Abstract
The mitogen-activated protein kinase (MAPK) cascades have been previously implicated in signal transduction during plant responses to various environmental stresses. As the convergent point of the MAPK cascades, MAPKKs play paramount roles in amplifying, integrating, and channeling information between the extracellular stimuli and intracellular responses. However, the functional role of MAPKKs in Lycium chinense has never been explored. In this study, a novel MAPKK gene, LcMKK, in L. chinense belonging to group A MAPKKs was isolated and functionally characterized. The transcript level of LcMKK rapidly increased in L. chinense after drought treatments. Overexpression of LcMKK in tobacco conferred dehydration and drought tolerance. Under dehydration and drought conditions, the transgenic tobacco lines exhibited better water status, less accumulation of reactive oxygen species (ROS), higher levels of germination rate and antioxidant enzyme activity than the wild type. In addition, overexpression of LcMKK enhanced the expression of ROS-related and stress-responsive genes under drought conditions. Taken together, these data demonstrate that LcMKK acts as a positive regulator in dehydration/drought stress responses by either regulating ROS homeostasis through the activation of the cellular antioxidant defense system or modulating transcriptional levels of a variety of stress-associated genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are constantly exposed to various harsh terrestrial environmental conditions, among which drought has been shown to be the most devastating one that limit the growth and productivity of diverse crop plants. On the other hand, during the long process of evolution, plants have developed a variety of elaborate mechanisms to perceive stress signals and express adaptive responses to protect themselves (Umezawa et al. 2006). Protein phosphorylation is one of the major mechanisms that are responsible for transducing stress signals and regulating expression of stress-responsive genes. Although several families of proteins can orchestrate protein phosphorylation, mitogen-activated protein kinase (MAPK) cascades play crucial roles in the major transduction pathways that transfer the extracellular stimuli into intracellular responses (Tena et al. 2001).
The MAPK cascade typically consist of three functionally interlinked protein kinases: MAPK, MAPKK (MAPK kinase), and MAPKKK (MAPKK kinase). They are functionally linked and act as an important network for amplifying, integrating, and channeling a broad spectrum of signals from upstream receptors to the downstream cellular effectors, leading to an adaptive stress response at cellular and organismal levels (Nadarajah and Sidek 2010). MAPKK kinase phosphorylates a specific MAPK kinase at a conserved S/TXXXXXS/T motif, which in turn phosphorylates a MAPK on the threonine (T) and tyrosine (Y) residues located in the activation loop between subdomains VII and VIII of the kinase catalytic domain, the activated MAPK is often imported into the nucleus, where it can phosphorylate and active a variety of downstream signaling components such as transcription factors and other protein kinases (Stulemeijer et al. 2007). In Arabidopsis, there were fewer MAPKKs (10) than MAPKKKs (80) and MAPKs (20) (Hadiarto et al. 2006). These numbers suggest that various signal transduction pathways may converge at the level of MAPKKs in the MAPK cascades, and one type of MAPKKs may be involved in multiple MAPK cascades, carrying out various biological functions (Andreasson and Ellis 2010). In this sense, MAPKK, the crucial component of the MAPK cascade, is the major port of signal transduction from upstream to the target.
An increasing body of evidences has shown that MAPKKs display important functions in plant abiotic signal transduction pathways. In Arabidopsis, AtMKK1 can activate AtMPK3 to transmit cold, salt, and drought signals (Mizoguchi et al. 1998). In tobaccos, NtSIMKK can regulate salt stress signals by activating SIMK (Kiegerl et al. 2000). NtMEK2 can activate WIPK and SIPK to transmit osmotic stress signals (Mikołajczyk et al. 2000). Overexpression of GhMKK1 in Nicotiana benthamiana enhanced its tolerance to drought stress (Lu et al. 2013). Although many studies have focused on how MAPKKs function in response to abiotic stresses, the role of MAPKK signaling following drought stress is still an issue of great interest. Various stresses can cause plants to produce reactive oxygen species (ROS), such as hydrogen peroxide (H2O2) and superoxide anions (O2 −) (Chen et al. 2008). An imbalance in ROS concentrations can result in oxidative stress and cause irreversible damage to plants. Previous studies have showed that the MAPK pathway plays an important role in ROS homeostasis. Overexpression of AtMKK1 in Arabidopsis can decrease stress-associated ROS levels and increase drought tolerance; conversely, AtMKK1 deficiency results in elevated ROS generation as well as increased sensitivity to drought tolerance (Xing et al. 2008). How MAPKKs participate in ROS signaling to regulate drought tolerance of plants is a question that needs to be further pursued in the future.
Lycium chinense is a drought-resistant shrub or small tree that is extensively distributed in the northwestern areas of China and other warm and subtropical countries. However, previous studies on L. chinense have mainly focused on the extraction of active components, pharmacology and medical function, cultivation and so on (Dong et al. 2013; Kim et al. 2002). Although many MAPKK genes have been isolated and the roles of them in response to environmental stresses have been well studied in model and crop plants (Chen et al. 2012; Kong et al. 2012; Nicole et al. 2006; Rao et al. 2010), functional information on the MAPKKs present in L. chinense is scarce, therefore, we tried to clone an MAPKK gene from L. chinense and functionally characterize its role in dehydration/drought tolerance. Furthermore, accumulation of ROS and expression of stress-responsive genes in transgenic plants were illustrated.
Materials and methods
Plant materials, growth conditions and stress treatment
Lycium chinense seedlings were grown in greenhouse at 18–25 °C with a 12 h photoperiod and relative humidity of 60–75 %. Experiments were carried out using 8-month-old plants. Before drought treatment, uniform and healthy plants were carefully uprooted and transferred to Hoagland’s solution for 7 days under same conditions as described above. Then, the L. chinense plants were inserted in culture bottles wrapped with aluminum foil containing 20 % PEG 6000 Hoagland’s solution. Drought treatment was given for different time durations (0, 1, 3, 6, 12, 24 h). The samples at 0 h were used as controls. The fourth to fifth leaves from the top of the first lateral branch were harvested at the indicated times and immediately frozen in liquid nitrogen before further analysis. Ten independent plants at the same age were treated simultaneously, and the treatment was repeated at least three times independently.
Cloning procedure and sequence analysis of the LcMKK gene
Total RNA was extracted from the leaves of L. chinense using the RNeasy plant mini kit (QIAGEN). The isolated total RNA was used as the template for first-strand cDNA synthesis using a TaKaRa RNA PCR Kit (AMV) ver. 3.0. A gene-specific upstream prime (GSP) was designed based on the Unigene of the EST of L. chinense sequenced by BGI (Beijing Genomics Institute). The M13 Primer M4 in the TaKaRa RNA PCR Kit (AMV) ver. 3.0 was used as the downstream primer. Amplification was performed with L. chinense cDNA templates and PCR products were purified directly using a MiniBEST DNA Fragment Purification Kit ver.4.0 (TaKaRa). After being cloned into the pMD18-T vector using a pMD™ 18-T Vector Cloning Kit (TaKaRa), the LcMKK gene sequences were confirmed by sequencing three clones one time from three independent PCRs. The primers are shown in Table 1. The nucleotide and predicted amino acid sequences were analyzed using DNAMAN. The phylogenetic tree was constructed by the MEGA 4.1 software.
Expression analysis by quantitative real-time PCR (qRT-PCR)
The transcript level of LcMKK in L. chinense under drought stress and expression patterns of stress-responsive genes in tobaccos before and after drought treatment were analysed by qRT-PCR. RNA isolation was performed as mentioned above. Approximately 2 μg of total RNA was reverse transcribed using the TransScriptII one-step gDNA removal and cDNA synthesis SuperMix with random primers (TransGen Biotech). Each PCR reaction (25 μL) contained 12.5 μL 2× TransStart Top Green qPCR SuperMix (TransGen Biotech), 1 μL diluted cDNA, and 0.5 μL of gene-specific primers (GSP1, gene-specific primer for LcMKK). LcActin or NtUbiquitin was used as internal control to normalize the relative expression levels of the analyzed genes in L. chinense or tobacco. The thermal cycling conditions were 94 °C for 30 s followed by 40 cycles of 94 °C for 5 s, 60 °C for 30 s. Biological replicates were used and each PCR was performed in triplicates. The primers are listed in Table 1.
Construction binary vector and Nicotiana tabacum transformation
The open reading frame (ORF) of LcMKK (GenBank accession number: KF631314) was amplified by PCR using the specific primer (GSP2) including BamHI and SalI restriction sites. The fragment was inserted into the binary vector pCAMBIA2300 under the control of the Cauliflower mosaic virus (CaMV) 35S promoter. Then, the recombinant plasmid pCAMBIA2300-LcMKK was introduced into Agrobacterium tumefaciens (strain C58), and transformation of tobacco was performed using the leaf disc method (Horsch et al. 1985). Putative transgenic seedlings were screened on MS agar medium with 100 mg L−1 of kanamycin. The T1 seedlings with kanamycin-resistant were confirmed by PCR analysis using the specific primer of LcMKK (GSP3). The T3 homozygous transgenic lines were used in the present study. The wild type (WT) tobaccos were employed as controls. The primers are presented in Table 1.
Stress tolerance assays of the wild type (WT) and transgenic plants
Germination experiments
Seeds from WT and transgenic lines were surface sterilized and sown on MS agar medium containing different concentrations of mannitol (0 and 100 mM) for germination under a photoperiod of 16-h light/8-h dark at 22/25 °C. The germination rate was determined after 25 days.
Dehydration shock and drought tolerance of the transgenic plants
Seeds of WT and transgenic plants were surface sterilized and sown on MS agar medium for germination under a photoperiod of 16-h light/8-h dark at 22/25 °C. Some of the in vitro seedlings were subjected to dehydration and the others were transplanted to the pots filled with mixed soils (3:1 vermiculite: worm cast) for drought treatments. Potted plants were grown in greenhouse at 18–25 °C with a 12 h photoperiod and relative humidity of 60–75 %.
For dehydration analysis, the aerial parts of 4-week-old in vitro seedlings of WT and transgenic lines were put on clean filter papers to dry for up to 90 min in an ambient environment. The fresh weight of the seedlings was measured every 10 min to determine the rate of water loss relative to the initial value (fresh weight at 0 min). The leaves were sampled at the completion of dehydration and used to examine the electrolyte leakage and accumulation of H2O2 and O2 − as the methods described below.
For the drought stress evaluation, potted plants were kept on flat-bottom trays to grow for 8 weeks with regular irrigation until the drought treatment. After 7 days of withholding water, the third to fourth leaves from the top of the treated plants were collected for analysis of antioxidant enzyme activity, electrolyte leakage, total chlorophyll content and expression of stress-responsive genes. In order to investigate the survival rate under drought conditions, 4-week-old tobaccos grown in the pots were not watered for 21 days, followed by 3 days of re-watering. Plants were considered to be dead if all the leaves were brown and there was no regrowth 3 days after re-watering. Then the survival rate (number of surviving plants relative to total number of experimented plants) was calculated. Each treatment was repeated three times with three replicates in both the WT and transgenic plants composed of at least 10 independent plants for each.
In situ histochemical localization of H2O2 and O2 −
In situ accumulation of H2O2 and O2 − was examined based on histochemical staining by 3, 3′-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT), respectively. DAB and NBT staining protocols were performed as previously described (Lu et al. 2013).
Measurement of electrolyte leakage (EL) and total chlorophyll content
EL was measured as described in previous studies (Huang et al. 2010). The total chlorophyll content was measured according to Wintermans and De Mots (Wintermans and De Mots 1965).
Measurement of activity of antioxidant enzymes
For extraction of superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6) and peroxidase (POD, EC 1.11.1.7), 0.5 g of leaf samples were extracted on ice in 10 mL of 50 mM phosphate buffer, pH 7.8, containing 1 % polyvinylpyrrolidone. The homogenate was centrifuged at 12,000g for 10 min at 4 °C, and the supernatant was immediately used for the following antioxidant enzymes assays. The antioxidant enzyme activities were detected as previously described (Aebi 1984; Giannopolitis and Ries 1977).
Results
Isolation and sequence characterization of LcMKK
The LcMKK gene was isolated from L. chinense for the first time. The ORF of LcMKK is 1,074 bp, encoding a 357 amino acids polypeptide with a calculated molecule mass of about 39.55 kDa, and with pI of 5.20. Multiple protein sequence alignment against MAPKKs from various plant species was performed using the DNAMAN software. Consistent with other plant MAPKKs, LcMKK contains 11 conserved sub-domains, a catalytic loop (activation-loop), a consensus S/TxxxxxS/T motif for phosphorylation in the activation loop between the VII and VIII domains, and a docking site known to bind MAPKs or phosphatases (Supplementary Fig. 1A).
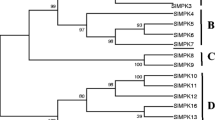
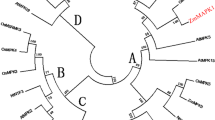
It has been proposed that plant MAPKKs can be classified into four distinct groups (A, B, C and D) from the homology analysis of the predicted proteins (Lu et al. 2013). The phylogenetic analysis showed that LcMKK exhibited a high similarity with members of the group A MAPKKs, such as AtMKK1 and AtMKK2. These results suggested that LcMKK was a member of MAPKK group A (Supplementary Fig. 1B).
Differential expression patterns of LcMKK in different organs and under drought stress
To investigate the expression patterns of LcMKK, we performed qRT-PCR analysis. The tissue-specific expression pattern of LcMKK in L. chinense was first examined. As shown in Fig. 1a, LcMKK mRNA was expressed in the root, stem and leaf, LcMKK transcript levels in the root and leaf were nearly the same, but the transcript level in the stem was higher than in the root and leaf. The results suggested that LcMKK was constitutively expressed in all three tested tissues. Furthermore, changes in LcMKK transcript levels in response to drought stress were investigated. Results from experiments indicated that LcMKK transcript was up-regulated by PEG treatment (Fig. 1b). LcMKK mRNA accumulation increased with time and reached a maximum at 6 h. The transcript level then decreased, but remained higher than that in the control (0 h).
Expression patterns of LcMKK in different tissues and under drought stress. a The tissue-specific expression of LcMKK under normal conditions. Total RNA was extracted from the roots, stems, and leaves of 8-month-old L. chinense plants and was subjected to qRT-PCR analysis. b Analysis of LcMKK transcription in the leaves of L. chinense plants under drought stress. Asterisks (* or **) above the column indicate significant differences in comparison with the control (*P < 0.05; **P < 0.01)
Identification of transgenic Nicotiana tabacum
To investigate the function of the LcMKK gene, we overexpressed its ORF under the control of the CaMV 35S promoter in tobacco. A total of 30 independent transgenic lines (T1) were selected by kanamycin-resistance screening, and then these transgenic lines were confirmed by PCR with primers specific to CaMV 35S and LcMKK (data not shown). Ten T2 LcMKK-overexpressing lines were randomly selected to detect the LcMKK expression levels in leaves by semi-quantitative RT-PCR and quantitative RT-PCR analysis. Six lines showed high expression of LcMKK; no LcMKK expression was detected in WT plants (Supplementary Fig. 2). The three representative LcMKK-overexpressing lines (OE-1, OE-3, OE-7) were selected for further functional analysis.
Overexpression of LcMKK confers tolerance to dehydration in transgenic plants
Because the expression of LcMKK in L. chinense was significantly induced by PEG treatment, we determined whether LcMKK could increase dehydration tolerance in transgenic tobaccos.
When the aerial parts of 4-week-old WT and transgenic tobacco seedlings were subjected to dehydration, fresh water loss was increased in both the WT and transgenic lines with the progressing of dehydration. However, the transgenic seedlings exhibited remarkably less water loss relative to the WT at any time point within 90 min of dehydration. At the end of dehydration, the water loss rate of the transgenic lines was 42.9, 40.7, and 34.9 % respectively, compared to 54.1 % in the WT (Fig. 2a). Additionally, morphological differences were prominent between the WT and transgenic lines after dehydration. The leaves of transgenic seedlings largely remained their turgor, but the WT leaves withered seriously after 40 min of dehydration (Fig. 2b). EL, an important indicator of membrane damage, was significantly higher in the WT (35.4 %) than in OE-1 (18.2 %), OE-3 (15.6 %) or OE-7 (16.5 %), suggesting that the WT suffered from more severe membrane injury (Fig. 2c). These findings indicated that the transgenic lines were more resistant to dehydration stress.
Phenotype and dehydration tolerance of WT and transgenic lines. a Time-course fresh water loss of WT and transgenic lines during a 90-min dehydration. b A representative photograph showing the WT and transgenic lines after 40 min of dehydration. c Electrolyte leakage of WT and transgenic lines after dehydration for 90 min. *, ** values of the transgenic lines were significantly different from those of WT at P < 0.05 and P < 0.01, respectively
Overexpression of LcMKK confers tolerance to drought in transgenic plants
Seeds from WT and transgenic lines were germinated to capacity on MS agar medium containing different concentrations of mannitol to mimic drought stress. There were no significant differences in seed germination between WT and transgenic lines without mannitol treatment. When the concentration of mannitol increased to 100 mM, although the germination of both the WT and transgenic lines was inhibited, the germination rate of OE-1 (57.4 %), OE-3 (65.5 %) and OE-7 (70.1 %) was significantly higher than the 43.6 % found in the WT (Supplementary Fig. 3). The results indicated that overexpression of LcMKK could enhance the drought tolerance of transgenic plants during seed germination period.
Apart from short-term dehydration, long-term drought tolerance of the potted plants (4-week-old, Fig. 3a) was also examined by water withholding. After 21 days without water, leaf wilting of WT was more obvious than that of OE-3 and OE-7, but the difference between WT and OE-1 was not prominent (Fig. 3b). After the resumption of watering, the transgenic plants grew better than the WT (Fig. 3c). Three days after re-watering and growth under normal conditions, the survival rate of transgenic plants was 64.8, 72.2 and 80.5 % respectively, which was significantly higher than that of the WT (32.3 %, Fig. 3e). In another experiment, 8-week-old potted plants were subjected to 7 days drought stress treatments. Similarly, phenotype differences were evident between the WT and transgenic plants; WT lost its turgor whereas transgenic plants grew better (Fig. 3d). EL and total chlorophyll content were measured in the samples collected after 7 days of drought. EL of OE-1 (26.8 %), OE-3 (20.9 %) and OE-7 (23.2 %) was significantly lower in comparison with WT (43.8 %, Fig. 3f). The total chlorophyll contents of transgenic plants (2.46 μg g−1 FW, 2.14 μg g−1 FW and 2.48 μg g−1 FW for OE-1, OE-3 and OE-7, respectively) were 1.3–1.5 times higher than that of the WT (1.55 μg g−1 FW, Fig. 3g). The above results indicated that overexpression of LcMKK confers tolerance to drought in transgenic plants.
Overexpression of LcMKK enhances drought tolerance in transgenic tobaccos. a–c Photograph of representative 4-week-old WT and transgenic plants grown in pots under drought conditions for 21 days, and then watered for 3 days to allow them to recover. a before drought treatment, b drought for 21 days, c re-watering for 3 days. d Photograph of representative 8-week-old WT and transgenic plants grown in pots under drought conditions for 7 days. e The survival rate in WT and transgenic plants after the 3 days re-watering following the drought. Electrolyte leakage f and total chlorophyll content g of the WT and transgenic lines after drought stress for 7 days. Each column represents the mean of three independent experiments, and bars indicate ± SDs. A significant difference from the WT is indicated by asterisks (*P < 0.05; **P < 0.01)
LcMKK mediates the accumulation of H2O2 and O2 − in transgenic plants
A lower EL level in transgenic lines implied that they might be subjected to less serious oxidative stress than the WT. Thus, it was of interest to evaluate the ROS accumulation in the tested lines under water stress. Histochemical staining by DAB or NBT was used to reveal in situ accumulation of H2O2 and O2 −, respectively. Before dehydration, slight DAB and NBT staining were detected in all tested lines. After dehydration, although DAB and NBT staining in all lines deepened relative to the normal conditions, the staining in transgenic lines were slighter than those in WT (Fig. 4a, b). Similarly, the transgenic lines exhibited less intense DAB and NBT staining in comparison with the WT under drought growth conditions with the exception of OE-1, which did not show detectable difference when compared with the WT in DAB staining (Fig. 4c). Taken together, these data indicated that the transgenic lines accumulated lower levels of ROS (H2O2 and O2 −) under water stress.
Histochemical staining by DAB and NBT to reveal accumulation of H2O2 and O2 − in leaves of WT and transgenic lines subjected to 90-min dehydration or 7 days drought stress. a–b Representative photographs showing accumulation of H2O2 (a) and O2 − (b) in leaves before (0 min, upper panel) and after (90 min, lower panel) dehydration. (c) Representative photographs showing accumulation of H2O2 (upper panel) and O2 − (lower panel) in leaves after 7 days drought stress
Overexpression of LcMKK increases the activity of antioxidant enzymes
The above-mentioned results showed that transgenic lines accumulated less ROS compared with the WT when they were simultaneously subjected to water stress. As antioxidant enzymes are known to play important roles in ROS scavenging, the activities of SOD, CAT and POD were measured in the transgenic lines and WT before and 7 days after drought treatments. As shown in Fig. 5, before drought stress, activities of the three antioxidant enzymes in transgenic lines were higher than those in the WT, but the difference was not significant with the exception of SOD, which was significantly higher in OE-3 and OE-7 compared with WT. After drought stress, activities of three antioxidant enzymes were augmented in all of the tested samples, while the transgenic plants had significantly higher activities than WT. SOD activity in OE-3 was 1.6 times of that in WT, and CAT activity in OE-7 was 1.7 times of that in WT. All of these results suggested that activities of the three antioxidant enzymes were higher in the transgenic plants than WT, in reverse proportion to the ROS accumulation of these lines.
Activity of antioxidant enzymes in transgenic lines and WT before and after drought treatment. a–c Activity of SOD (a), CAT (b) and POD (c) of WT and transgenic plants before and after 7 days of drought treatments. Each column represents the average of three replicates, and bars indicate ± SDs. ** and * indicate significant differences between WT and transgenic lines at P < 0.01 and P < 0.05, respectively
Expression analysis of ROS-related or stress-responsive genes in the WT and transgenic plants
To gain further insight into the molecular mechanisms underlying the enhanced drought resistance in transgenic tobacco lines, the transcript levels of five marker genes were examined in all lines before and after 7 days drought stress. These genes encode enzymes which are significant regulatory protein (NtDREB3), stress defensive protein (NtLEA5), or involved in direct ROS detoxification (NtSOD, NtCAT, and NtPOD). Under normal growth conditions, the expression levels of these genes in transgenic lines were significantly higher than those in the WT, except that the expression level of NtSOD in OE-1 was not significantly different from that in the WT. After drought stress, all the genes were induced at much higher levels in the transgenic lines as compared to WT (Fig. 6a–e). The results indicated that overexpression of LcMKK in tobacco enhanced the transcript levels of the ROS-related and stress-responsive genes.
Expression of ROS-related and stress-responsive genes in the WT and transgenic lines under normal and drought conditions. a–e Expression level of NtSOD (a), NtCAT (b), NtPOD (c), NtDREB3 (d) and NtLEA5 (e) of WT and transgenic lines under normal and drought conditions. Total RNA was isolated from the third to fourth leaves of the WT and transgenic lines. Data represent the means of three replicates. Asterisks show that the values are significantly different between transgenic lines and the WT under the same growth conditions, (**P < 0.01; *P < 0.05)
Discussion
Abiotic stress responses require the concerted and coordinated action of a myriad of important signaling members, including the MAPK cascades. As the nodal point of the MAPK cascades, MAPKKs play paramount roles in amplifying, integrating, and channeling information between the extracellular stimuli and intracellular responses (Kong et al. 2011a; Lu et al. 2013). L. chinense has great capability for environmental adaption. All the findings prompted the cloning and characterization of the function of a MAPKK gene from L. chinense, in which the MAPK cascade has never been explored.
LcMKK shares a high degree of sequence similarity with other plant MAPKKs. It contains 11 conserved sub-domains that may assume biological functions related to substrate specificity or protein interactions (Agarwal et al. 2010). Phylogenetic analysis showed that the MAPKKs were classified into four distinct groups, in line with earlier reports (Lu et al. 2013). LcMKK belongs to group A, which consists of several well-characterized MAPKK genes, including AtMKK2, NtSIMKK, and GhMKK1. It has been documented that MAPKK members in groups A are predominantly implicated in abiotic stress responses (Kiegerl et al. 2000). Clustering of these genes in the same group suggested that they may have a similar or even the same biological functions in abiotic stress responses.
The strong induction of the LcMKK transcript level by PEG led to the elucidation of its function in water stress tolerance by gene overexpression approach in tobaccos. The stress tolerance assay revealed that the transgenic lines enhanced tolerance to either dehydration or drought as compared with the WT. Our results agreed with earlier reports, in which overexpression of MAPKK genes showed improved tolerance to multiple stresses in transgenic lines (Cai et al. 2014), implying that LcMKK hold great potential for enhancement of stress tolerance via genetic modification.
Although transformation of LcMKK led to improvement of water stress tolerance, the physiological mechanism underling the tolerance needs to be further examined. This promoted us to carry out more work to explore physiological differences between the transgenic plants and WT under water stress. It is well known that ROS accumulation is related to physiological perturbation and ROS level can reflect the degree of damage to cellular components (Foyer and Shigeoka 2011). Histochemical staining by NBT or DAB indicated that transgenic tobaccos may accumulate less H2O2 and O2 − than WT under dehydration or drought stress conditions. As ROS level depends greatly on the homeostasis between the generation and concurrent scavenging under stress conditions (Pitzschke et al. 2009), accumulation of less ROS in transgenic tobaccos implied that scavenging systems in transgenic plants might work more effectively in comparison with WT. Plant possesses a complex antioxidant system for ROS detoxification, in which several enzymes play important roles in scavenging ROS and protecting the cells against oxidative stress (Miller et al. 2010). Of these enzymes, SOD provides the first line of defense against ROS by catalyzing the dismutation of O2 − to H2O2 and O2, which were then scavenged by coordinated action of CAT and POD (Samuel and Ellis 2002). In this study, although the difference was not significant with the exception of SOD, activities of the three enzymes in transgenic lines were higher than WT under normal conditions, suggesting that overexpression of the LcMKK gene facilitated the activation of the antioxidant system even in the absence of stresses. Under water stress conditions, the antioxidant system was activated in both the WT and transgenic plants, which may be due to a dramatically elevated rate of ROS production. Activities of the three antioxidant enzymes in the transgenic lines were significantly higher than those in WT, indicating that the transgenic lines had more robust ROS scavenging capacity during stresses, which agreed with the remarkable reduction of ROS levels and ROS-associated membrane damage, i.e. lower electrolyte leakage. This provide convincing evidence to show that the LcMKK functions in dehydration and drought tolerance by, at least partially, the activation of the antioxidant system. These results were consistent with earlier reports working on MAPKK genes in other plants (Kong et al. 2011b; Lu et al. 2013). Taken together, Overexpression of LcMKK confers dehydration and drought tolerance in transgenic tobaccos, which may be a result of enhanced ROS-scavenging enzyme activity under stress conditions.
Earlier studies have showed that the MAPK cascades regulate the expression of a set of ROS-related genes in maize and tobacco (Samuel and Ellis 2002; Zhang et al. 2006). To gain a deeper understanding of the function of LcMKK in stress tolerance, transcript levels of the ROS-related and stress-responsive genes were analyzed. The transcript levels of the genes encoding ROS-scavenging enzymes were significantly higher in the transgenic plants than WT under normal or drought conditions, except that the difference in NtSOD expression between OE-1 and WT was not significantly under normal conditions. This may explain the activation of the antioxidant enzymes and the lower ROS levels in the transgenic plants.
Previous studies have showed that dehydration-responsive element-binding proteins (DREBs) play an important role in regulating stress responses. Overexpression of DREB genes could confer drought tolerance in various plants (Chen et al. 2008; Ravikumar et al. 2014). In this study, the transcript level of NtDREB3, a DREB family member, was higher in the transgenic plants than in the WT, suggesting that overexpression of LcMKK has a stimulatory impact on the expression level of the DREB gene.
NtLEA5 encodes a group 5 late embryogenesis abundant (LEA) protein, Circumstantial evidence has showed that LEA proteins function in dehydration tolerance by binding water, stabilizing labile enzymes, and protecting cellular and macromolecular structures (Hundertmark and Hincha 2008). In this study, qRT-PCR analysis showed that mRNA level of NtLEA5 in LcMKK-overexpressing lines was significantly higher than that in WT after drought stress, indicating that this gene was more intensely induced by drought in the transgenic lines. Greater induction of NtLEA5 demonstrated that more LEA proteins might be synthesized in the transgenic plants, which was partially supported by the greater water retention and less extensive membrane damage.
In summary, a novel group A MAPKK gene, LcMKK, was successfully isolated from L. chinense, whose mRNA level was dramatically induced by drought. Overexpression of LcMKK enhanced tolerance to both dehydration and drought in transgenic tobaccos. The enhanced stress tolerance may be ascribed to activation of antioxidant enzymes and the genes encoding these enzymes, leading to efficient scavenging of excess ROS. Meanwhile, several stress-responsive genes were found to be up-regulated by LcMKK overexpression. The results presented in this study indicate that LcMKK may be a candidate gene with potential application to enhance abiotic stress tolerance in plants.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Agarwal PK, Gupta K, Jha B (2010) Molecular characterization of the Salicornia brachiata SbMAPKK gene and its expression by abiotic stress. Mol Biol Rep 37:981–986
Andreasson E, Ellis B (2010) Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci 15:106–113
Cai G, Wang G, Wang L, Pan J, Liu Y, Li D (2014) ZmMKK1, a novel group A mitogen-activated protein kinase kinase gene in maize, conferred chilling stress tolerance and was involved in pathogen defense in transgenic tobacco. Plant Sci 214:57–73
Chen J-Q, Meng X-P, Zhang Y, Xia M, Wang X-P (2008) Over-expression of OsDREB genes lead to enhanced drought tolerance in rice. Biotechnol Lett 30:2191–2198
Chen L et al (2012) Genome-wide identification and analysis of MAPK and MAPKK gene families in Brachypodium distachyon. PLoS One 7:e46744
Dong JZ et al (2013) Selenium increases chlorogenic acid, chlorophyll and carotenoids of Lycium chinense leaves. J Sci Food Agric 93:310–315
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155:93–100
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59:309–314
Hadiarto T et al (2006) Activation of Arabidopsis MAPK kinase kinase (AtMEKK1) and induction of AtMEKK1–AtMEK1 pathway by wounding. Planta 223:708–713
Horsch R, Fry J, Hoffmann N, Eichholtz D, Rogers SA, Fraley R (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Huang X-S, Liu J-H, Chen X-J (2010) Overexpression of PtrABF gene, a bZIP transcription factor isolated from Poncirus trifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. BMC Plant Biol 10:230
Hundertmark M, Hincha DK (2008) LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics 9:118
Kiegerl S et al (2000) SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress–induced MAPK, SIMK. Plant Cell 12:2247–2258
Kim HP et al (2002) Zeaxanthin dipalmitate from Lycium chinense fruit reduces experimentally induced hepatic fibrosis in rats. Biol Pharm Bull 25:390–392
Kong X et al (2011a) ZmMKK4, a novel group C mitogen-activated protein kinase kinase in maize (Zea mays), confers salt and cold tolerance in transgenic Arabidopsis. Plant Cell Environ 34:1291–1303
Kong X, Sun L, Zhou Y, Zhang M, Liu Y, Pan J, Li D (2011b) ZmMKK4 regulates osmotic stress through reactive oxygen species scavenging in transgenic tobacco. Plant Cell Rep 30:2097–2104
Kong F, Wang J, Cheng L, Liu S, Wu J, Peng Z, Lu G (2012) Genome-wide analysis of the mitogen-activated protein kinase gene family in Solanum lycopersicum. Gene 499:108–120
Lu W, Chu X, Li Y, Wang C, Guo X (2013) Cotton GhMKK1 Induces the tolerance of salt and drought stress, and mediates defence responses to pathogen infection in transgenic Nicotiana benthamiana. PLoS One 8:e68503
Mikołajczyk M, Awotunde OS, Muszyńska G, Klessig DF, Dobrowolska G (2000) Osmotic stress induces rapid activation of a salicylic acid–induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell 12:165–178
Miller G, Suzuki N, CIFTCI-YILMAZ S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Mizoguchi T, Ichimura K, Irie K, Morris P, Giraudat J, Matsumoto K, Shinozaki K (1998) Identification of a possible MAP kinase cascade in Arabidopsis thaliana based on pairwise yeast two-hybrid analysis and functional complementation tests of yeast mutants. FEBS Lett 437:56–60
Nadarajah K, Sidek HM (2010) The green MAPKS. Asian J Plant Sci 9:1
Nicole M-C, Hamel L-P, Morency M-J, Beaudoin N, Ellis B, Séguin A (2006) MAP-ping genomic organization and organ-specific expression profiles of poplar MAP kinases and MAP kinase kinases. BMC Genomics 7:223
Pitzschke A, Djamei A, Bitton F, Hirt H (2009) A major role of the MEKK1–MKK1/2–MPK4 pathway in ROS signalling. Mol Plant 2:120–137
Rao KP, Richa T, Kumar K, Raghuram B, Sinha AK (2010) In silico analysis reveals 75 members of mitogen-activated protein kinase kinase kinase gene family in rice. DNA Res 17:139–153
Ravikumar G et al (2014) Stress-inducible expression of AtDREB1A transcription factor greatly improves drought stress tolerance in transgenic indica rice. Transgenic Res 23:421–439
Samuel MA, Ellis BE (2002) Double jeopardy both overexpression and suppression of a redox-activated plant mitogen-activated protein kinase render tobacco plants ozone sensitive. Plant Cell 14:2059–2069
Stulemeijer IJ, Stratmann JW, Joosten MH (2007) Tomato mitogen-activated protein kinases LeMPK1, LeMPK2, and LeMPK3 are activated during the Cf-4/Avr4-induced hypersensitive response and have distinct phosphorylation specificities. Plant Physiol 144:1481–1494
Tena G, Asai T, Chiu W-L, Sheen J (2001) Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol 4:392–400
Umezawa T, Fujita M, Fujita Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Curr Opin Biotechnol 17:113–122
Wintermans J, De Mots A (1965) Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol. BBA 109:448–453
Xing Y, Jia W, Zhang J (2008) AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J 54:440–451
Zhang A, Jiang M, Zhang J, Tan M, Hu X (2006) Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol 141:475–487
Acknowledgments
This work was supported financially by the National Science and Technology Key Project of China on GMO cultivation for new varieties (No. 2014ZX08003-002B) and the National Natural Science Foundation of China (No. 31271419, No. 31271793 and No. 31401391).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, D., Ji, J., Wang, G. et al. LcMKK, a novel group A mitogen-activated protein kinase kinase gene in Lycium chinense, confers dehydration and drought tolerance in transgenic tobacco via scavenging ROS and modulating expression of stress-responsive genes. Plant Growth Regul 76, 269–279 (2015). https://doi.org/10.1007/s10725-014-9998-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-014-9998-5