Abstract
Plants encounter various abiotic stresses during the whole life cycle. The mitogen-activated protein kinase kinase (MAPKK) acts as the convergent point of MAPK cascade in stress signaling pathway, which has not widely been studied so far. In the present study, we isolated a group A MAPKK gene (named as CaMKK1) from Chenopodium album, a salt-tolerant plant species in Chenopodiaceae. Quantitative RT-PCR analysis indicates that CaMKK1 was significantly up-regulated and displayed a fast and transient activation, which was similar with MAPK expression pattern in C. album under salt and drought stress. Ectopic expression of CaMKK1 in Escherichia coli BL21 (DE3) strains showed that overexpression of CaMKK1 could increase the salt and drought tolerance to bacterium. Overexpression of CaMKK1 in transgenic tobacco exhibited attenuated salt and PEG sensitivity by means of improving germination percentage and seedling growth. Furthermore, transgenic tobacco lines significantly accumulated organic osmoprotectants, and increased antioxidant enzyme activities which effectively relieved the accumulated reactive oxygen species under stress; the water loss rate was reduced and net photosynthetic rate was significantly increased in transgenic lines compared to non-transgenic plant. The more worth mentioning is that the transcripts of downstream transcription factors (NtDREB2, NtDREB3, NtDREB4) were quickly and dramatically accumulated (from 15 to 224 folds) in transgenic lines under stress. In conclusion, our results indicate that CaMKK1 is a positive regulator in response to salt and drought stress in plants, which should provide new data for further analyzing the function of plant MAPK pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants constantly encounter different stresses in growth and developmental process because of sessility (Long et al. 2013). To cope with, they have developed a variety of mechanisms, including morphological, physiological and molecular aspects. Out of these, signal transduction pathway is the particularly important strategy for plant adaptation to harsh circumstances (Shinozaki et al. 2003; Huang et al. 2012). Mitogen-activated protein kinase (MAPK) cascade is one of the well-known signaling pathways of plant in response to adversity (Smekalova et al. 2014), which comprises three functionally linked protein kinases, MAPK kinase kinase (MAPKKK), MAPK kinase (MAPKK) and MAPK. Following successive phosphorylation, signals can be transferred from upstream to a variety of downstream components (Hirt 1997; MAPK group 2002; Rodriguez et al. 2010). Plant MAPKKs are present with the least members in the MAPK signaling modules (Takahashi et al. 2009), e.g. in Arabidopsis, only ten MAPKK genes were identified compared to 20 MAPK and more than 60 MAPKKK. So far, a variety of MAPKKs have been characterized from poplar (Hamel et al. 2006), rice (Singh et al. 2012), maize (Kong et al. 2013), etc. Generally, signals perceived by MAPKKKs are integrated and transduced by MAPKKs, and the specificity of cellular response regulated by MAPK module is mainly determined by MAPKK, suggesting that MAPKKs play critical role in transforming extracellular stimuli into cellular responses in MAPK cascade (Andreasson and Ellis 2010). Transgenic plants with MAPKK overexpression provide further evidence in plant response to cold, salt, drought, heat, etc. stresses (Agarwal et al. 2010; Kiegerl 2000; Liu et al. 2015; Mikołajczyk et al. 2000; Mizoguchi et al. 1998; Wu et al. 2015). Site-direction mutagenesis of OsMKK6 from rice (Oryza sativa) makes the salt tolerance function of this gene constitutively active (Kumar and Sinha 2013). Not all members of MAPKKs respond to stress positively, some of them apply opposite effect. Overexpression of GhMKK1 in cotton enhances while GhMKK5 reduces salt and drought tolerance (Lu et al. 2013; Zhang et al. 2012). Analysis of BdMKK6.2 (Brachypodium distachyon) reveals a negative regulation of drought tolerance by influencing reactive oxygen species (ROS) homeostasis and transcription of stress-responsive genes (Sun et al. 2016).
It has been reported that a cross-talk between MAPK cascade and ROS signaling is involved in plant response to various abiotic stresses, e.g. MEKK1-MKK1/MKK2-MPK4 cascade in ROS homeostasis in A. thaliana, because some redox-related genes failed to express in mekk1 or mpk4 mutants (Nakagami et al. 2006). Previous studies indicated that AtMKK1 induced CAT1 (catalase) expression by triggering H2O2 production in response to drought and salt stress (Xing et al. 2007). In addition, several transcription factors which act downstream of MAPK cascade in ROS response have been identified, e.g. transcription factor AtMYB44 (Persak and Pitzschke 2014), NbWRKY8 (Ishihama et al. 2011), etc. However, currently other downstream transcription factor, e.g. DREB, etc. in interaction with MAPK pathway components has not been well-documented.
Chenopodium album is a salt tolerant species (Hamidov et al. 2007) and widely distributes in the semi-arid area in Xinjiang province, China (Commissione Redactorum Florae Xinjiangensis 1994). It shows very strong adaptability to harsh environments, however, no specialized structures, e.g. assimilation shoot, succulent or degenerated leaf, etc. are employed by C. album in dealing with the heterogeneous circumstances. We speculate that it must have some special way (e.g. physiological or molecular mechanism) in adaption of the unpredictable conditions, so, in the present study, we cloned the coding sequence of MAPKK from C. album, and characterized its responses to salt and drought stress in transgenic tobacco. In comparison with non-transgenic plants, we found that overexpression of CaMKK1 reduced the sensitivity to salt and drought in seed germination, seedling and adult plant growth, which corresponded to changes of various physiological parameters, e.g. organic osmoprotectant contents, anti-oxidant enzyme activities, water loss rate, net photosynthesis rate, etc. More interestingly, CaMKK1 overexpression dramatically increased the expression level of three transcription factors-DREB2, DREB3, DREB4 (dehydration response element binding factors, DREB), which is the first report on the quick and remarkable response of downstream DREB transcription factors to MAPKK. Our data suggest that CaMKK1 is a positive regulator which promotes the expression of downstream transcription factor in MAPK cascade and confers stress tolerance to transgenic tobacco.
Materials and methods
Plant cultivation and treatments
Seeds of C. album were sown in pots containing a 3:1 mixture of vermiculite:perlite (v/v) in the growth chamber under a 16 h light/8 h dark photoperiod, 25 °C, 10–20% relative humidity. Plants were cultivated for 4–6 weeks [well-watered and with Hoagland solution supplying at an interval of 2–3 weeks] before next use.
For quantitative RT-PCR (qPCR) analysis of CaMKK1, the above plants were treated with Hoagland solution containing 50, 100, 300 mM NaCl (for NaCl stress), or 0, 5, 10, 15% polyethylene glycol (PEG) 6000 (for mimic drought stress), then sampling at 0, 0.5, 1, 2, 5, 12, 24, 48 h time points during treatment, using Hoagland solution only as control. For qRT-PCR analysis of CaMPK, 300 mM NaCl or 20% PEG was applied for stress treatment. For qPCR analysis of stress-related genes in transgenic tobacco lines, seeds of non-transgenic (NT) and transgenic (T1) tobacco lines were surface-sterilized and sown on MS medium only (for NT) and MS containing 250 mg L−1 Kanamycin (for T1), seedlings grew till two leaves (1–2 cm in diameter) were present and NT seedlings would become yellowish. The green seedlings were transferred into fresh MS medium for 1 week and subjected to PCR identification, the positive seedlings grew for another 1 month till four leaves were present and the height reached to 10 cm, then transferred into vermiculite:perlite (3:1, v/v) mixture and managed as mentioned above. For assays, four samples were collected from young fresh leaves in the upper part of the plants, and immediately frozen in liquid nitrogen until use.
Total RNA isolation
Young fresh leaves (0.12 g) were harvested and total RNA was extracted by using the HP Plant RNA Kit (R6837-02, Omega, US). Each reverse transcription reaction was performed with total RNA (1.5 μg) by using the M-MLV reverse transcriptase kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions.
Cloning of CaMKK1 and construction of prokaryotic and plant expression vectors
The cDNA fragment of MAPKK open reading frame (ORF) from C. album was amplified using the above cDNA template with the primers shown in Table 1. The PCR product was cloned into the pMD-18-T vector (TaKaRa, Dalian, China) and sent for sequencing. The CaMAPKKcDNA with correct sequence (named as CaMKK1) was submitted to GenBank and received an accession number JQ994336. Consequently it was recombined into the prokaryotic expression vector as pET-28a-CaMKK1 and plant expression vector pCAMBIA2300 under the control of the CaMV 35S promoter (shortened as 35S::CaMKK1).
Generation of the transgenic tobacco lines
Germfree tobacco seedlings (5–6 week-old) were transformed with Agrobacterium strain EHA105 in which 35S::CaMKK1 was harboring by using tobacco leaf disc transformation method (Horsch et al. 1985). Regeneration tobacco plantlets with 2–3 leaves [non-transgenic plant (NT) or CaMKK1 transformed] were transplanted in soil and cultivated in greenhouse with a 16 h light/8 h dark photoperiod, 25 °C, 20% relative humidity. The seeds from regenerated plants of PCR positive (T0) were harvested and screened on MS medium containing 250 mg L−1 kanamycin for 10–15 days. Kanamycin-resistant seedlings were identified by PCR and RT-PCR to confirm the gene integration in genome (T1). Three representative CaMKK1-overexpressed lines (OE1, OE2, OE3) of T1 generation tobacco were selected for further functional assays. For analysis of seed germination and seedling growth, the transgenic seeds of T2 generation were harvested from the three lines of T1 generation and applied in the measurement. For PCR identification, genomic DNA from transgenic plant was extracted according to instruction of Tiangen Plant Genomic DNA Extraction Kit (Tiangen, Beijing, China) by using primers described in Table 1 [CaMKK1 (ORF)], PCR was performed in the following conditions: 94 °C 5 min; 35 cycles at 94 °C 30 s, 56 °C 30 s and 72 °C 1 min, followed by a final incubation at 72 °C for 10 min.
Analysis of salt and drought tolerance with CaMKK1 overexpression
Effect of recombinant CaMKK1 on prokaryotic cell growth E. coli
BL21 (DE3) strains with recombinant plasmid (pET-28a-CaMKK1) and control vector (pET-28a) were inoculated in LB liquid medium containing 100 mg L−1 kanamycin and grown at 37 °C overnight. The cultures were then re-inoculated to fresh LB medium containing 100 mg L−1 kanamycin (1% culture in 20 mL LB in a 100 mL conical flask), which continued to grow for 4 h (about 0.5 OD600), then were induced with 0.5 mM IPTG to express recombinant protein for another 4 h. Each culture (200 μL of 0.8 OD600) was inoculated into 20 mL fresh LB medium (with 100 mg L−1 kanamycin) containing 0, 50, 100, 200 mM NaCl, or 0, 5, 10, 15% PEG 6000, and incubated at 37 °C. Cultures (1.0 mL) were removed at an interval of 1 h for a total of 6 h and the optical density value at 600 nm (OD600) was recorded by spectrophotometer (Benchmark Plus microplate, Bio-Rad, USA). Three replicates were applied with each treatment.
Effect of CaMKK1 overexpression in transgenic tobacco lines
To characterize salt or drought-stress sensitivity, we performed a germination and seedling growth assay. A total of 30 surface-sterilized seeds from homozygous T2 transgenic lines and non-transgenic (NT) tobacco plants were germinated on MS medium containing different concentrations of NaCl (0, 100, 200, 300 mM) or PEG 6000 (0, 5, 10, 15%) at 25 °C, with a 16 h light/8 h dark photoperiod. Each experiment was repeated at least three times with similar results. Germination percentage was recorded every 24 h during a 2-week period. The seedling growth was measured by calculating ten seedlings of the root length at the end of 2-week germination.
Measurement of physiological responses of CaMKK1 overexpressed tobacco lines to abiotic stresses
Three-month-old tobacco transgenic lines (OE1, OE2, OE3; T1 generation) and NT plants were treated with 15% PEG 6000 or 300 mM NaCl for 48 h, respectively. For assay of the organic osmoprotectants-proline, soluble sugar (SS) or glycine betaine (GB), young fresh leaves (0.15–0.2 g) were used in extraction. Proline concentration was determined by using aqueous sulfosalicylic acid to extract and acidic ninhydrin to develop the color according to Zhao et al. method (Zhao et al. 2009); GB was determined by using Lokhande et al. method (Lokhande et al. 2010); the concentration of SS was determined by using anthrone-sulphuric acid colorimetric method described by Palma et al. (2009). For measuring O2 − production rate, H2O2 concentration or the amount of malondialdehyde (MDA), young fresh leaves (0.15–0.2 g) were homogenized with different extraction buffers. O2 − level was examined by using hydroxylamine oxidization method, H2O2 concentration was determined by titanium sulfate colorimetric method and the amount of MDA was determined by the thiobarbituric acid reaction according to Weydert and Cullen (2010). For measurement of activity of superoxide dismutase (SOD), peroxidase (POD) or catalase (CAT), young fresh leaves (0.15 g) were homogenized in ice-cold PBS buffer, after being filtered and centrifuged, the supernatant of the homogenate was used as crude enzyme immediately for analysis. SOD activity was determined by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium chloride (NBT), as described by Basu et al. (2009); POD or CAT activity was defined as guaiacol increase at 470 nm or H2O2 decrease at 240 nm, according to the method of Yu et al. (2015).
Water loss experiment
Young fresh leaves of tobacco transgenic lines-OE1, OE2, OE3 and NT plants (3-month-old) were detached and placed on filter paper at room temperature, the fresh weight of each detached leaf was determined at 0, 10, 30, 60, 90, 120, 150, 180, 210 and 240 min, respectively, then the leaves were photographed when the dehydration was completed. The experiment was repeated at least for three times with the similar results.
Detection of H2O2 and superoxide anion accumulation by 3,3′-diaminobenzidine (DAB) and nitro blue tetrazolium (NBT) staining
Non-transgenic plants and transgenic lines were subjected to 200 mM NaCl or 15% PEG 6000 treatment for 2 h before test. For H2O2 detection, young fresh leaves were incubated in DAB aqueous solution (1 mg mL−1, pH 3.2) for overnight at 25 °C in the dark according to Kumar et al. (2013) method; superoxide anion was detected via NBT staining based on Kumar et al. (2013) method. In NBT assay, young fresh leaves were incubated in 0.2% NBT solution [0.1 g NBT in 50 mL 50 mM sodium phosphate buffer (pH 7.5)] for 24 h at 25 °C in the dark. Thereafter, the leaves (from the above two methods) were soaked in 95% ethanol and heated in a boiling water-bath for 10 min (or more if necessary, with intermittent shaking) to remove chlorophyll. At least three leaves from each treatment were stained in the experiment. The staining result was determined by doing statistical analysis with MATLAB software (vision 7.0) based on graphical user interface.
Measurement of the net photosynthetic rate and intercellular CO2 concentration in transgenic tobacco
The net photosynthetic rate (Anet) and intercellular CO2 concentration (Ci) of NT and three T1 transgenic lines (OE1, OE2, OE3) were measured before and after NaCl or PEG treatment for 2 h, 24 and 48 h by using a portable infrared gas analyzer-based photosynthesis system (Li-Cor 6400; Li-Cor, Lincoln, NE, USA) according to the method described by Zhang et al. (2014). For measurement, the third fully expanded leaf from the top of a plant with the least area of 5 × 5 cm2 was taken into use. At least ten repeat readings of each leaf and three leaves from three plants of each line were recorded.
Quantitative RT-PCR analysis of CaMKK1 and CaMPK in C. album and the stress-related genes in CaMKK1-overexpressed transgenic tobacco lines under abiotic stresses
Total RNA was isolated from the young fresh leaves of C. album or tobacco (NT and transgenic lines) under different stresses, and the cDNA was synthesized according to the above procedure. Quantitative RT-PCR was carried out by using the ABI 7500 Real time PCR system (Applied Biosystem, US). Detection of qRT-PCR product was performed by staining with QuantiNova SYBR Green PCR Kit (Cat. 208054; Qiagen, Germany). For expression pattern of CaMKK1 and CaMPK in C. album, the specific primers were shown in Table 1. The relative amplification of β-actin of C. album was used for normalization. For expression profile of the stress-related genes in tobacco transgenic lines, NtMPK4, three dehydration response element binding factor (DREB) genes (NtDREB2, NtDREB3, NtDREB4), stress response genes-group five late embryogenesis abundant protein (NtLEA5), arginine decarboxylase (NtADC2) and superoxide dismutase (NtSOD) were analyzed in the present study. The primer sequences were shown in Table 1. The relative amplification of β-actin of tobacco was used for normalization. The above amplification was performed in the following conditions: 95 °C 2 min followed by 40 cycles of 95 °C 5 s, 60 °C 30 s. Four samples (biological replicates) of each treatment were duplicated (technical replicates) in qRT-PCR experiment. The relative expression level of genes was quantified according to the \({\text{R = 2}}^{{ - {{\Delta \Delta }}{\text C}_{{\text{T}}} }}\) mathematical model (Shi and Chiang 2005), where ∆∆CT = ∆CT target sample − ∆CT control sample, ∆CT sample = CT test gene − CT reference gene. The final value of relative quantification was described as fold change of gene expression in the test sample compared to control.
Statistical analysis
All data were expressed as mean ± standard error. For seed germination, percentage was arcsine transformed from four replicates before statistical analysis; for root length, ten seedlings were measured; for physiological parameter assay, four replicates were measured; for qRT-PCR, four biological replicates and two technical replicates of each treatment were applied. Microsoft Excel and the software of GraphPad Prism 5.0 were employed in data analysis. One-way ANOVA Post Hoc Duncan’s multiple range test was used in multiple variables comparison. Any significant difference compared to control at P < 0.05 or P < 0.01 was given.
Results
Cloning and characterization of CaMKK1 in C. album
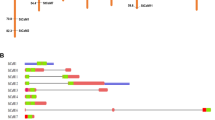
A cDNA fragment of CaMKK1 open reading frame (ORF) was obtained from C. album, which is 1062 bp long and encodes 353 putative amino acids with an estimated molecular weight of 36 kDa. Alignment of the deduced amino acid sequence of CaMKK1 with MAPKKs from other plant species indicates that CaMKK1 possesses all 11 conserved subdomains (I–XI) and contains a conserved S/TXXXXXS/T motif as the phosphorylation site between VII and VIII domains, and a docking site known as binding MAPKs or phosphatases (Fig. 1a).
Alignment and phylogenetic analysis of CaMKK1 and other MAPKK family members and expression patterns of CaMKK1 in C. album under salt and drought stress. a Alignment of CaMKK1 amino acid sequence with the representative closely related amino acid sequences-AtMKK1 (AEE85154.1), NtMKK1 (AAF67262.1), OsMKK1 (AAG40578.1), LeMKK (NP_001234588.1), BdMKK2 (NP_001289805.1) and BvMKK2 (XP_010696552.1) using DNAMAN software. The conserved subdomains are shown with Roman numbers (I–XI) at the top of the sequences, the numbers on both ends of the conserved sequence represent the starting and ending sites of amino acid in the complete sequence. b The phylogenetic tree between CaMKK1 and other plant MAPKK proteins. CaMKK1 is in bold and with a dot in front. Different groups (A, B, C, D) of MAPKKs are marked on very beginning of the branch on the left. Numbers above or below branches indicate bootstrap values from 1000 replicates. The protein sequences of the MAPKKs used for construction of the tree are acquired from GenBank database. The accession numbers are listed below: AtMKK1 (AEE85154.1), AtMKK2 (AEE85679.1), AtMKK3 (AED94548.1), AtMKK4 (AEE32696.1), AtMKK5 (AEE76478.1), AtMKK6 (AED96783.1), AtMKK7 (AEE29706.1), AtMKK8 (NP_187274.1), AtMKK9 (AEE35470.1), NtMKK1(AAF67262.1), NtMKK2 (AAG53979.1), OsMKK1 (AAG40578.1), OsMKK6 (ABG45894.1), ZmMKK3 (AET74070.1), GmMKK2 (NP_001236577.1), GhMKK2 (ADI52621.1), BnMKK1 (ADZ75456.1), BvMKK2 (XP_010696552.1), BdMKK2 (NP_001289805.1), BdMKK6.1 (AFS18269.1) and LeMKK (NP_001234588.1). c Expression patterns of CaMKK1 and CaMPK in C. album and NtMPK4 in transgenic tobacco line (OE3) under salt and drought stress by quantitative RT-PCR. Total RNA was isolated from C. album fresh leaves at the indicated treatment time points. NaCl: NaCl treatment; PEG: polyethylene glycol 6000 treatment. Values are means ± SE of eight replicates. Different lowercase letters above the columns indicate significant difference (P < 0.05) according to Duncan’s multiple range test
To reveal the evolutionary relationship among MAPKKs from various plant species, we constructed a phylogenetic tree using amino acid sequences derived from the GenBank database (Fig. 1b). Blastp analysis showed that CaMKK1 shared a high degree of sequence similarity with BvMKK2 (XP_010696552.1) from Beta vulgaris subsp. Vulgaris, and which is a member of group A MAPKK.
To investigate the expression pattern of CaMKK1 under abiotic stresses, we examined the transcript accumulation of CaMKK1 in C. album by qRT-PCR. As shown in Fig. 1c (top), both NaCl and PEG treatments could induce expression of CaMKK1. For NaCl, the transcripts accumulated significantly from 50 to 300 mM concentration and reached to the highest level at a short time (1 h); PEG treatment revealed a little different expression pattern, in which a sharp increase of CaMKK1 transcription at 10% PEG was observed at 0.5 h, while that of the other concentrations reached to the highest level at 5 h. These results suggest that CaMKK1 can respond actively to abiotic stress.
To further explore the expression pattern of downstream component of CaMKK1, we analyzed the transcriptional level of CaMPK under 300 mM NaCl or 20% PEG treatment. The responses of CaMPK to salt and drought showed similar trends with those of CaMKK1, in which CaMPK was quickly induced under salt stress, and the transcripts were accumulated significantly to the highest level at 1 h (Fig. 1c, middle); upon exposure to 20% PEG, the expression level of CaMPK gradually increased and reached to the maximum at 2 h, which also showed a quick induction pattern. Meanwhile, we also analyzed the expression of NtMPK4 in CaMKK-overexpressed transgenic tobacco line (OE3), which is a homolog of CaMPK in N. tabacum and can positively respond to stress (Zhang et al. 2013). Results revealed that the NtMPK4 was quickly up-regulated by NaCl or PEG treatment, and the increment was significantly higher than that of the NT plant under stress (Fig. 1c, bottom). All these results suggest that CaMKK1, CaMPK and NtMPK4 all respond to stress fast and transiently, and the former one can positively activate the downstream component-MPK expression in C. album and transgenic tobacco.
Overexpression of CaMKK1 enhances salt tolerance to bacterium and transgenic tobacco
To investigate the function of salt tolerance, CaMKK1 was over-expressed in tobacco. Genomic PCR and RT-PCR results showed that CaMKK1 was transformed into tobacco plants and expressed at transcriptional level (Fig. 2a, left and middle), moreover, the expression level of CaMKK1 in different transgenic lines (OE1, OE2, OE3) was slight different, i.e. OE3 was the higher, OE1 was in the middle, OE2 was the lower (Fig. 2a, right). Three transgenic lines which exhibited 3:1 segregation ratio between the antibiotic-resistant and non-resistant seedlings were selected for further experiments.
Overexpression of CaMKK1 enhances salt tolerance in transgenic tobacco. a PCR, RT-PCR, or qPCR analysis of CaMKK1 transgenic tobacco. Left Genomic DNA PCR, middle RT-PCR, Right qPCR. M DL2000, 1–4 T1 transgenic tobacco lines, C+ positive control, NT non-transgenic tobacco plant. b Effect of NaCl stress on the growth of recombinant E. coli strain pET28a-CaMKK1 (BL21). c Seed germination of NT and T2 transgenic tobacco lines (OE1, OE2, OE3) on MS medium containing different concentrations of NaCl. OE1, OE2, OE3: T2 generation transgenic tobacco lines 1, 2, 3. d Germination percentage of NT and T2 transgenic lines on MS medium containing different concentrations of NaCl. e Root length of 12 days-old seedlings on MS medium containing different concentrations of NaCl. f Seedling development of NT and T2 transgenic lines on MS medium containing different concentrations of NaCl. In (d) and (f), values are means ± SE of four (germination) or ten (root) replicates. Different lowercase letters above the columns indicate significant difference (P < 0.05) according to Duncan’s multiple range test
Before the transgenic tobacco lines were analyzed, the performance of ectopic expression of CaMKK1 in E. coli was tested. The time course of growth of recombinant E. coli strain harboring with pET-28a-CaMKK1 was measured under NaCl treatment (Fig. 2b). Results showed that the cells grew similarly well under non-stress condition, whereas the recombinant strain grew much better than that of the control strain when being applied with salt stress (50–200 mM NaCl).
To further understand the role of CaMKK1 in response to stress, we generated transgenic tobacco lines of CaMKK1. Seed germination and seedling growth of T2 generation revealed that no significant difference was observed between NT and transgenic lines without NaCl treatment (Fig. 2c), however, with NaCl concentration rising, the germination percentage of three T2 transgenic tobacco lines (OE1, OE2, OE3) was significantly higher than that of NT plants (P < 0.05) (Fig. 2d). The seedling and root growth of transgenic lines were significantly greater than that of NT plants in the presence of 100–300 mM NaCl (Fig. 2e, f).
Overexpression of CaMKK1 confers drought tolerance to bacterium and transgenic tobacco
To gain a better understanding of CaMKK1 in drought tolerance, we carried out a series of experiments to investigate the performance. First, recombinant E. coli strain harboring with pET-28a-CaMKK1 was measured under PEG treatment (Fig. 3a). Compared to the control, the recombinant strain could tolerate much broader range of different stresses and grow much better when being applied with drought stress.
Overexpression of CaMKK1 confers drought tolerance to transgenic tobacco. a Effect of PEG stress on the growth of recombinant E. coli strain pET28a-CaMKK1 (BL21). b Seed germination of NT and T2 transgenic lines on MS medium containing different concentrations of PEG. NT non-transgenic tobacco, OE1, OE2, OE3 T2 transgenic tobacco lines 1, 2, 3. c Germination percentage of NT and T2 transgenic lines on MS medium containing different concentrations of PEG. d Root length of 12 days-old seedlings of NT and T2 transgenic lines on MS medium containing different concentrations of PEG. e Seedling development of NT and T2 transgenic lines on MS medium containing different concentrations of PEG. f The water loss rate. The rate of water loss was calculated by the loss of fresh weight of the samples. g The phenotype of detached leaves of NT and transgenic lines at the indicated times. In (c) and (d), values are means ± SE of four (germination) or ten (root) replicates. Different lowercase letters above the columns indicate significant difference (P < 0.05) according to Duncan’s multiple range test
Furthermore, seed germination was conducted under PEG 6000 (5, 10, 15%) to mimic drought stress. No obvious difference existed between NT and transgenic lines without PEG treatment (Fig. 3b). However, when treated with PEG, the germination percentage of OE lines were significantly higher than that of NT plant (P < 0.05) (Fig. 3c). The root length in OE lines was greater compared to that of NT plants in the presence of PEG (Fig. 3d, e).
To further investigate the drought tolerance of transgenic plants, water loss assay with fresh leaves of 3-month-old plants was performed. Lower water loss rate was detected in transgenic leaves in comparison with the control under dehydration treatment (Fig. 3f), and NT leaves appeared to be more withered than that of transgenic tobacco leaves after dehydration in air for 4 h (Fig. 3g). These results indicate that overexpression of CaMKK1 can improve the drought tolerance in transgenic tobacco.
Organic osmoprotectants contribute to salt and drought tolerance of CaMKK1 transgenic tobacco lines
The elevated tolerance of CaMKK1 transgenic plants was correlated to changes of the physiological and biochemical indexes. In the present study, we found that low level of free proline was detected and no significant difference observed between NT and OE lines under normal condition. However, salt and drought stresses promoted the accumulation of free proline, especially in transgenic plants under 15% PEG stress (Fig. 4a). Soluble sugar content of some transgenic line was significantly higher than that of the NT plant under 15% PEG or 300 mM NaCl treatment (Fig. 4b). The glycine betaine content of transgenic lines was significantly higher than that of NT plants under 15% PEG rather than NaCl treatment (Fig. 4c). There had no significant change of the total soluble protein in NT or transgenic plant under PEG or NaCl treatment (Fig. 4d). These data indicate that overexpression of CaMKK1 may increase the accumulation of organic osmoprotectants, which should contribute to enhance the drought or salt tolerance.
Analysis of organic osmoprotectants in transgenic tobacco. a Proline content, b soluble sugar content, c betaine content, d protein content of NT and transgenic lines under NaCl and PEG treatment. NT non-transgenic tobacco, OE1, OE2, OE3 T1 transgenic tobacco lines 1, 2, 3. Values are means ± SE of ten replicates. Different lowercase letters above the columns indicate significant difference (P < 0.05) according to Duncan’s multiple range test
Overexpression of CaMKK1 in tobacco alleviated ROS accumulation under drought or salt stress
To investigate whether CaMKK1 expression can elevate tolerance to oxidative stress or not, in the present study, DAB and NBT staining were used to measure the accumulation of H2O2 and O2 − in freshly detached leaves from NT and OE lines under PEG and NaCl treatment. Under normal condition, DAB staining (H2O2 content) had no significant difference between NT and OE lines. Following treatment with NaCl or PEG, the DAB staining was found to be much lighter in the transgenic plants than that of the control plants, which means that the level of H2O2 was considerably lower in the transgenic plants (Fig. 5a). The NBT staining (for O2 − content) of three OE lines was significantly weaker than that of NT plants upon NaCl or PEG stress, which revealed that there was less O2 − accumulation in OE lines after treatment with NaCl or PEG (Fig. 5b). These data indicate that the transgenic plant accumulates lower level of ROS, and overexpression of CaMKK1 can either inhibit ROS production or stimulate ROS scavenging.
Analysis of ROS accumulation and activity of antioxidant enzymes in NT and transgenic lines in response to salt and drought stress. a, b Left histochemical staining by DAB or NBT to reveal accumulation of H2O2 or O2 − in leaves of NT and transgenic lines, right statistical analysis with the Image G and GraphPad Prism 5.0. Staining area (%) represents the ratio between the stained area and the whole leaf area. c Changes of ROS level and MDA content and d the activity of antioxidant enzyme in NT and transgenic lines after treatment with NaCl and PEG. NT non-transgenic tobacco, OE1, OE2, OE3 T1 transgenic tobacco lines 1, 2, 3. Values are means ± SE of four replicates. Different lowercase letters above the columns indicate significant difference (P < 0.05) according to Duncan’s multiple range test
To quantitatively analyze the accumulation of ROS in plant, we measured H2O2 and O2 − level in transgenic lines under drought and salt stress. Results showed that H2O2 level or O2 − production in NT increased significantly than that of OE lines under NaCl or PEG treatment (Fig. 5c). MDA content of OE lines and NT plants all increased under stress, there were a 87% increase of MDA content under 300 mM NaCl and a 71% increase under 15% PEG stress of NT plants, which were significantly higher than that of OE lines (50% and 33%) (Fig. 5c). Moreover, the activity of antioxidant enzymes-SOD, POD and CAT increased significantly in some of the transgenic lines in comparison to that of NT plants under NaCl or PEG treatment (Fig. 5d). These data suggest that overexpression of CaMKK1 in transgenic tobacco may result in ROS effective scavenging by regulating the activity of antioxidant enzymes.
Overexpression of CaMKK1 enhances net photosynthetic rate to transgenic tobacco
The net photosynthetic rate (Anet) and intercellular CO2 concentration (Ci) of transgenic lines and NT plants were measured to clarify the improvement of the photosynthesis. Before NaCl or PEG treatment, the Anet and Ci showed no significant difference between NT plants and transgenic lines. After treatment, the Anet was significantly increased in OE1 and OE3 compared to NT plants; while the Ci was significantly lower in transgenic lines (except for OE2) than that of NT plants (Fig. 6).
Analysis of net photosynthetic rate (Anet) and the intercellular CO2 concentration (Ci) in NT and transgenic lines in response to salt and drought stress. a NaCl treatment. b PEG treatment. NT non-transgenic tobacco, OE1, OE2, OE3 T1 transgenic tobacco lines 1, 2, 3. Values are means ± SE of ten replicates. Different lowercase letters above the columns indicate significant difference (P < 0.05) according to Duncan’s multiple range test
Overexpression of CaMKK1 enhances stress-responsive genes expression
To investigate the effects of CaMKK1 overexpression on the downstream genes, we performed quantitative RT-PCR analysis with some typical stress responsible genes: NtDREB2, NtDREB3, NtDREB4, a class of DREB/CBF transcription factors that bind to drought response cis-element; LEA5, SOD, ADC2 (stress-related genes). When exposed to NaCl stress, overexpression of CaMKK1 in transgenic tobacco lines (especially in OE3) dramatically enhanced the expression level of NtDREB2 with 100-fold more at 24 h (P < 0.0001) and 150-fold more at 48 h (P < 0.0001), NtDREB3 almost 20-fold at 24 h (P < 0.0001) and 125-fold at 48 h (P < 0.0001), and NtDREB4 10-fold at 24 h (P < 0.0001) and 83-fold at 48 h (P < 0.0001) compared to NT plants; under PEG treatment, the increased expression levels of CaMKK1 in OE lines with 100-fold at 24 h (P < 0.0001) in NtDREB2 and 50-fold at 48 h (P < 0.0001), 50-fold at 24 h or 48 h (P < 0.0001) of NtDREB3, 47-fold at 24 h (P < 0.0001) and ninefold at 48 h (P < 0.0001) of NtDREB4 were observed, which suggests that these DREB transcription factors may be regulated by MAPKK and contribute to enhancement of stress tolerance (Fig. 7).
Expression patterns of stress-related genes under NaCl and PEG treatment in NT and transgenic lines by quantitative RT-PCR. a NaCl treatment. b PEG treatment. NtDREB2, NtDREB3, NtDREB4 dehydration response element binding factor 2/3/4, NtSOD superoxide dismutase, NtADC2 arginine decarboxylase, NtLEA5 group 5 late embryogenesis abundant protein. These genes are all from N. tabacum. NT non-transgenic tobacco, OE1, OE2, OE3 T1 transgenic tobacco lines 1, 2, 3. Values are means ± SE of eight replicates. Different lowercase letters above the columns indicate significant difference (P < 0.05) according to Duncan’s multiple range test
The expression of NtSOD, NtADC2 and NtLEA5 in transgenic lines had no significant change compared to that of the control under normal condition. However, the expression of NtSOD significantly elevated in the transgenic lines compared to NT plants under NaCl or PEG treatment for 48 h (Fig. 7) (P < 0.0001 for NaCl; P = 0.0009 for PEG). The expressions of NtADC2 (P = 0.0018 for NaCl and P = 0.0028 for PEG) and NtLEA5 (P = 0.0269 for NaCl and P = 0.0349 for PEG) in transgenic lines were also significantly higher than that of NT plant under NaCl or PEG treatment for 48 h.
Discussion
A large amount of evidence indicates that MAPK modules are closely related to plant adaptability to environmental stresses (Sun et al. 2014). As a signal convergence point in the hierarchical organization of MAPK cascades, MAPKK plays crucial role in integrating and passing signals, finally transferring extracellular stimuli into intracellular responses (Xu et al. 2016). In the present study, we characterized the stress tolerance function of a group A MAPKK from C. album (shortened as CaMKK1). Based on the molecular cloning and identifying CaMKK1, overexpression of which could efficiently scavenge the excess ROS, and improve various stress-responsive physiological parameters in transgenic tobacco under stress conditions, e.g. increased content of osmoprotectants, activity of antioxidant enzymes, net photosynthesis rate, etc. It is noteworthy that transcription factor genes-DREB2, DREB3, DREB4 in CaMKK1 transgenic tobacco were remarkably up-regulated in response to salt and drought stress. These findings not only provide the indication of involvement of CaMKK1 in abiotic stress response, but suggest a crosstalk in the signaling pathway between MAPK cascade and ROS signaling.
Phylogenetic analysis of amino acid sequences in our work revealed that CaMKK1 belongs to group A MAPKK and possesses all 11 conserved domains or motifs. It has been reported that AtMKK1, AtMKK2, and AtMKK6 (group A MAPKK) participate in defense response and mediate phytohormone (e.g. ABA, GA) signaling transduction, to increase the tolerance of abiotic stress (Gao et al. 2008; Xing et al. 2009). OsMKK1 (group A) or OsMKK6 (group A) is involved in the regulation of drought or salinity stress tolerance (Kumar et al. 2008). Besides positively response to salt and drought stress, cotton GhMKK1 (group A) also mediates defense response to pathogen infection in transgenic tobacco (Lu et al. 2013). These previous reports suggest a role of group A MAPKKs in plant stress responses.
Salinity and drought are major abiotic factors which affect plant growth and development (Liu et al. 2015). MAPK signaling pathway is well-known in mediating stress-responsive signaling (Colcombet and Hirt 2008). In the present study, CaMKK1 (the crucial component in the MAPK cascade) was up-regulated under salinity and drought stress in C. album. It is consistent with the reports of TaMAPKK in T. aestivum and LcMKK in L. chinense (Wen et al. 2015; Wu et al. 2015); whereas AtMKK9 (A. thaliana), BdMKK6.2 (B. distachyon) or GhMKK5 (G. hirsutum) applied negative effect at the same conditions (Xu et al. 2008; Zhang et al. 2012; Sun et al. 2016). It is suggested that different members of MAPKKs may play diverse roles in response to stress (Colcombet and Hirt 2008). In the present study, ectopic expression of CaMKK1 in prokaryote displayed much better growth under salt and drought stress; moreover, CaMKK1 overexpression in tobacco not only improved seed germination and seedling growth, but also enhanced the expression of downstream gene-NtMPK4 in transgenic lines under stress condition. Similar result shows that overexpression of ZmMKK4 in Arabidopsis had a significantly higher germination rate and better seedling growth under salt stress (Kong et al. 2011), while a T-DNA insertion mutation of Arabidopsis MKK2 gene confers hypersensitivity to salt stress in germination (Teige et al. 2004). In addition, MAPK is the last component of MAPK cascade and plays crucial roles in linking upstream MAPKKs and downstream components in stress signaling (Agarwal et al. 2010; Wang et al. 2015). In the present study, we found that CaMPK in C. album and NtMPK4 in CaMKK-overexpressed transgenic tobacco could actively respond to salt and drought stress, and all of them displayed similar fast and transient transcription pattern. Taken together, it suggests that CaMKK1 should be a positive and important regulator in MAPK cascade in response to salt and drought stress.
Proline and soluble sugars play an important role in adaptation to adversity in plants. When countering the environmental stimulations, proline in particular increases the water-control capacity and helps to prevent water loss, avoid protein denaturation, and minimize disruption to cellular metabolism (Liu and Zhu 1997). Accumulation of soluble sugars and glycine betaine not only reduces the solute potential in plant cells, but also assists the scavenging and regulation of ROS (Couée et al. 2006; Fan et al. 2012). In the present study, water loss rate was lower in the transgenic tobacco lines than NT plants under dehydration treatment, and the compatible organic molecules-proline, soluble sugar and glycine betaine in CaMKK1-overexpressed tobacco lines were significantly accumulated compared to that of NT plants. This result was corresponding to the improved net photosynthesis rate of transgenic tobacco lines in our experiment. Avoiding excessive water loss is very important for various physiological activities, especially the photosynthesis (Chaves et al. 2009). Our results suggest that overexpression of CaMKK1 in tobacco protects transgenic plant from losing water probably by accumulating the osmoprotectant content, which then contributes to improving photosynthesis and adaptation to salt and drought stress.
It has been reported that ROS can activate MAPK, whereas disturbing MAPK cascades can modulate ROS production and action (Liu and He 2017). In the present study, we found that overexpression of CaMKK1 significantly decreased O2 −, H2O2 and MDA accumulation in transgenic lines under salt and drought stress, which was corresponding to the histochemical staining of DAB or NBT with a significant reduction of H2O2 or O2 − in transgenic lines. ROS and MAPK-associated signaling pathways play a crucial role in stimulating the response to protect against oxidative stress (Liu and He 2016). A great deal of research has shown that induction of the antioxidant enzymes is important for protection against various stresses (Sinha et al. 2011). Overexpression of AtMKK1 or AtMKK2 adjusts ROS metabolism by regulating the catalase genes which encode the H2O2 scavenging enzymes (Pitzschke et al. 2009). Our further analysis showed that overexpression of CaMKK1 alleviated the accumulation of ROS by improving the activity of ROS scavenging enzymes-SOD, POD and CAT under salt and drought stress. Our results were consistent with earlier reports on MAPKK genes in other plants (Kong et al. 2011; Lu et al. 2013; Wu et al. 2015). SOD is the first line of defense against ROS (Samuel and Ellis 2002) by catalyzing the dismutation of O2 − to oxygen and H2O2, which plays an important role in protecting cells against superoxide radicals. In our study, in accord to the enzyme activity of SOD, the transcription level of NtSOD in transgenic tobacco increased significantly compared to that of NT plants under stress condition. Our data suggest that CaMKK1 can regulate the expression and activity of these antioxidant enzymes, which may lead to enhancement of ROS scavenging and protection against salt and drought stresses.
The dehydration response element binding proteins are important transcription factors in regulation of plant response to abiotic stresses (Kamioka et al. 2016). In the present study, three transcription factors-NtDREB2, NtDREB3, NtDREB4 were sharply up-regulated in transgenic tobacco lines compared to that of NT plants under NaCl or PEG treatments, i.e. at least 15-fold and at most near 224-fold (with an average of more than 30-fold) of the transcript accumulation of NtDREB2, NtDREB3, NtDREB4 were induced. Compared to other genes in the present study, the DREB genes responded to the stress much quicker and stronger in CaMKK1-overexpressed tobacco. It suggests that MAPKK might regulate the expression of DREB transcription factors directly in the MAPK signaling pathway in response to stress. It has been reported that NtDREB2A was up-regulated in ZmMKK4-overexpression transgenic tobacco under salt stress (Kong et al. 2011). LEA5 is a group 5 late embryogenesis abundant protein, which can accumulate substantially in plants under high-salt stress and dehydration (Liu et al. 2009). In the present study, NtLEA5 was up-regulated in transgenic tobacco lines compared to that of NT plants under stress. Arginine decarboxylase (ADC) is a rate-limiting enzyme that catalyzes the first step of polyamine biosynthesis, which is involved in abiotic stress responses, e.g. salinity, heat and low temperature (Urano et al. 2005). The expression of NtADC2 in the present study was significantly induced in transgenic lines under stress. The high expression of these stress-related genes should contribute to salt and drought tolerance in CaMKK1-overexpressed tobacco lines, and which might be involved in the regulation of the signaling from CaMKK1 to DREBs.
The significant involvement of MAPKKs in ROS-related signaling pathways had been described previously. Stimuli cause the activation of AtMEKK1, then activate AtMEK1-AtMPK4 module, which is the first identified cascade pathway in drought response (Ichimura et al. 1998; Mizoguchi et al. 1998). Later, MEKK1-MKK2-MPK4/MPK6 module related to enhancement of salt tolerance is characterized (Matsuoka et al. 2002; Teige et al. 2004). In the present study, CaMKK1 shared high similarity with AtMKK1 and AtMKK2 in group A, based on which we speculate that the MAPK cascade in C. album responds to stress similar to AtMKK1 and AtMKK2 in Arabidopsis. When ROS is generated upon salt or drought stress, which may activate MAPK signal transduction pathway, and the CaMKK1, as a key point of MAPK cascade, may in turn activate the CaMPK, and then modulate the expression of downstream transcription factor (TF) and stress-related genes, e.g. DREB TF. The quick and high expression of NtDREBs in CaMKK1 transgenic tobacco under stress in the present study may present an evidence for our speculation. However, further studies are necessary to characterize MAPK-related cascade of C. album in response to abiotic stress.
Conclusion
A group A MAPKK gene-CaMKK1 was identified and characterized from a salt-tolerant species-C. album. Overexpression of CaMKK1 in prokaryote or in transgenic tobacco lines improved the salt and drought tolerance, which reflects in the transgenic tobacco lines with significant improvement of physiological activities, e.g. increased proline, soluble sugar or glycine betaine contents; reduced water loss and improved net photosynthesis rate, etc. under salt and drought stresses. Besides, the improved stress tolerance of CaMKK1 overexpressed lines could partially be related to activation of antioxidant genes/enzymes, which lead to a more efficient scavenging of ROS under stress conditions. Meanwhile, stress-responsive genes were upregulated by CaMKK1 overexpression, especially the DREB transcription factors. NtDREB2, NtDREB3, NtDREB4 quickly and dramatically responded to stress in transgenic tobacco, suggesting that CaMKK1 may transfer signal to CaMPK which then interacts with downstream TF components. The results of the present study suggest that CaMKK1 acts as a positive regulator in stress responsible signaling pathway. The present study will no doubt broaden our knowledge on further understanding of the molecular mechanism of MAPK signal transduction pathway in response to stress.
References
Agarwal PK, Gupta K, Jha B (2010) Molecular characterization of the Salicornia brachiata SbMAPKK gene and its expression by abiotic stress. Mol Biol Rep 37:981–986
Andreasson E, Ellis B (2010) Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci 15:106–113
Basu S, Roychoudhury A, Saha PP, Sengupta DN (2009) Differential antioxidative responses of indica rice cultivars to drought stress. Plant Growth Regul 60:51–59
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Colcombet J, Hirt H (2008) Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem J 413:217–226
Couée I, Sulmon C, Gouesbet G, Amrani AE (2006) Involvement of soluble sugars in reactive oxygen species balamnce and responses to oxidative stress in plants. J Exp Bot 57:449–459
Fan W, Zhang M, Zhang H, Zhang P (2012) Improved tolerance to various abiotic stresses in transgenic sweet potato (Ipomoea batatas) expressing spinach betaine aldehyde dehydrogenase. PLoS ONE 7:e37344
Gao M, Liu J, Bi D, Zhang Z, Cheng F, Chen S, Zhang Y (2008) MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res 18:1190–1198
Hamel LP, Nicole MC, Sritubtim S, Morency MJ, Ellis M, Ehlting J, Beaudoin N, Barbazuk B, Klessig D, Lee J, Martin G, Mundy J, Ohashi Y, Scheel D, Sheen J, Xing T, Zhang S, Seguin A, Ellis BE (2006) Ancient signals: comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci 11:192–198
Hamidov A, Beltrao J, Neves A, Khaydarova V, Khamidov M (2007) Apocynum lancifolium and Chenopodium album: potential species to remediate saline soils. WSEAS Trans Environ Dev 3:123–128
Hirt H (1997) Multiple roles of MAP kinases in plant signal transduction. Trends Plant Sci 2:11–14
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Huang GT, Ma SL, Bai LP, Zhang L, Ma H, Jia P, Liu J, Zhong M, Guo ZF (2012) Signal transduction during cold, salt, and drought stresses in plants. Mol Biol Rep 39:969–987
Ichimura K, Mizoguchi T, Irie K, Morrisd P, Giraudate J, Matsumotoc K, Shinozaki K (1998) Isolation of ATMEKK1 (a MAP kinase kinase Kinase): interacting proteins and analysis of a MAP kinase cascade in Arabidopsis. Biochem Biophys Res Commun 253:532–543
Ishihama N, Yamada R, Yoshioka M, Katou S, Yoshioka H (2011) Phosphorylation of the Nicotiana benthamiana WRKY8 transcription factor by MAPK functions in the defense response. Plant Cell 23:1153–1170
Kamioka M, Takao S, Suzuki T, Takie K, Higashiyama T, Kinoshita T, Nakamichi N (2016) Direct repression of evening genes by CIRCADIAN CLOCK-ASSOCIATED 1 in the Arabidopsis circadian clock. Plant Cell 28:696–711
Kiegerl S, Cardinale F, Siligan C, Gross A, Baudouin E, Liwosz A, Eklöf S, Till S, Laszlo Bögre L, Hirt H, Meskiene I (2000) SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK. Plant Cell 12:2247–2258
Kong X, Pan J, Zhang M et al (2011) ZmMKK4, a novel group C mitogen-activated protein kinase kinase in maize (Zea mays), confers salt and cold tolerance in transgenic Arabidopsis. Plant Cell Environ 34:1291–1303
Kong X, Lv W, Zhang D, Jiang S, Zhang S, Li D (2013) Genome-wide identification and analysis of expression profiles of maize mitogen-activated protein kinase kinase kinase. PLoS ONE 8:e57714
Kumar K, Sinha AK (2013) Overexpression of constitutively active mitogen activated protein kinase kinase 6 enhances tolerance to salt stress in rice. Rice 6:25
Kumar K, Rao KP, Sharma P, Sinha AK (2008) Differential regulation of rice mitogen activated protein kinase kinase (MKK) by abiotic stress. Plant Physiol Biochem 46:891–897
Kumar D, Yusuf MA, Singh P, Sardar M, Sarin NB (2013) Modulation of antioxidant machinery in α-tocopherol-enriched transgenic Brassica juncea plants tolerant to abiotic stress conditions. Protoplasma 250:1079–1089
Liu Y, He C (2016) Regulation of plant reactive oxygen species (ROS) in stress responses: learning from AtRBOHD. Plant Cell Rep 35:995–1007
Liu Y, He C (2017) A review of redox signaling and the control of MAP kinase pathway in plants. Redox Biol 11:192–204
Liu J, Zhu JK (1997) Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol 114:591–596
Liu X, Wang Z, Wang L, Deng X (2009) LEA 4 group genes from the resurrection plant Boea hygrometrica confer dehydration tolerance in transgenic tobacco. Plant Sci 176:90–98
Liu Y, Xie L, Liang X, Zhang S (2015) CpLEA5, the late embryogenesis abundant protein gene from Chimonanthus praecox, possesses low temperature and osmotic resistances in prokaryote and eukaryotes. Int J Mol Sci 16:26978–26990
Lokhande VH, Nikam TD, Penna S (2010) Biochemical, physiological and growth changes in response to salinity in callus cultures of Sesuvium portulacastrum L. Plant Cell Tiss Organ Cult 102:17–25
Long L, Gao W, Xu L, Liu M, Luo X, He X, Yang X, Zhang X, Zhu L (2013) GbMPK3, a mitogen-activated protein kinase from cotton, enhances drought and oxidative stress tolerance in tobacco. Plant Cell Tissue Organ Cult 116:153–162
Lu W, Chu X, Li Y, Guo X (2013) Cotton GhMKK1 induces the tolerance of salt and drought stress, and mediates defence responses to pathogen infection in transgenic Nicotiana benthamiana. PLoS ONE 8:e68503
MAPK group (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7:301–308
Matsuoka D, Nanmori T, Sato K, Fukami Y, Kikkawa U, Yasuda T (2002) Activation of AtMEK1, an Arabidopsis mitogen-activated protein kinase kinase, in vitro and in vivo: analysis of active mutants expressed in E. coli and generation of the active form in stress response in seedlings. Plant J 29:637–647
Mikołajczyk M, Awotunde OS, Muszyńska G, Klessig DF, Dobrowolska GY (2000) Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell 12:165–178
Mizoguchi T, Ichimura K, Irie K, Morris P, Giraudat J, Matsumoto K, Shinozaki K (1998) Identification of a possible MAP kinase cascade in Arabidopsis thaliana based on pairwise yeast two-hybrid analysis and functional complementation tests of yeast mutants. FEBS Lett 437:56–60
Nakagami H, Soukupová H, Schikora A, Zárský V, Hirt H (2006) A Mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J Biol Chem 281:38697–38704
Palma F, Lluch C, Iribarne C, García-Garrido JM, Tejera GNA (2009) Combined effect of salicylic acid and salinity on some antioxidant activities, oxidative stress and metabolite accumulation in Phaseolus vulgaris. Plant Growth Regul 58:307–316
Persak H, Pitzschke A (2014) Dominant repression by Arabidopsis transcription factor MYB44 causes oxidative damage and hypersensitivity to abiotic stress. Int J Mol Sci 15:2517–2537
Pitzschke A, Schikora A, Hirt H (2009) MAPK cascade signalling networks in plant defence. Curr Opin Plant Biol 12:421–426
Rodriguez MCS, Petersen M, Mundy J (2010) Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol 61:621–649
Samuel M a, Ellis BE (2002) Double jeopardy: both overexpression and suppression of a redox-activated plant mitogen-activated protein kinase render tobacco plants ozone sensitive. Plant Cell 14:2059–2069
Shi R, Chiang VL (2005) Facile means for quantifying microRNA expression by real-time PCR. Biotechniques 39:519–524
Shinozaki K, Yamaguchi-Shinozaki K, Seki M (2003) Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol 6:410–417
Singh R, Lee MO, Lee JE, Choi J, Park JH, Kim EH, Yoo RH, Cho JI, Jeon JS, Rakwal R, Agrawal GK, Moon JS, Jwa NS (2012) Rice mitogen-activated protein kinase interactome analysis using the yeast two-hybrid system. Plant Physiol 160:477–487
Sinha AK, Jaggi M, Raghuram B, Tuteja N (2011) Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal Behav 6:196–203
Smekalova V, Doskocilova A, Komis G, Samaj J (2014) Crosstalk between secondary messengers, hormones and MAPK modules during abiotic stress signalling in plants. Biotechnol Adv 32:2–11
Sun Y, Wang C, Yang B, Wu F, Hao X, Liang W, Niu F, Yan J, Zhang H, Wang B, Deyholos MK, Jiang YQ (2014) Identification and functional analysis of mitogen-activated protein kinase kinase kinase (MAPKKK) genes in canola (Brassica napus L.). J Exp Bot 65:2171–2188
Sun J, Zhou R, Li Y, Hu W, Qiu D, Wang X, Wang Q, Feng Z, Wang L, Zhou Y, He G, Yang G (2016) A Brachypodium distachyon MAPKK gene BdMKK6.2 negatively regulates drought stress tolerance in transgenic tobacco plants. J Plant Growth Regul 35:121–134
Takahashi F, Ichimura K, Shinozaki K, Shirasu K (2009) Plant mitogen-activated protein kinase cascades in signaling crosstalk. In: Signal crosstalk in plant stress responses. Wiley-Blackwell, Hoboken, pp 23–42
Teige M, Scheikl E, Eulgem T, Dóczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H (2004) The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell 15:141–152
Urano K, Hobo T, Shinozaki K (2005) Arabidopsis ADC genes involved in polyamine biosynthesis areb essential for seed development. FEBS Lett 579:1557–1564
Wang NN, Zhao LL, Lu R, Li Y, Li XB (2015) Cotton mitogen-activated protein kinase4 (GhMPK4) confers the transgenic Arabidopsis hypersensitivity to salt and osmotic stresses. Plant Cell Tissue Organ Cult 123:619–632
Wen Y, Li X, Guo C, Ma C, Duan W, Lu W, Xiao K (2015) Characterization and expression analysis of mitogen-activated protein kinase cascade genes in wheat subjected to phosphorus and nitrogen deprivation, high salinity, and drought. J Plant Biochem Biotechnol 24:184–196
Weydert CJ, Cullen JJ (2010) Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5:51–66
Wu D, Ji J, Wang G, Guan W, Guan C, Jin C, Tian X (2015) LcMKK, a novel group A mitogen-activated protein kinase kinase gene in Lycium chinense, confers dehydration and drought tolerance in transgenic tobacco via scavenging ROS and modulating expression of stress-responsive genes. Plant Growth Regul 76:269–279
Xing Y, Jia W, Zhang J (2007) AtMEK1 mediates stress-induced gene expression of CAT1 catalase by triggering H2O2 production in Arabidopsis. J Exp Bot 58:2969–2981
Xing Y, Jia W, Zhang J (2009) AtMKK1 and AtMPK6 are involved in abscisic acid and sugar signaling in Arabidopsis seed germination. Plant Mol Biol 70:725–736
Xu J, Li Y, Wang Y, Liu H, Lei L, Yang H, Liu G, Ren D (2008) Activation of MAPK kinase 9 induces ethylene and camalexin biosynthesis and enhances sensitivity to salt stress in Arabidopsis. J Biol Chem 283:26996–27006
Xu J, Meng J, Meng X, Zhao Y, Liu J, Sun T, Liu Y, Wang Q, Zhang S (2016) Pathogen-responsive MPK3 and MPK6 reprogram the biosynthesis of indole glucosinolates and their derivatives in Arabidopsis immunity. Plant Cell 28:1144–1162
Yu L, Yan J, Yang Y, Zhu W (2015) Overexpression of tomato mitogen-activated protein kinase SlMPK3 in tobacco increases tolerance to low temperature stress. Plant Cell Tissue Organ Cult 121:21–34
Zhang L, Li Y, Lu W, Meng F, Wu C, Guo X (2012) Cotton GhMKK5 affects disease resistance, induces HR-like cell death, and reduces the tolerance to salt and drought stress in transgenic Nicotiana benthamiana. J Exp Bot 63:3935–3951
Zhang X, Cheng T, Wang G, Yan Y, Xia Q (2013) Cloning and evolutionary analysis of tobacco MAPK gene family. Mol Biol Rep 40:1407–1415
Zhang DY, Yang HL, Li XS, Li HY, Wang YC (2014) Overexpression of Tamarix albiflonum TaMnSOD increases drought tolerance in transgenic cotton. Mol Breed 34:1–11
Zhao X, Tan HJ, Liu YB, Li XR, Chen GX (2009) Effect of salt stress on growth and osmotic regulation in Thellungiella and Arabidopsis callus. Plant Cell Tissue Organ Cult 98:97–103
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31260037; 31460043; 30660012); Initial Project of 973 Program from the Ministry of Science and Technology of China (2012CB722204). The authors thank the anonymous reviewers and all of the Editors for their helpful comments and suggestions on this manuscript.
Author contributions
HYL designed the project and the experiments. JW, XXL, SXJ, YLM, SYZ, YL and XRL performed the experiments. JW, XXL and SXJ analyzed the data. JW and HYL wrote and revised the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Juan Wang, Xinxin Lan and Shengxiu Jiang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, J., Lan, X., Jiang, S. et al. CaMKK1 from Chenopodium album positively regulates salt and drought tolerance in transgenic tobacco. Plant Cell Tiss Organ Cult 130, 209–225 (2017). https://doi.org/10.1007/s11240-017-1216-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1216-5