Abstract
A protocol was developed to induce and identify imazapyr tolerance in sugarcane, which involved induction of somaclonal variation via exposure to 8 or 16 mM ethyl methanesulfonate for 4 h, followed by a stepwise increase in imazapyr concentration in the medium from 0.08 to 0.16 μM. The regenerated plantlets were then acclimatized for 3 mo after which they were sprayed with 182 g a.i. ha−1 imazapyr, and the above-ground biomass was determined after 47 d. Following a 1-mo waiting period for the putative tolerant plants to regrow, acetohydroxyacid synthase (AHAS; EC 2.2.1.6) enzyme assays of the plants that survived and showed a normal growth pattern were undertaken. Based on the enzymatic I 50 values, three imazapyr-tolerant genotypes were identified with an AHAS activity of 2.8 to 4.0 times that in sensitive sugarcane plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The application of herbicides to control weeds is a well-established necessity in sugarcane plantations and is most crucial during the establishment of plant cane and the regeneration of the subsequent ratoon crops (Campbell 2008). Weeds can reduce cane and sugar yields by more than 40 % (Richard 1990; Lencse and Griffin 1991; Millhollon 1992, 1995) and cause unnecessary harvesting expenses. However, the misuse of herbicides has considerable negative impacts on both the environment and human health (Bennett et al. 2004). Furthermore, herbicides must be carefully selected and applied as they function by disrupting essential processes (e.g., photosynthesis and amino acid biosynthesis) which can be shared by both crops and weeds (Sandhu et al. 2002; Mulwa and Mwanza 2006). In sugarcane, herbicide application is especially difficult as most of the weeds infesting plantations are also graminaceous species such as Cynodon dactylon, Cynodon plectostachyus, and Digitaria longifolia (Landrey et al. 1993; Campbell et al. 2008).
One approach to herbicide selectivity is the development of cultivars with tolerance to existing broad-spectrum herbicides (Sandhu et al. 2002). In sugarcane, as in most crops, this can be achieved by conventional plant breeding and biotechnological approaches, which include genetic transformation (Gallo-Meagher and Irvine 1996; Falco et al. 2000; Leibbrandt and Snyman 2003) and induced mutagenesis (Irvine et al. 1991; Ali et al. 2007; Kenganal et al. 2008). However, in many parts of the world, neither conventional breeding nor genetic transformation are simple options for sugarcane modification. Sugarcane breeding is often restricted by unfavorable climatic conditions for fertile pollen production (Brett 1950), whereas transgenic sugarcane release for commercial use has been limited due to acceptance by international markets and intellectual property restrictions (Snyman et al. 2008).
Selection of somaclonal variants produced with or without induced mutagenesis, followed by in vitro plant regeneration, has proven successful for obtaining plants tolerant to a variety of stresses (Haughn and Somerville 1986; Irvine et al. 1991; Zambrano et al. 1999; Zambrano et al. 2003a; Ali et al. 2007; Punyadee et al. 2007; Kenganal et al. 2008; Rai et al. 2011). Although physical mutagenesis (radiation) has been used most frequently in sugarcane (Saif-Ur-Rasheed et al. 2001; Zambrano et al. 2003b; Patade et al. 2006; Ali et al. 2007; Patade and Suprasanna 2008; Patade et al. 2008; Khan and Khan 2010), chemical mutagens lead to more specific and predictable mutations (Luan et al. 2007), are easier to administer, and do not require specialized, expensive equipment. Ethyl methanesulfonate (EMS) is one of the preferred mutagens because it causes a high frequency of mutations (primarily point mutations) (Schy and Plewa 1989) at random sites (Sung 1976) with a low frequency of chromosome aberrations (Van Harten 1998). Exposure of Arabidopsis to EMS resulted in mutant plants up to 300-fold more tolerant to the herbicide chlorsulfuron (which inhibits the same enzyme as the imidazolinone herbicides) than the wild-type plant (Haughn and Somerville 1986). EMS is most effective when applied to dividing cells, such as rapidly proliferating callus, because the probability of incorrect repair of the mutation is the highest when the cells are engaged in DNA replication (Kilbey and Hunter 1983). The base pair changes that are generally produced by EMS mutagenesis are G:C to A:T transitions (Jander et al. 2003). In sugarcane, this mutagen has been used to produce salt-tolerant plants (Kenganal et al. 2008), and preliminary work suggests that in combination with an appropriate selection protocol, it can be used for inducing imazapyr tolerance (Koch et al. 2010).
The imidazolinone herbicides such as imazapyr affect a wide spectrum of grass and broadleaf weeds at low application rates, and they possess a favorable environmental profile, including low mammalian toxicity (Tan et al. 2005; Senseman 2007). These herbicides act by inhibiting acetohydroxyacid synthase (AHAS), also referred to as acetolactate synthase (ALS), a key enzyme in the biosynthesis of essential branched-chain amino acids in plants (Haughn and Somerville 1986; Subramanian et al. 1990; Webster and Masson 2001; Kolkman et al. 2004; Tan et al. 2005; Senseman 2007). A reduction in target-site sensitivity, which confers tolerance to imidazolinone herbicides, has been reported to result from one of several single-base-pair changes (Saari et al. 1994; Webster and Masson 2001; Tan et al. 2005). These mutations occur in a number of highly conserved regions in the AHAS enzyme (Webster and Masson 2001). Imidazolinone tolerance has been obtained through in vitro mutagenesis and selection in maize, wheat, rice, oilseed rape, and sunflower (Tan et al. 2005).
The aim of this study was to establish a protocol for the in vitro induction and subsequent regeneration of imazapyr-tolerant sugarcane plants, using EMS as the mutagen. The sugarcane cultivar N12 was selected because it is commonly cultivated by South African growers and has low tolerance to the herbicides currently in use.
Materials and Methods
Standard culture procedures and acclimation.
The indirect somatic embryogenesis protocol of Snyman (2004) was employed. Briefly, leaf disks of immature leaf whorls from sugarcane cultivar N12 were decontaminated in 70 % ethanol for 5 min and then cultured on callus induction medium (CIM) containing MS basal salts and vitamins (Murashige and Skoog 1962) (Highveld Biological, Johannesburg, Republic of South Africa), 3 mg l−1 2,4-dichlorophenoxyacetic acid (2,4-D), 20 g l−1 sucrose, and 8 g l−1 agar (Agar Agar; Saarchem, Johannesburg, Republic of South Africa), pH 5.8, at 26 ± 1°C in the dark. Tissues were subcultured every 2 wk for 6–10 wk. Embryogenic callus (types 3 and/or 4 callus as described by Taylor et al. (1992)) were then transferred to embryo germination medium (EGM) (CIM without 2,4-D), subcultured twice monthly, and maintained in a growth room at 16:8-h light/dark, 160 μE m−2 s−1 photon flux density for 6–8 wk. Following embryo germination, plantlets with shoots greater than 20 mm in height were transferred to Magenta® vessels (Sigma-Aldrich, St. Louis, MO) containing 80 ml of plantlet establishment medium (PEM) (1/2 MS salts and vitamins, 5 g l−1 sucrose, 8 g l−1 agar, pH 5.8) for at least 4 wk.
Once established, in vitro plantlets (50–100 mm shoot height) were transferred to polymer-coated, 105-place (40 × 120 mm) polystyrene Speedling® trays (HygroTech (PTY) Ltd., Pretoria, Republic of South Africa) containing a substrate consisting of one part peat moss/one part vermiculite (v v −1) enhanced with 5 g dolomitic agricultural lime (7.0 % Si, 33.0 % Ca, 5.69 % Mg; Calmasil®, Middelburg, Republic of South Africa) per 10 kg mix. The trays were placed in a misting chamber in the greenhouse (22–30°C, 80 % relative humidity; 180 μE m−2 s−1) and watered with a fine mist sprayer (1 min every 6 h; 1.5 l min−1) during daylight hours for 3 d. Ex vitro plants were then transferred to a polytunnel with a clear polycarbonate roof and shaded cloth sides (17–35°C, 54–60 % relative humidity) with natural daylight and watered by automatic sprayers for 5 min twice a d (600 ml min−1). Plants were fertilized monthly (5:1:5 N/P/K granules, 0.1 g per plant; Grovida, Durban, Republic of South Africa) and remained under these conditions for 90 d before herbicide application.
Mutagenic treatment of embryogenic callus.
Individual pieces (0.2 g) of embryogenic callus were immersed for 4 h in centrifuge tubes containing 10 ml of filter-sterilized mutagen solutions containing 8–96.6 mM EMS (C3H8O3S, 9.7 M, Sigma-Aldrich) made in 1/2-strength liquid CIM. Tubes were inverted every 30 min during the incubation period. The callus pieces were rinsed three times with 5 ml 1/2-strength liquid CIM (with 2,4-D), air-dried on sterile filter paper for 5 min, and transferred to 90-mm Petri dishes containing 30 ml CIM. The cultures were maintained in the dark (26 ± 1°C) for 4 wk, after which the callus pieces were transferred to Petri dishes containing 30 ml EGM and placed in the growth room for 8 wk. Callus pieces were subcultured every 2 wk. The controls were not exposed to EMS.
Imazapyr selection conditions.
Imazapyr PESTANAL® (100 mg, Sigma-Aldrich) was prepared in 15 ml 10 mM potassium phosphate buffer (pH 7.5), filter-sterilized, and added to 30 ml (Petri dishes) or 80 ml (Magenta® vessels) of autoclaved EGM (≥50°C), as required. Medium that contained imazapyr was used within 24 h of being prepared due to photodegradation (Senseman 2007). After preliminary investigations to establish lethal values, 0.025–0.1 μM imazapyr was incorporated into the medium for selection and screening of embryogenic callus and plantlets.
The commercial herbicide Arsenal® (250 g l−1 imazapyr; BASF, Agro BV Arnhem, Zürich Switzerland) was used for spraying acclimated plants. The herbicide (9.25–262.5 g a.i. ha−1) was applied over the top of the plants (ground speed of 1 m s−1) using a hand-held gas-regulated sprayer at a pressure of 200 KPa, fitted with an Albuz(R) APE Flat Fan Nozzle (110°) (Coors Tek, Golden, CO).
Combined EMS and imazapyr treatments.
For each treatment and replication, approximately 0.2 g embryogenic callus was weighed under sterile conditions and exposed to 8 or 16 mM EMS for 4 h. The mutagenized callus pieces were transferred to Petri dishes containing 30 ml CIM and maintained in the dark (26 ± 1°C) for 3 d. Nine different imazapyr selection regimes (IP1–IP9) were tested, using a stepwise increase in imazapyr concentration (0.042–0.16 μM), with five replicates per regime (Table 1). Cultures were maintained on CIM (in the dark) for 4 wk followed by transfer to EGM (under 16-h photoperiod conditions) for 8 wk with 2 wk subcultures. Surviving plantlets were transferred to Magenta® vessels containing PEM and imazapyr and then PEM without imazapyr, for 4 wk each. The control cultures followed the same protocol but were not exposed to EMS or imazapyr. Plantlets that survived the various regimes were transferred to Speedling trays, acclimated for 3 mo and then sprayed with imazapyr (182 g a.i. ha−1). Above-ground biomass was recorded 47 d after application, and re-growth was recorded 1 mo later.
Molecular analyses.
Genomic DNA was extracted for amplified fragment length polymorphism (AFLP) analysis from plants surviving either EMS or herbicide exposure, using a DNeasy Plant Mini Kit (QIAGEN® Ltd., West Sussex, UK) according to the manufacturer’s recommendations. AFLP analysis was conducted (AFLP Analysis System Kit, Life Technologies Gibco-BRL, InvitrogenTM) using eight AFLP primer combinations (EcoRI/MseI: EAAG/MCAA, EAAG/MCAT, EAAG/MCTA, EAAG/MCTC, EAAG/MCTG, EAAG/MCTT, EAAG/MCAC, and EAAG/MCAG). PCR and electrophoresis were conducted as previously described (Watt et al. 2009). Polymorphic bands were identified and scored as either present or absent and recorded for each primer combination used. The polymorphism rate was calculated as a percentage of the total number of monomorphic bands.
Acetohydroxyacid synthase activity.
Inhibition of AHAS activity by imazapyr was measured by the production of acetolactate, after its conversion by decarboxylation to acetoin (Osuna et al. 2003). AHAS assays were conducted on imazapyr-sensitive plants (control, i.e., no EMS or imazapyr treatment) and on putative imazapyr-tolerant plants (i.e., following EMS exposure and in vitro and ex vitro imazapyr treatments). Young leaf tissue (1.2 g) from shoots that were at least 30 cm in height was ground in liquid nitrogen, then placed in extraction buffer (5 ml g−1 fresh weight) and polyvinylpolypyrrolidone (0.25 g). The extraction buffer contained 0.1 M (pH 7.5) potassium phosphate, 1 mM sodium pyruvate, 0.5 mM magnesium chloride, 0.05 mM thiamine pyrophosphate, 1 μM flavin adenine dinucleotide (FAD), 1.2 mM dithiothreitol, and glycerol (1:9 v v −1). The homogenate was filtered through one layer of cheesecloth and centrifuged at 23,200×g for 15 min. The protein fraction, including the AHAS enzyme, was precipitated from the crude extract at 50 % saturation of (NH4)2SO4 (3,000×g for 17 min). The pellet was resuspended in 0.3 ml extraction buffer and desalted using a PD MiniTrap G-10 column (GE Healthcare, Piscataway, NJ), previously equilibrated with elution buffer (0.1 M potassium phosphate, 20 mM sodium pyruvate, 0.5 mM MgCl2, pH 7.5). The method of Bradford (1976) was used to determine total protein content using bovine serum albumin as the standard, and the protein extract was immediately used to determine AHAS enzyme activity.
Preliminary investigations using control plants established that the optimum substrate concentration was 20 mM. Furthermore, strict Michaelis–Menten kinetics of AHAS with respect to pyruvate was observed (K m value of 2.028 mM and V max value of 0.0137 μmol mg−1 protein h−1). AHAS activity was assayed using 0–100 μM imazapyr to determine the imazapyr concentration required to inhibit AHAS activity by 50 %. AHAS activity was assayed, in a 96-well plate, by adding 22 μl of enzyme extract (1 μg μl−1) to 44 μl freshly prepared assay buffer (0.08 M potassium phosphate, 20 mM sodium pyruvate, 1.5 mM MgCl2, 16 μM FAD, and 0–100 μM technical-grade imazapyr, pH 7.5). A final reaction volume of 110 μl was achieved by the addition of 0.04 M potassium phosphate (pH 7.0). After incubation at 37°C for 1 h, the reaction was stopped by the addition of 22 μl 3 M H2SO4. The reaction plate was then heated at 60°C for 15 min to facilitate decarboxylation of acetolactate to acetoin. The addition of 109 μl creatine (5 g l−1, freshly prepared in dH2O) and 109 μl α-naphthol (50 g l−1, freshly prepared in 2.5 M NaOH), followed by incubation at 60°C for 15 min (Westerfield 1945), enabled the detection of acetoin as a colored complex (A 520 nm). Background was determined by stopping the reaction before incubation and subtracting that value from the corresponding assay value. The level of tolerance was defined by the imazapyr concentration required to inhibit AHAS enzyme activity by 50 % (I 50). The I 50 values were calculated from the nonlinear regression analysis of log [inhibitor] vs. response (GraphPad Prism 5.0, GraphPad Software Inc., San Diego, CA).
Data collection and statistical analyses.
The statistical program Genstat, version 11 (VSN International Ltd., Hertfordshire, UK), was used for all analyses (excluding enzyme assay data), and data were initially tested for normality using the Kolmogorov–Smirnov test (P < 0.05). GraphPad Prism 5.0 was used for all enzyme assay data.
Results and Discussion
Effect of callus exposure to EMS on plantlet regeneration.
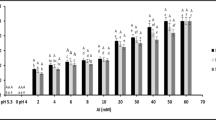
A significant difference in callus fresh mass among treatments was detected after 8 wk in culture, which became more distinct at 10 and 12 wk (repeated-measures ANOVA followed by a Holm–Sidak test, P < 0.05) (Fig. 1A ). Callus fresh mass was inhibited at EMS concentrations higher than 25 mM and 16.1 mM EMS at 10 and 12 wk, respectively, compared with the control. Based on statistical analysis of the data, at 12 wk, the range of EMS concentrations tested formed three distinct groups with respect to response severity: low (8 and 16.1 mM), medium (25, 32.2, and 40 mM), and high (48.3, 64.4, and 96.6 mM). Low-severity treatments (8 and 16.1 mM EMS) did not significantly inhibit the increase in callus fresh mass when compared with the untreated control (Fig. 1B ). The high-severity treatments resulted in callus necrosis.
The effect of 0–96.6 mM ethyl methanesulfonate (EMS) for 4 h on callus fresh mass. (A) Change in embryogenic callus fresh mass over 12 wk. (B) Average fresh mass increase at 12 wk. Different letters denote statistically significant differences between treatments at a specific time (repeated-measures ANOVA and Holm–Sidak test, P < 0.05, n = 8–26, mean ± SE). Data for EMS concentrations of 40, 48.3, and 64.4 mM are omitted from (A) for clarity.
Ultimately, the desired result of exposure to EMS was phenotypically normal plants carrying mutations at any of several locations of the ALS gene (Saari et al. 1994; Webster and Masson 2001; Tan et al. 2005). Our initial objective was to determine an EMS exposure level that was sufficient to generate useful mutations, without excessive killing of cells and/or inhibition of embryo germination and while still allowing the regeneration of a sufficiently large number of phenotypically normal plantlets. Toward this end, 6- to 8-wk-old embryogenic callus was exposed to a range of EMS concentrations (8–96.6 mM) for 4 h before being subjected to induction of somatic embryogenesis. In this study, a longer (4 h) exposure time was used to facilitate even absorption of the mutagen by the cells (Omar and Novak 1990). The chemical mutagen EMS has been successfully used in vitro in many plant species to increase the frequency of useful mutations, at a concentration range and exposure time of 8–40 mM and 1–3 h, respectively (Sung 1976; Masrizal et al. 1991; Hofmann et al. 2004; Luan et al. 2007; Kenganal et al. 2008). In sugarcane, 40 mM EMS for 2.5 h was the reported optimum treatment for production of salt-tolerant plants from callus (Kenganal et al. 2008).
Based on the total number of plants produced (regardless of their phenotypic status), a significant reduction in plantlet production compared with the untreated control was observed at 32.2 to 96.6 mM EMS, with no plantlet development from callus exposed to 96.6 mM EMS (Fig. 2). A reduction in callus proliferation, embryo induction, germination, and plantlet development concurs with other workers that have used EMS (Svetleva and Crinó 2005; Luan et al. 2007; Muthusamy et al. 2007). Tetrazolium tests were performed on the embryogenic callus pieces to determine their viability (results not shown); the reduction in plant yield may have been due to the decrease in viable embryogenic callus production. Significant differences in plantlet development were observed between the control and treated cultures for total number of plants (at 32.2 mM EMS or higher); number of phenotypically normal, green plants (at 40 mM or higher); and number of abnormal plants (at 48.3 mM or higher) (Fig. 2). Plant regeneration was significantly inhibited by EMS above 32.2 mM, and although not significantly different from the control, 16.1 mM EMS resulted in a 50 % decrease in the production of normal plants. The acclimation success of these plants was highly variable and not related to EMS concentration, ranging from 5 to 100 % (average 27 % ± 6.6), whereas 96 % of the control plants were successfully acclimated (results not shown).
In conclusion, although a significant reduction in cell proliferation was recorded compared with the untreated control (Fig. 1), 32.2 mM EMS was considered the highest concentration for treating the callus because (a) the percentage of callus that produced only roots (i.e., abnormal embryo development) was not significantly higher than the untreated control (results not shown), (b) the number of normal plantlets that developed was not significantly lower than the control (Fig. 2), and (c) the number of abnormal plantlets that were produced was not significantly higher than the untreated control (Fig. 2).
Screening treatments for imazapyr tolerance in vitro and ex vitro.
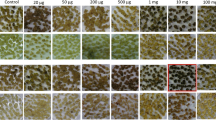
In this study, the dose–response to imazapyr was investigated for the selection of imazapyr-tolerant cell lines of sugarcane; these tests were performed on callus not exposed to the mutagen. Selection was applied to embryogenic callus by incorporating the herbicide (0.025–0.1 μM imazapyr) into the culture medium throughout the 12-wk period of embryo germination and plantlet establishment. Plant numbers declined with increasing imazapyr concentrations (Fig. 3), with 50 and 90 % inhibition of plantlet regeneration calculated to occur on media containing 0.042 (LD50) and 0.08 μM (LD90) imazapyr, respectively. In addition, imazapyr incorporation into the EGM resulted in abnormal plantlet development, with an increase in the severity of abnormalities being directly related to herbicide concentration. The abnormal plants were either albino or stunted, with thickened stems and leaves that had a mottled appearance (Fig. 4), as reported for alfalfa and Coleus cultures exposed to herbicides (Srinivasan et al. 2006). However, the majority of the plants grew normally during acclimation, i.e., once the in vitro selective pressure had been removed. The acclimation success of these plants ranged from 83 (control) to 45 % (0.05 μM imazapyr) (results not shown).
Examples of abnormal (A) and normal (B) plantlets regenerated from embryogenic callus grown on embryo germination medium that contained imazapyr at (A) 0.1 μM and (B) 0 μM. The extensive root system and the mottled, thickened, and stunted leaves (A) were noted in the high-imazapyr-concentration treatments.
Plantlets produced on imazapyr-containing medium had a more developed root system (Fig. 4A ) than those that regenerated in the absence of the herbicide (Fig. 4B ). This observation has not been previously reported in other in vitro studies involving herbicides, but it has been observed with date palm and cotton as a response to in vitro chemical mutagen applications (Omar and Novak 1990; Ganesan et al. 2005). Thus, stimulation of root system growth could be a consequence of in vitro stress and/or an advantage for successful plantlet acclimation (Ganesan et al. 2005). The significance of the observed in vitro root response to imazapyr regarding tolerance in the field to aerial herbicide spraying is intriguing and should be investigated further.
Screening callus on imazapyr-containing media allowed for the dose–response to be determined with respect to plant regeneration (Fig. 3). A high R 2 value was achieved when testing 0.025–0.1 μM imazapyr, indicating a low amount of variation within these treatment levels. The I 50 and I 90 values obtained in this study (0.043 and 0.08 μM, respectively) are similar to the results obtained by other workers. For example, 50 % inhibition of germination and plantlet development was recorded for 0.05 μM imazapyr in wheat callus (Heering et al. 1992) and for 0.06 μM imazapyr in sugarcane cell suspension cultures (Punyadee et al. 2007).
Heering et al. (1992) found that callus was less sensitive to imazapyr than plants, which might be due to the absence of a vascular system in the callus. Cellular absorption of imazapyr occurs by ion trapping (Little et al. 1994), and once absorbed, the herbicide dissociates to a charged form due to high cell cytoplasmic pH (Heering et al. 1992). Imazapyr in this form is less lipophilic, making diffusion into neighboring cells difficult and resulting in the accumulation of imazapyr in the cells directly in contact with the medium; a concentration gradient is thus established from the basal to apical cells of the callus (Heering et al. 1992). As this allows the possibility of plantlets developing from apical cells that are not truly tolerant of imazapyr, a screening step after acclimation was, therefore, deemed a necessity in the present study.
The effect of imazapyr on shoot meristems was assessed by recording re-growth 1 and 2 mo after removal of aboveground biomass (Fig. 5). There were no significant differences between control and imazapyr treatments up to and including 51 g a.i. ha−1 in the percentage of plants that regrew (Fig. 5). Imazapyr applications of 102 and 262.5 g a.i. ha−1 resulted in a significant reduction in regrowth compared with all other treatments (Fig. 5). In the present study, 182 g a.i. ha−1 (midway between the two highest applications) was the dosage considered sufficient to screen 3-mo-old plants (Fig. 5), and as there was no significant difference between the results obtained at each of the sampling times, it was concluded that assessing re-growth after 1 mo was adequate (Fig. 5). Greenhouse tests are often used to score plants for their susceptibility to a particular herbicide (Newhouse et al. 1991). However, the herbicide concentrations used for greenhouse tests are usually lower than in field trials because plants are generally more sensitive to herbicides under controlled environmental conditions than in the field (Pozniak and Hucl 2004).
Production and analysis of imazapyr-tolerant plants.
In this study, a stepwise imazapyr selection regime was employed to ensure a stringent selection process, so that only plantlets with high tolerance levels to imazapyr should survive (Punyadee et al. 2007). The choice of low EMS concentrations (8 or 16 mM) rather than the maximum concentration identified in the preliminary experiments (32.2 mM) was due to the predicted greater stress levels caused by the combination of EMS mutation (Hofmann et al. 2004; Luan et al. 2007; Muthusamy et al. 2007; Kenganal et al. 2008) and imazapyr screening (Newhouse et al. 1991; Heering et al. 1992; Punyadee et al. 2007). Callus pieces were exposed to 8 or 16 mM EMS for 4 h before being placed on CIM. All cultures were given at least a 3-d recovery period on medium without imazapyr, in a manner similar to that used for selection of transgenic sugarcane plants (Snyman 2004). Nine different selections regimes (IP1–IP9; Table 1) were devised based on the results obtained from the initial callus screening. The imazapyr concentration in the medium ranged from 0.042 (LD50) to 0.16 μM (lethal dose). Each selection regime (other than IP7) included a step-wise increase in imazapyr concentration over time. In the first three regimes (IP1–IP3), imazapyr was incorporated into the medium 4 wk after EMS exposure, whereas in all the others callus was transferred to the imazapyr-containing medium 3 d after EMS exposure. The initial imazapyr dose was low in IP4–IP6 (LD50; 0.042 μM) and high in IP7–IP9 (LD90; 0.08 μM). The numbers of normal (green) and abnormal (albino and visually chimeric) plantlets were recorded for each treatment, 12 wk after EMS exposure (Table 2).
In general, there was a decrease in the number of normal plants regenerated with increasing severity of the mutagen and selection regimes (Table 2). The number of normal plants regenerated from callus exposed to 8 mM EMS and imazapyr regimes IP3, IP5, IP6, IP8, and IP9 was significantly reduced when compared with the control. A similar result was found for the 16-mM EMS treatment, which also produced a significant inhibition of normal plant production under regime IP7. No significant differences in the percentage of abnormal plants between treatments were detected (restricted maximum likelihood analysis) due to high variation within treatments (Table 2).
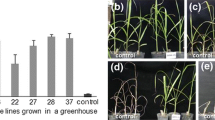
All green (visually normal) plants were transferred to Magenta® vessels containing PEM and the same imazapyr concentration to which they had been exposed at week 12. After 1 mo, the surviving plantlets were transferred to PEM without imazapyr for a second month. Thereafter, plantlets were acclimated for 3 mo, at which time plantlet survival ranged from 0 % (IP5 following 8 mM EMS and IP7 following 16 mM EMS) to 100 % (IP3 following 8 mM EMS) (Fig. 6A ). In general, greater numbers of surviving plants were obtained from the 16-mM EMS treatment compared with the 8-mM EMS treatment (Fig. 6A ), indicating that the higher EMS concentration may have induced more mutations that enabled the plants to survive exposure to imazapyr and subsequent acclimation. The imazapyr selection regimes that produced the most normal plants, following the exposure of callus to 16 mM EMS, were IP4 (40 plants survived per 0.2 g callus), IP1 (25 plants survived per 0.2 g callus), and IP8 (19 plants survived per 0.2 g callus) (Fig. 6A ).
The percentage of plants that survived acclimation (A) and that regrew after subsequent application of imazapyr (182 g a.i. ha−1) and aboveground biomass removal (B). Numbers above each bar represent actual plant numbers. Plants were initially exposed to either 8 or 16 mM EMS and cultured on imazapyr selection regimes IP1–IP9, in vitro.
The plants that survived acclimation were sprayed with 182 g a.i. ha−1 imazapyr (midway between 102 and 262.5 g a.i. ha−1; Fig. 5), after which their above-ground biomass was removed and re-growth recorded (Fig. 6B ). The treatments from which most plants survived were IP4 (16 mM EMS; 24 plants), IP1 (16 mM EMS; 17 plants), IP1 (8 mM EMS; 14 plants), and IP8 (16 mM EMS; 14 plants) (Fig. 6B ). Control plants obtained through conventional indirect somatic embryogenesis protocol were considered “sensitive,” while those that were exposed to EMS and screened via imazapyr selection regimes IP1–IP9 were considered “tolerant” to imazapyr. When imazapyr (182 g a.i. ha−1) was applied to these 165 plants, 106 survived and regrew (Fig. 6B ). This represents a survival rate of 64 % compared with 12 % for sensitive control plants that survived the same treatment (Fig. 6B ). Based on this, we contend that the level of escapes from the in vitro selection protocol developed in this paper is low.
Further tests were conducted on these plants to determine the enzymatic tolerance to imazapyr. Of the tolerant plants, 21 were subjected to enzymatic analyses based on their growth and tillering ability: Only shoots that were at least 30 cm in height after 3 mo were selected for subsequent enzyme analysis. The average I 50 value for the control plants was 2.155 μM. However, as each tolerant plant was considered to be a unique genotype, a wide range of I 50 values were obtained, with 86 % (18 plants) at least twice as resistant to imazapyr as the control N12 (data not shown). The three genotypes with the highest I 50 values are displayed in Table 3, and their AHAS I 50 values were between 2.8 and 4.0 times that of the sensitive N12 control. Further work on these three plants is being undertaken by the South African Sugarcane Research Institute. Punyadee et al. (2007) found that tolerant sugarcane cells had an AHAS I 50 value 6.5 times that of the sensitive cells. The levels found in this study are similar.
In vitro generation of herbicide tolerance as an agronomically valuable trait has been successful in many plant species (Chaleff and Parsons 1978; Miller and Hughes 1980; Chaleff and Ray 1984; Nafziger et al. 1984; Anderson and Georgeson 1989; Newhouse et al. 1991; Heering et al. 1992; Zambrano et al. 1999; Zambrano et al. 2003a; Punyadee et al. 2007) and that approach has yielded imidazolinone-tolerant mutants (Anderson and Georgeson 1989; Swanson et al. 1989; Saxena and King 1990; Subramanian et al. 1990; Newhouse et al. 1991; Heering et al. 1992; Punyadee et al. 2007).
There are three mechanisms that can confer resistance to herbicides in crop plants: prevention of the herbicide from reaching the site of uptake, metabolic detoxification, and resistance at the site of action (Tan et al. 2005). Although a mechanism involving uptake may be ruled out based on the enzyme assays, further studies will be needed to determine the mode of resistance in the three genotypes with the highest I 50 values identified in this study (designated R1-N12, R2-N12, and R3-N12).
Ongoing work includes vegetative propagation of the tolerant plants to increase plant numbers for phenotypic analyses (stalk height, stalk diameter, number of stalks per stool, mass per stool, and sucrose yield; Ramburan et al. 2007). Conventional sexual propagation followed by screening will determine the inheritance and stable expression of the imazapyr-tolerance trait. The imazapyr-tolerant plants will be useful not only for future genotype development programs but also in the breeding of imazapyr-tolerant sugarcane varieties. Although this protocol was established for the N12 cultivar, it should be easily adapted to other sugarcane cultivars.
Genetic integrity assessments.
The protocol for plantlet regeneration via indirect somatic embryogenesis used in our laboratories has been shown to generate low levels of DNA polymorphism (Sweby et al. 1994; Watt et al. 2009). However, other workers (Heinz and Mee 1971; Lourens and Martin 1987; Irvine et al. 1991; Burner and Grisham 1995; Taylor et al. 1995; Zucchi et al. 2002) have found high levels of genetic variation regardless of the morphogenesis route, so AFLP analyses were undertaken on genomic DNA from in vitro regenerated plants. Using eight primer combinations that are routinely employed in our laboratory, embryogenic callus exposed to 8–64.4 mM EMS yielded between 0 and 0.3 % polymorphic bands. In regenerated plants before and after acclimation, no significant differences were recorded for the number of polymorphic bands among the treatments (results not shown).
Conclusions
Prior to the current study, the only method to introduce herbicide tolerance into sugarcane was transgenic (Gallo-Meagher and Irvine 1996; Falco et al. 2000; Leibbrandt and Snyman 2003). However, there is notable public resistance to accepting transgenic crops, and much effort continues in an attempt to convince consumers and politicians otherwise (Jain 2001). Additionally, international markets and intellectual property restrictions have prevented transgenic sugarcane from being released commercially. Consequently, sugarcane generated through in vitro mutagenesis, such as presented here, may be more readily accepted than transgenic sugarcane at present.
References
Ali A, Naz S, Alam SS, Iqbal J (2007) In vitro induced mutation for screening of red rot (Colletotrichum falcatum) resistance in sugarcane (Saccharum officinarum). Pakistan J Bot 39:1979–1994
Anderson PC, Georgeson M (1989) Herbicide-tolerant mutants of corn. Genome 31:994–999
Bennett R, Phipps R, Strange A, Grey P (2004) Environmental and human health impacts of growing genetically modified herbicide-tolerant sugarbeet: a life-cycle assessment. Plant Biotechnol J 2:273–278
Bradford MM (1976) A rapid and sensitive method of the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Brett PGC (1950) Flowering and pollen fertility in relation to sugarcane breeding in Natal. Proc S Afr Sugarcane Technol Ass 7:43–56
Burner DM, Grisham MP (1995) Induction and stability of phenotypic variation in sugarcane as affected by propagation procedure. Crop Sci 35:875–880
Campbell PL (2008) Efficacy of glyphosate, alternative post-emergence herbicides and tillage for control of Cynodon dactylon. S Afr J Plant Soil 25:220–228
Campbell PL, Smith MT, Sewpersad C, van den Berg M (2008) Image analysis to quantify herbicide efficacy for Cynodon dactylon control. S Afr J Plant Soil 25:229–235
Chaleff RS, Parsons MF (1978) Direct selection in vitro for herbicide-resistant mutants of Nicotiana tabacum. Proc Nat Acad Sci USA 75:5104–5107
Chaleff RS, Ray TB (1984) Herbicide-resistant mutants from tobacco cell cultures. Science 16:1148–1151
Falco MC, Neto AT, Ulian EC (2000) Transformation and expression of a gene for herbicide resistance in a Brazilian sugarcane. Plant Cell Rep 19:1188–1194
Gallo-Meagher M, Irvine JE (1996) Herbicide resistant transgenic sugarcane plants containing the bar gene. Crop Sci 36:1367–1374
Ganesan M, Bhanumathi P, Jayabalan N (2005) Mutagenic effect of sodium azide on somatic embryo regeneration and root growth of cotton (Gossypium hirsutum L. CV. SVPR2). J Agri Technol 1:365–380
Haughn GW, Somerville C (1986) Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Gent 204:430–434
Heering DC, Guenzi AC, Peeper TF, Claypool PL (1992) Growth response of wheat (Triticum aestivum) callus to imazapyr and in vitro selection for resistance. Weed Sci 40:174–179
Heinz DJ, Mee GWP (1971) Morphologic, cytogenetic, and enzymatic variation in Saccharum species hybrid clones derived from callus tissue. Amer J Bot 58:257–262
Hofmann NE, Raja R, Nelson RL, Korban SS (2004) Mutagenesis of embryogenic cultures of soybean and detecting polymorphisms using RAPD markers. Biol Plant 48:173–177
Irvine JE, Benda GTA, Legendre BL, Machado GR Jr (1991) The frequency of marker changes in sugarcane plants regenerated from callus cultures II. Evidence for vegetative and genetic transmission, epigenetic effects and chimera disruption. Plant Cell Tissue Organ Cult 26:115–125
Jain SM (2001) Tissue culture-derived variation in crop improvement. Euphytica 118:153–166
Jander G, Baerson SR, Hudak JA, Gonzalez KA, Gruys KJ, Last RL (2003) Ethyl methanesulfonate saturation mutagenesis in Arabidopsis to determine frequency of herbicide resistance. Plant Physiol 131:139–146
Kenganal M, Hanchinal RR, Nadaf HL (2008) Ethyl methane sulfonate (EMS) induced mutation and selection for salt tolerance in sugarcane in vitro. Indian J Plant Physiol 13:405–410
Khan SJ, Khan MA (2010) Application of in vitro mutation techniques for sugarcane improvement. J Agr Res 48:429–435
Kilbey B, Hunter F (1983) Factors affecting mutational yield from EMS exposures of yeast (Saccharomyces cerevisiae). Mutation Res 122:35–38
Koch AC, Snyman SJ, Ramgareeb S, Rutherford RS, Watt MP (2010) An in vitro induced mutagenesis protocol for the production of sugarcane tolerant to imidazolinone herbicides. Proc Int Soc Sugar Cane Technol 27:122
Kolkman JM, Slabaugh MB, Bruniard JM, Berry S, Bushman BS, Olungu C, Maes N, Abratti G, Zambelli A, Miller JF, Leon A, Knapp SJ (2004) Acetohydroxyacid synthase mutations conferring resistance to imidazolinone or sulfonylurea herbicides in sunflower. Theor Appl Genet 109:1147–1159
Landrey OP, Eichler GG, Chedzey J (1993) Control of creeping grasses in small grower cane in the Umbumbulu district. Proc S Afr Sugarcane Technol Ass 67:33–38
Leibbrandt NB, Snyman SJ (2003) Stability of gene expression and agronomic performance of a transgenic herbicide-resistant sugarcane line in South Africa. Crop Sci 43:671–677
Lencse RJ, Griffin JL (1991) Itchgrass (Rottboellia cochinchinensis) interference in sugarcane (Saccharum sp.). Weed Technol 5:396–399
Little DL, Shaner DL, Ladner DW, Tecle B, Ilnicki RD (1994) Root absorption and translocation of 5-substituted analogs of the imidazolinone herbicide, imazapyr. Pestic Sci 41:161–169
Lourens AG, Martin FA (1987) Evaluation of in vitro propagated sugarcane hybrids for somaclonal variation. Crop Sci 27:793–796
Luan Y-S, Zhang J, Gao X-R, An L-J (2007) Mutation induced by ethyl methanesulfonate (EMS), in vitro screening for salt tolerance and plant regeneration of sweet potato (Ipomoea batatas L.). Plant Cell Tissue Organ Cult 88:77–81
Masrizal, Simonson RL, Baenziger PS (1991) Response of different wheat tissues to increasing doses of ethyl methanesulfonate. Plant Cell Tissue Organ Cult 26:141–146
Miller OK, Hughes KW (1980) Selection of paraquat-resistant variants of tobacco from cell cultures. In Vitro 16:1085–1091
Millhollon RW (1992) Effect of itchgrass (Rottboellia cochinchinensis) interference on growth and yield of sugarcane (Saccharum spp. hybrids). Weed Sci 40:48–53
Millhollon RW (1995) Growth and yield of sugarcane as affected by johnsongrass (Sorghum halepense) interference. J Amer Soc Sugarcane Technol 15:32–40
Mulwa RMS, Mwanza LM (2006) Biotechnology approaches to developing herbicide tolerance/selectivity in crops. Afr J Biotechnol 5:396–404
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Muthusamy A, Vasanth K, Sivasankari D, Chandrasekar BR, Jayabalan N (2007) Effects of mutagens on somatic embryogenesis and plant regeneration in groundnut. Biol Plant 51:430–435
Nafziger ED, Widholm JM, Steinrücken HC, Killmer JL (1984) Selection and characterization of a carrot cell line tolerant to glyphosate. Plant Physiol 76:571–574
Newhouse K, Singh B, Shaner D, Stidham M (1991) Mutations in corn (Zea mays L.) conferring resistance to imidazolinone herbicides. Theor Appl Genet 83:65–70
Omar MS, Novak FJ (1990) In vitro regeneration and ethylmethanesulphonate (EMS) uptake in somatic embryos of date palm (Phoenix dactylifera L.). Plant Cell Tissue Organ Cult 20:185–190
Osuna MD, Fischer AJ, De Prado R (2003) Herbicide resistance in Aster squamatus conferred by a less sensitive form of acetolactate synthase. Pest Manag Sci 59:1210–1216
Patade VY, Suprasanna P (2008) Radiation induced in vitro mutagenesis for sugarcane improvement. Sugar Tech 10:14–19
Patade VY, Suprasanna P, Bapat VA, Kulkarni UG (2006) Selection for abiotic (salinity and drought) stress tolerance and molecular characterization of tolerant lines in sugarcane. BARC Newsletter 273:244–257
Patade VY, Suprasanna P, Bapat VA (2008) Gamma irradiation of embryogenic callus cultures and in vitro selection for salt tolerance in sugarcane (Saccharum officinarum L.). Agri Sci China 7:1147–1152
Pozniak CJ, Hucl PJ (2004) Genetic analysis of imidazolinone resistance in mutation-derived lines of common wheat. Crop Sci 44:23–30
Punyadee P, Throngros M, Pornprom T (2007) Biochemical mechanism of resistance to imazapyr in sugarcane cell selections. Thai J Agri Sci 40:133–141
Rai MK, Kalia RK, Singh R, Gangola MP, Dhawan AK (2011) Developing stress tolerant plants through in vitro selection—an overview of the recent progress. Environ Exp Biol 71:89–98
Ramburan S, Redshaw KA, van den Berg M (2007) Variety evaluation in the South African sugarcane industry: an overview. Proc S Afr Sugarcane Technol Ass 26:558–602
Richard EP Jr (1990) Timing effects on johnsongrass (Sorghum halepense) with asulam in sugarcane (Saccharum sp.). Weed Technol 4:81–86
Saari LL, Cotterman JC, Thill DC (1994) Resistance to acetolactate synthase inhibiting herbicides. In: Powles SB, Holtum JAM (eds) Herbicide resistance in plants, biology and biochemistry. Lewis, Boca Raton, pp 83–139
Saif-Ur-Rasheed M, Asad S, Zafar Y, Waheed RA (2001) Use of radiation and in vitro techniques for development of salt tolerant mutants in sugarcane and potato. IAEA 1227:51–60
Sandhu SS, Bastos CR, Azini LE, Neto AT, Colombo C (2002) RAPD analysis of herbicide-resistant Brasilian rice lines produced via mutagenesis. Genet Mol Res 1:359–370
Saxena PK, King J (1990) Lack of cross-resistance of imidazolinone-resistant cell lines of Datura innoxia P. Mill. to chlorsulfuron. Plant Physiol 94:1111–1115
Schy WE, Plewa MJ (1989) Molecular dosimetry studies of forward mutation induced at the yg2 locus in maize by ethyl methanesulfonate. Mutation Res 211:231–241
Senseman SA (2007) Herbicide handbook, 9th edn. Weed Science Society of America, USA, pp 84–86
Snyman SJ (2004) Transformation of sugarcane. In: Curtis IS (ed) Transgenic crops of the world–essential protocols. Kluwer Academic, Dordrecht, pp 103–114
Snyman SJ, Baker C, Huckett BI, McFarlane SA, van Antwerpen T, Berry S, Omarjee J, Rutherford RS, Watt DA (2008) South African Sugarcane Research Institute: embracing biotechnology for crop improvement research. Sugar Tech 10:1–13
Srinivasan M, Nachiappan V, Rajasekharan R (2006) Potential application of urea-derived herbicides as cytokinins in plant tissue culture. J Biosci 3:599–605
Subramanian MV, Hung H-Y, Dias JM, Miner VW, Butler JH, Jachetta JJ (1990) Properties of mutant acetolactate synthase resistant to triazolopyrimidine sulfonanilide. Plant Physiol 94:239–244
Sung ZR (1976) Mutagenesis of cultured plant cells. Genetics 84:51–57
Svetleva DL, Crinó P (2005) Effect of ethyl methanesulfonate (EMS) and N-nitrose-N′-ethyl urea (ENU) on callus growth of common bean. Cent Eur J Agri 6:59–64
Swanson EB, Herrgesell MJ, Arnoldo M, Sippell DW, Wong RSC (1989) Microspore mutagenesis and selection: canola plants with field tolerance to the imidazolinones. Theor Appl Genet 78:525–530
Sweby DL, Huckett BI, Botha FC (1994) Minimising somaclonal variation in tissue cultures of sugarcane. Proc S Afr Sugarcane Technol Ass 68:29–37
Tan S, Evans RR, Dahmer ML, Singh BK, Shaner DL (2005) Imidazolinone-tolerant crops: history, current status and future. Pest Manag Sci 61:246–257
Taylor PWJ, Ko H-L, Adkins SW (1992) Factors affecting protoplast isolation and the regeneration of shoot-like structures from protoplast-derived callus of sugarcane (Saccharum spp. hybrids). Aust J Bot 40:863–876
Taylor PWJ, Geijskes JR, Ko HL, Fraser TA, Henry RJ, Birch RG (1995) Sensitivity of random amplified polymorphic DNA analysis to detect genetic changes in sugarcane during tissue culture. Theor Appl Genet 90:1169–1173
Van Harten AM (1998) Mutation breeding: theory and practical applications. Cambridge University Press, London
Watt MP, Banasiak M, Reddy D, Albertse EH, Snyman SJ (2009) In vitro minimal growth storage of Saccharum spp. hybrid (genotype 88H0019) at two stages of direct somatic embryogenic regeneration. Plant Cell Tissue Organ Cult 96:263–271
Webster EP, Masson JA (2001) Acetolactate synthase-inhibiting herbicides on imidazolinone-tolerant rice. Weed Sci 49:652–657
Westerfield WW (1945) A colorimetric determination of blood acetoin. J Biol Chem 161:495–502
Zambrano AY, Demey JR, González V (1999) Selection of an ametryn tolerant sugarcane cellular line. J Agri U Puerto Rico 83:47–54
Zambrano AY, Demey JR, Fuchs M, González V, Rea R, De Sousa O, Gutierrez Z (2003a) Selection of sugarcane plants resistant to SCMV. Plant Sci 165:221–225
Zambrano AY, Demey JR, González V (2003b) In vitro selection of a glyphosate-tolerant sugarcane cellular line. Plant Mol Biol Rep 21:365–373
Zucchi MI, Arizono H, Morais VA, Fungaro MHP, Vieira MLC (2002) Genetic instability of sugarcane plants derived from meristems cultures. Genet Mol Biol 25:91–96
Acknowledgments
We wish to thank the South African Sugarcane Research Institute, the University of KwaZulu-Natal, the National Research Foundation (RSA), and the Deutscher Akademischer Austausch Dienst for continued support. We are also grateful to E. Albertse, N. Pillay, N. Keeping, G. Meyer, and D. Watt for technical assistance and helpful discussions; N. Sewpersad for assisting with statistical analyses; and P. Campbell for performing herbicide applications.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Todd Jones
Rights and permissions
About this article
Cite this article
Koch, A.C., Ramgareeb, S., Rutherford, R.S. et al. An in vitro mutagenesis protocol for the production of sugarcane tolerant to the herbicide imazapyr. In Vitro Cell.Dev.Biol.-Plant 48, 417–427 (2012). https://doi.org/10.1007/s11627-012-9448-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-012-9448-x