crit Rev Plant Sci 32(1):21–52. https://doi.org/10.1080/07352689.2012.696461
Wang L, Wu J, Lan F, Gao P (2020) Morphological, cytological and molecular variations induced by gamma rays in Chrysanthemum morifolium ‘Donglinruixue.’ Folia Horticult 32(1):87–96. https://doi.org/10.2478/fhort-2020-0009
Wani AA (2009) Mutagenic effectiveness and efficiency of gamma rays, ethyl methane sulphonate and their combination treatments in chickpea (Cicer arietinum L.). Asian J Plant Sci 8(4):318
Wannajindaporn A, Kativat C, Tantasawat PA (2016) Mutation induction of dendrobium ‘Earsakul’ using sodium azide. HortScience 51(11):1363–1370. https://doi.org/10.21273/hortsci10860-16
Xi M, Sun L, Qiu S, Liu J, Xu J, Shi J (2012) In vitro mutagenesis and identification of mutants via ISSR in lily (Lilium longiflorum). Plant Cell Rep 31(6):1043–1051. https://doi.org/10.1007/s00299-011-1222-8
Yoosumran V, Ruamrungsri S, Duangkongsan W, Kanjana S (2018) Induced mutation of Dendranthemum grandiflora through tissue culture by ethyl methanesulphonate (EMS). Int J Agricult Technol 14(1):73–82
ZakyZayed M, Ho W-S, Pang S-L, Ahmad FB (2014) EMS-induced mutagenesis and DNA polymorphism assessment through ISSR markers in Neolamarckia cadamba (kelampayan) and Leucaena leucocephala (petai belalang). Eur J Exp Biol 4(4):156–163
Zalewska M, Lema-Rumińska J, Miler N (2007) In vitro propagation using adventitious buds technique as a source of new variability in chrysanthemum. Sci Hortic 113(1):70–73. https://doi.org/10.1016/j.scienta.2007.01.019
Zalewska M, Miler N, Tymoszuk A, Drzewiecka B, Winiecki J (2010) Results of mutation breeding activity on Chrysanthemum × grandiflorum (Ramat.) Kitam. in Poland. Electr J Pol Agric Univ 13(4):27
Zalewska M, Tymoszuk A, Miler N (2011) New chrysanthemum cultivars as a result of in vitro mutagenesis with the application of different explant types. Acta Sci Pol Hortorum Cultus 10(2):109–123
Acknowledgements
The authors acknowledge the Vice-Chancellor of Academic Affairs, University of Kurdistan for financial support.
Author information
Authors and Affiliations
Contributions
YV, HZ and AKM participated in its design and coordination, and guaranteed of integrity of the entire study. FN carried out the experiment. YV and FN performed statistical analysis. YV and FN were involved in drafting the manuscript, evaluated the statistical analysis and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Communicated by M. I. Beruto.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nasri, F., Zakizadeh, H., Vafaee, Y. et al. In vitro mutagenesis of Chrysanthemum morifolium cultivars using ethylmethanesulphonate (EMS) and mutation assessment by ISSR and IRAP markers. Plant Cell Tiss Organ Cult 149, 657–673 (2022). https://doi.org/10.1007/s11240-021-02163-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02163-7