Abstract
An effective plant regeneration system via somatic embryogenesis and synthetic seeds was developed for Mondia whitei, an endangered medicinal plant. Friable embryogenic callus was induced by culturing leaf explants on solid Murashige and Skoog (MS) medium containing various concentrations and combinations of sucrose and plant growth regulators. The highest frequency of somatic embryogenesis (100 %) and production of all developmental stages of somatic embryos were obtained on MS medium with 40 g l−1 sucrose, 8 g l−1 agar, 20 μM 2,4-dichlorophenoxyacetic acid (2,4-D) and 1 μM thidiazuron. This was followed by establishment in MS medium with 20 g l−1 sucrose, 8 g l−1 agar, 0.5 μM meta-topolin riboside (mTR) and 0.25 μM indole-3-acetic acid (IAA). All the embryos germinated and produced healthy plantlets on the same medium. Somatic embryos at the heart, torpedo and cotyledonary-stages were collected from media (EDM) containing MS medium plus 20 g l−1 sucrose, 8 g l−1 agar, 0.5 μM mTR and 0.25 μM IAA. The embryos were encapsulated with liquid MS medium plus different concentrations of sodium alginate (SA) and calcium chloride (CaCl2·2H2O) with a 10 min exposure. A combination of 3 % SA and 100 mM CaCl2·2H2O provided higher survival (95.7 %) and germination (73 %) frequencies of synthetic seeds. Germination frequency of synthetic seeds was 51.6 % after 50 days of storage at 4 °C. Somatic embryos and synthetic seed-developed plantlets were successfully acclimatized in the greenhouse with 90 % survival ex vitro. Application of the protocol provides a relatively simple and rapid system for conservation of natural populations for germplasm conservation. Analysis of bioactive compounds and genetic transformation studies can also be performed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mondia whitei (Hook.f.) Skeels (Apocynaceae) is a woody climber with a tuberous root stock. It is a truly versatile South African medicinal plant and is reported as endangered in the Red Data List of South African plants (SANBI 2013). It is sold in Traditional Medicinal markets in South Africa (McCartan and Crouch 1998; SANBI 2013). It produces various bioactive compounds such as epinephrine, norepinephrine, dopamine, serotonin, γ-aminobutyric acid and coumarinolignans (Bunel et al. 2014). The roots are used as spices, aphrodisiacs and in the treatment of urinary tract infections, jaundice, headache, easing of flatulence, abdominal pains, constipation, bilharzia, diarrhoea and gonorrhoea (Gerstner 1941; Kokwaro 1976; Gelfand et al. 1985; Noumi et al. 1998) and a plethora of other ailments including hypertension, stroke, anemia, asthma, hangover, mastitis and allergies, to improve sleep, ease birth pains, mouth freshener, antacid, indigestion, and to stimulate appetite (Oketch-Rabah 2012). It also contains androgenic properties (Watcho et al. 2004). In addition, the plant is used to treat fits in children and for stress and tension in adults. The leaves serve as animal fodder, human vegetables and food supplement (Oketch-Rabah 2012). Accordingly, wild populations of M. whitei are over-exploited and are becoming threatened with extinction. In this context, mass propagation of M. whitei through in vitro regeneration is essential for conservation and commercial production. Although a micropropagation protocol has been developed for M. whitei (McCartan and Crouch 1998), high frequency rapid in vitro plant regeneration for mass propagation and conservation remains a major challenge. An efficient in vitro plant regeneration through somatic embryogenesis and production of synthetic seeds could play an important role in M. whitei including fulfilling the rapid mass propagation, conservation and traditional medicine demands.
Plant somatic cells are able to produce somatic embryos (bipolar structures) through somatic embryogenesis (Bhojwani and Razdan 1996). The somatic embryo develops into characteristic morphological stages (globular, heart, torpedo and cotyledonary) leading to plantlet formation. Various novel and innovative ideas like optimization of plant growth regulators and adjustment of osmotic conditions have been used to improve somatic embryogenesis frequency in plants (Karami et al. 2006; Deo et al. 2010; Baskaran and Van Staden 2014). Somatic embryogenesis holds great promise for en masse propagation, bioactive compound production, the improvement of the species by genetic engineering, germplasm cryopreservation and production of artificial or synthetic seeds (Fowler 1983; Mathews et al. 1992; Wilde et al. 1992; Blakesley et al. 1995; Manjkhola et al. 2005). Artificial or synthetic seeds are considered as analogous to natural seeds. Synthetic seeds consist of somatic embryos surrounded by an artificial layer creating a capsule (Murashige 1977). The first successful report of synthetic seed production was reported for carrot (Kitto and Janick 1982). The technology has been extended to a wide variety of agricultural, ornamental and medicinal plants (Redenbaugh 1993; Ghosh and Sen 1994; Janeiro et al. 1997; Pattnaik and Chand 2000; Utomo et al. 2008; Cheruvathur et al. 2013). Synthetic seed technology provides an opportunity for easy handling, storage, shipping, ex situ conservation of the germplasm of elite and endangered plant species, exchange of axenic plant materials between laboratories and pharmaceutical industries (Rai et al. 2009; Cheruvathur et al. 2013) and propagation of rare hybrids, elite genotypes, and genetically engineered plants for which the seeds are either very expensive or not available. In addition, the technology is an alternative system for in situ conservation and is also useful to avoid environmental disasters. Therefore, the aim of the current study was to develop a simple and effective, rapid in vitro plant regeneration system through somatic embryogenesis, synthetic seed production and synthetic seed storage for the conservation and large-scale commercial production of M. whitei from leaf explants.
Materials and methods
Plant material and somatic embryogenesis
Expanding young leaves of Mondia whitei were collected from the Botanical Garden, University of KwaZulu-Natal, Pietermaritzburg, South Africa. Leaves were washed under running tap water for 15 min to remove loose dirt, washed with Tween® 20 for 1 min and then decontaminated with 0.2 % aqueous HgCl2 for 7 min. Leaves were then rinsed five times with sterile distilled water. Leaf explants (approximately, 10 × 5.0 mm) of M. whitei were excised and cultured on MS (Murashige and Skoog 1962) solid (8 g l−1 agar) medium with 30–50 g l−1 sucrose and different concentrations of plant growth regulators [PGRs: 2,4-dichlorophenoxyacetic acid (2,4-D), picloram, benzyladenine (BA), meta-topolin riboside (mTR), kinetin (Kin) and thidiazuron (TDZ)] alone or in combination for induction of somatic embryos (SEs) for 8 weeks. This was followed by establishment of individual treatments in MS medium with 20 g l−1 sucrose and 8 g l−1 agar for SE maturation and plantlet formation. The PGR treatments are indicated in Tables 1 and 2. Friable embryogenic callus (FEC) with somatic embryoids produced from an optimized somatic embryogenesis medium (MS medium plus 40 g l−1 sucrose, 20 µM 2,4-D and 1 µM TDZ) were transferred to MS medium containing 20 g l−1 sucrose, 8 g l−1 agar and different concentrations and combinations of PGRs [BA, mTR, TDZ, Kin, indole-3-acetic acid (IAA) and naphthaleneacetic acid (NAA)] to enhance somatic embryogenesis and plantlet development. The PGR treatments are as indicated in Table 3. The SEs (globular, heart, torpedo and cotyledonary) and degree of plantlet development were recorded after 12 weeks from culture initiation. All embryo stages were photographed under a Leica M Stereo Microscope (JVC-Digital Camera: KY-F 1030U type; 0.5×, Wayne, NJ, USA). In all experiments, the medium lacking PGRs served as control. The chemicals used were of analytical grade (Biolab, South Africa; Oxoid, England and Sigma, USA). All media were adjusted to pH 5.8 with 0.1 N NaOH before gelling with 8 g l−1 agar and autoclaved at 121 °C for 20 min. The cultures were maintained at a temperature of 25 ± 2 °C and light irradiance of 40 µmol m−2 s−1 provided by cool white fluorescent tubes (OSRAM L 58 W/740, South Africa) under a 16 h photoperiod. All the plantlets (approximately, 70–90 mm) from somatic embryos were transferred to a plastic box containing a 1:1 (v/v) vermiculite and soil mixture and placed in the greenhouse (25 ± 2 °C under natural photoperiod conditions and a midday PPF of 950 ± 50 µmol m−2 s−1) for acclimatization ex vitro and established in the greenhouse.
Synthetic seed production, storage and germination
Somatic embryos (heart, torpedo and cotyledonary) were collected equally from an optimized embryo development medium (EDM: MS medium plus 40 g l−1 sucrose, 8 g l−1 agar, 20 µM 2,4-D and 1 µM TDZ followed by establishment in MS medium plus 20 g l−1 sucrose, 8 g l−1 agar, 0.5 µM mTR and 0.25 IAA) and were suspended in a capsule matrix of liquid MS medium containing sodium alginate (SA: 1–4 % w/v) dropped into a complexing agent solution of calcium chloride (CaCl2·2H2O: 75–125 mM). Each drop-containing embryo was maintained and shaken gently by hand in CaCl2·2H2O for 10 min for proper bead formation. The beads were washed in sterile distilled water 3 times to remove traces of CaCl2 and then placed on sterilized filter paper to remove excess water. After optimization of the capsule matrix (3 % SA) and complexing agent (100 mM CaCl2), experiments were conducted further for synthetic seeds production from SEs (heart, torpedo and cotyledonary stages) derived from different EDM to study their ability to germinate. The EDM is indicated in Table 4. The synthetic seeds stored at 4 °C were evaluated for viability over 10–50 days through survival and germination tests for germplasm conservation. Non-stored synthetic seeds were used as a control. Synthetic seeds were incubated in MS medium containing 30 g l−1 sucrose and 8 g l−1 agar at 25 ± 2 °C with a 16-h photoperiod for germination. The survival and germination of synthetic seeds were recorded after 6 weeks of culture. The experiments were conducted in aseptic conditions. The plantlets (approximately, 70–90 mm) were successfully acclimatized in 1:1 (v/v) vermiculite:soil mixture and established in the greenhouse.
Statistical analysis
All experiments were repeated at least three times with 25 replicates for somatic embryogenesis and 50 replicates for synthetic seed germination per treatment. Data were statistically analyzed using analysis of variance (ANOVA), and are presented as mean ± standard error of three independent experiments. Treatment means were separated using Duncan’s multiple range test at the 5 % probability level and analyzed using SPSS Windows version 21 (SPSS Inc., Chicago, IL, USA).
Results and discussion
Development of somatic embryos and plantlets
Somatic embryogenesis and plantlet development was achieved from leaf explants on MS medium containing different concentrations of sucrose with various PGRs alone or in combination. The leaf explants enlarged and initiated profuse friable embryogenic calli (FEC) at wounding sites during 2 weeks of culture. Later calli (whitish or whitish green) covered the explants on different media treatments (Fig. 1a). The FEC were differentiated into white globular embryoids in all treatments except the control after 6 weeks (Fig. 1b). Similarly, embryoids were achieved from callus in Rhinacanthus nasutus (Cheruvathur et al. 2013). In this study, FEC promoted different stages of somatic embryos (SEs: globular, heart and torpedo) in all treatments after 8 weeks of culture (Fig. 1c). Increasing sucrose concentrations enhanced induction of FEC as well as SEs. This indicates that sucrose at higher concentrations may create both nutritional and excess osmotic stress that helps to improve somatic embryogenesis. Therefore, it is suggested that, nutrient and osmotic effects of sucrose may cause normal development of somatic embryos. The positive effect of high osmolarity may mimic the osmolarity alterations that occur surrounding the embryo in nature (Merkle et al. 1995). Different developmental stages (globular, heart, torpedo and cotyledonary) of SEs and plantlet formation from all treatments improved when transferred to MS medium with 20 g l−1 sucrose and 8 g l−1 agar. The frequency of embryogenesis and mean number of SEs and plantlets varied with treatment after 12 weeks from culture initiation (Table 1). The frequency (98 %) and number of heart-stage embryos (13.6) increased significantly on MS medium containing 40 g l−1 sucrose and 20 μM picloram, while the globular, torpedo and cotyledonary-stage embryos and plantlet number were increased on media containing 50 g l−1 sucrose and 20 μM 2,4-D or picloram (Table 1; Fig. 1d). However, no significant difference were observed between the two treatments (20 μM 2,4-D or picloram) in formation of torpedo-stage embryos (Table 1). Therefore, higher concentrations of 2,4-D or picloram and sucrose were essential in enhancement of M. whitei somatic embryogenesis.
Somatic embryogenesis from leaf explants of M. whitei: a Induction of whitish green FEC on MS medium containing 40 g l−1 sucrose, 20 μM 2,4-D and 1 μM TDZ. b Formation of embryoids in MS medium containing 40 g l−1 sucrose, 20 μM 2,4-D and 1 μM TDZ. c Induction of globular, heart, torpedo and cotyledonary stage embryos on MS medium containing 40 g l−1 sucrose, 20 μM 2,4-D and 1 μM TDZ. d Photograph of developmental stages of SEs and plantlets (×5). In c and d, arrows indicate the heart and torpedo stage embryos
Combination of 2,4-D or picloram and Kin or TDZ or mTR or BA was more effective in production of SEs and plantlet development (Table 2). The combined favorable influence of auxin and cytokinin observed in the present system were in accordance with the culture response of other Apocynaceae species (Sudha and Seeni 2006; Siddiqui et al. 2011; Yuan et al. 2011; Dipti et al. 2014). Whitish and whitish-green SEs and plantlets were produced varying in number with each treatment (Table 2; Fig. 2a). The SEs and plantlet development were increased by 40 g l−1 sucrose, 20 µM 2,4-D or picloram and 1 µM TDZ or mTR or BA (Table 2; Fig. 2b). However, among the different combinations, the medium containing 40 g l−1 sucrose, 20 µM 2,4-D and 1 µM TDZ markedly increased the number of globular and torpedo-stage embryos and plantlet numbers (Table 2; Fig. 2c). Induction of heart and cotyledonary stage embryos was enhanced in 40 g l−1 sucrose, 20 µM picloram and 1 μM BA or mTR (Table 2). These results reveal that sucrose at higher concentration and in combination with 2,4-D and TDZ or picloram and BA or mTR were important for significant SEs and plantlet production in M. whitei.
In vitro plant regeneration by somatic embryogenesis from leaf explants of M. whitei. a Proliferation of whitish green somatic embryos and plantlets on MS medium containing 40 g l−1 sucrose, 20 μM 2,4-D and 1 μM TDZ. b Development of somatic embryos to plantlet formation (×5). c Proliferation of plantlets from somatic embryos on MS medium containing 40 g l−1 sucrose, 20 μM 2,4-D and 1 μM TDZ
Enhancement of somatic embryos and plantlets
The prolific SEs and plantlets from optimized somatic embryogenesis medium were significantly multiplied on MS media plus 20 g l−1 sucrose and 8 g l−1 agar containing various treatments of PGRs singly and/or in combination (Table 3). Somatic embryos were induced rapidly in all the treatments after one week of culture compared to control. Different stages of SEs were observed simultaneously on the same medium treatments indicating that somatic embryo production in M. whitei is an asynchronous phenomenon. Similar findings have been reported for other plant species (Karami et al. 2006; Baskaran and Van Staden 2014). Clearly, globular-shaped embryos were further developed into heart, torpedo and cotyledonary-stage embryos and plantlet development were observed after 10 weeks of culture (Fig. 3a). However, SEs improved on medium containing 0.5 µM BA or mTR (Table 3). Similarly, addition of cytokinin in the medium has been reported to improve somatic embryogenesis in other plant species (Karami et al. 2006; Siddiqui et al. 2011; Dipti et al. 2014). In this study, addition of IAA or NAA with cytokinin was even more effective in inducing SEs and plantlet formation (Table 3; Fig. 3b). Medium containing 0.5 μM BA and 0.25 μM IAA produced the highest number of globular-stage embryos (27.2), while the heart (20.2), torpedo (18.6) and cotyledonary-shaped embryos (16.0) and plantlets (20.4) were higher from medium containing 0.5 μM mTR and 0.25 μM IAA (Table 2). In addition, combination of 0.5 μM mTR and 0.25 μM IAA induced longer plantlet (approximately, 2–3 cm shoot and 5–6 cm radicle) compared to other treatments (Fig. 3c). Accordingly, meta-topolin riboside (mTR) in combination with tested auxins was effective in inducing somatic embryogenesis in M. whitei. The aromatic cytokinin, meta-Topolin is well documented for in vitro cultures in many plant species (Aremu et al. 2012; Baskaran et al. 2012). The present study indicated that SE production in M. whitei is dependent on the type and combination of PGRs. Similar phenomena have also been reported for other plant species (Sudha and Seeni 2006; Baskaran and Van Staden 2014; Dipti et al. 2014). Well-developed plantlets (approximately, 70–90 mm) (Fig. 3d) were separated and then transferred to a plastic box containing a 1:1 (v/v) vermiculite:soil mixture. The plantlets were successfully acclimatized and established in the greenhouse with 90 % survival rate (Fig. 3e, f).
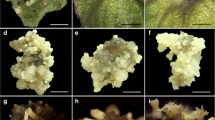
In vitro plant regeneration from somatic embryos (SEs) and synthetic seeds from leaf explants of M. whitei. a Conversion of somatic embryos to plantlet formation on MS medium containing 20 g l−1 sucrose and 0.5 μM mTR. b Proliferation of plantlets from SEs on MS medium containing 20 g l−1 sucrose, 0.5 μM mTR and 0.25 μM IAA. c Development of longer plantlets on MS medium containing 20 g l−1 sucrose, 0.5 μM mTR and 0.25 μM IAA. d Well developed plants from somatic embryo. e Acclimatized plants of M. whitei in the greenhouse after 3 months. f Ex vitro plants of M. whitei after 6 months. g Production of synthetic seeds from 3 % SA and 100 mM CaCl2·2H2O. h Germination of synthetic seed after 1 week. i Development of plantlets from synthetic seeds. j Acclimatized plants from synthetic seeds after 4 months
Synthetic seed production, storage and germination
Somatic embryos (SEs: heart, torpedo and cotyledonary) from different embryo development media (EDM) were used for synthetic seed production and viability testing in various concentrations of sodium alginate (SA: 1, 2, 3 and 4 %) and calcium chloride (CaCl2·2H2O: 75, 100 and 125 mM for 10 min exposure) (Table 4). Ideal concentrations and combinations of SA (3 %) and CaCl2·2H2O (100 mM) were optimized (Fig. 3g) from EDM (0.5 μM mTR and 0.25 μM IAA)-derived embryos. The standardized concentrations of SA and CaCl2·2H2O were essential to obtain the most uniform beads with a hard enough coat for survival as well as viability of synthetic seeds. Germination of synthetic seeds was initiated after 1 week culture (Fig. 3h). The survival and germination frequencies varied with concentration combination of SA and CaCl2·2H2O after 6 weeks (Table 4). Among the different concentrations of SA and CaCl2·2H2O used, 3 % SA and 100 mM CaCl2·2H2O proved to be the best for production of most uniform beads, and supported significantly higher survival (95.7) and germination (73 %) frequencies (Table 4; Fig. 3i). Increasing concentrations of SA (>3 %) and CaCl2·2H2O (>100 mM) with 10 min exposure resulted in hard beads with lower survival and germination frequencies (Table 4). These results are in agreement with other reports and suggest the direct relation between higher frequency of germination of synthetic seed with lower concentration and exposure time to CaCl2·2H2O (Malabadi and Van Staden 2005; Cheruvathur et al. 2013). High concentration or excessive exposure time of CaCl2·2H2O leads to more absorption and penetration to the embryos which generates inhibition of germination, growth and development in the field (Redenbaugh et al. 1986; Malabadi and Van Staden 2005). In this study, germination of synthetic seed from heart, torpedo and cotyledonary stage embryos was rapid, with survival and germination frequencies significantly higher in embryos derived from EDM containing 0.5 μM mTR and 0.25 μM IAA (Table 4). Therefore, the medium for production of embryos is essential for healthy and viable synthetic seeds.
The synthetic seeds were stored at 4 °C for different time periods to test viability. Thereafter, synthetic seeds were transferred to MS medium containing 30 g l−1 sucrose and 8 g l−1 agar for testing competence of germination. The present study provided promising results for survival and germination frequencies of synthetic seeds; however, it was significantly lower than control (non-stored synthetic seed) (Table 4). Similar results were also reported for other plant species (Ipekci and Gozukirmizi 2003; Krishna Kumar and Thomas 2012). Poor viability of stored synthetic seeds may be related to both oxygen deficiency in the gel bead and rapid drying (Redenbaugh et al. 1991). All the plantlets (approximately, 70–90 mm) produced from synthetic seeds were successfully acclimatized in 1:1 (v/v) vermiculite:soil mixture and established in the greenhouse with a survival rate of 90 % (Fig. 3j).
In conclusion, this is the first report on somatic embryogenesis and synthetic seed production in M. whitei using leaf explant-derived FEC. Promising plant regeneration from somatic embryogenesis was influenced markedly by sucrose at higher concentrations and combinations of PGRs. The synthetic seeds of M. whitei could be stored at low temperature (4 °C) for 50 days. This facilitates transportation and exchange of germplasm across international borders. The system can be used for conservation, production of bioactive compounds and genetic transformation.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- BA:
-
6-Benzyladenine
- EDM:
-
Embryo development medium
- FEC:
-
Friable embryogenic callus
- IAA:
-
Indole-3-acetic acid
- Kin:
-
Kinetin
- MS:
-
Murashige and Skoog medium
- mTR:
-
6-(-3-Hydroxybenzylamino)-9-β-D-ribofuranosylpurine
- NAA:
-
α-Naphthaleneacetic acid
- PGRs:
-
Plant growth regulators
- PPF:
-
Photosynthetic photon flux
- SA:
-
Sodium alginate
- SEs:
-
Somatic embryos
- TDZ:
-
Thidiazuron
References
Aremu AO, Bairu MW, Doležal K, Finnie JF, Van Staden J (2012) Topolins: a panacea to plant tissue culture challenges? Plant Cell Tissue Organ Cult 108:1–16
Baskaran P, Van Staden J (2014) Plant regeneration via somatic embryogenesis in Drimia robusta. Plant Cell Tissue Organ Cult 119:281–288
Baskaran P, Ncube B, Van Staden J (2012) In vitro propagation and secondary product production by Merwilla plumbea (Lindl.) Speta. Plant Growth Regul 67:235–245
Bhojwani SS, Razdan MK (1996) Plant tissue culture: theory and practice. Elsevier, Amsterdam, pp 1–766
Blakesley D, Al-Mazrooci S, Graham GH (1995) Cryopreservation of embryogenic tissue of sweet potato (Ipomea batatus): use of sucrose and dehydration of cryoprotection. Plant Cell Rep 15:259–263
Bunel V, Hamel M, Duez P, Stevigny C (2014) Artifactual generation of an alkaloid in the course of Mondia whitei (Hook.f.) Skeels roots extraction: a clue to endogenous-formed bioactive compounds? Phytochem Lett 10:101–106
Cheruvathur MK, Kumar GK, Thomas TD (2013) Somatic embryogenesis and synthetic seed production in Rhinacanthus nasutus (L.) Kurz. Plant Cell Tissue Organ Cult 113:63–71
Deo PC, Taylor M, Harding RM, Tyagi AP, Becker DK (2010) Initiation of embryogenic cell suspensions of taro (Colocasia esculenta var. esculenta) and plant regeneration. Plant Cell Tissue Organ Cult 100:283–291
Dipti L, Fatima S, Mujib A (2014) Morphological anomalies in somatic embryo structure in Catharanthus roseus: improving embryo germination by amending plant growth regulators, activated charcoal and sucrose level. Br Biotechnol J 4:10–20
Fowler MW (1983) Commercial application and economic aspects of mass plant cell culture. In: Smith H, Mantell SH (eds) Plant Biotechnology. Cambridge University Press, Cambridge, pp 3–38
Gelfand M, Mavi S, Drummond RB, Ndemera B (1985) The traditional medical practitioner in Zimbabwe. Mambo Press, Gweru, p 411
Gerstner J (1941) A preliminary check list of Zulu names of plants with short notes. Bantu Stud 13:277–301
Ghosh B, Sen S (1994) Plant regeneration from alginate encapsulated somatic embryos of Asparagus cooperi Barker. Plant Cell Rep 13:381–385
Ipekci Z, Gozukirmizi N (2003) Direct somatic embryogenesis and synthetic seed production from Paulownia elongate. Plant Cell Rep 22:16–24
Janeiro LV, Ballester A, Vieitez AM (1997) In vitro response of encapsulated somatic embryos of camellia. Plant Cell Tissue Organ Cult 51:119–126
Karami O, Deljou A, Ashari ME, Ahmadi PO (2006) Effect of sucrose concentrations on somatic embryogenesis in carnation (Dianthus caryophyllus L.). Sci Hortic 110:340–344
Kitto SL, Janick J (1982) Polyox as an artificial seed coat for a sexual embryos. Hortic Sci 17:448
Kokwaro JO (1976) Medicinal plants of East Africa. African Literature Bureau, Nairobi, p 384
Krishna Kumar G, Thomas TD (2012) High frequency somatic embryogenesis and synthetic seed production in Clitoria ternatea Linn. Plant Cell Tissue Organ Cult 110:141–151
Malabadi R, Van Staden J (2005) Storability and germination of sodium alginate encapsulated somatic embryos derived from the vegetative shoot apices of mature Pinus patula trees. Plant Cell Tissue Organ Cult 82:259–265
Manjkhola S, Dhar U, Joshi M (2005) Organogenesis, embryogenesis, and synthetic seed production in Arnebia euchroma—a critically endangered medicinal plant of the Himalaya. In Vitro Cell Dev Biol Plant 41:244–248
Mathews H, Litz RE, Wilde HD, Merkle SA, Wetzstein HY (1992) Stable integration and expression of β-glucuronidase and NPT II genes in mango somatic embryos. In Vitro Cell Dev Biol Plant 28:172–178
McCartan SA, Crouch NR (1998) In vitro culture of Mondia whitei, a threatened Zulu medicinal plant. S Afr J Bot 64:313–314
Merkle SA, Parrott WA, Flin BS (1995) Morphogenic aspect of somatic embryogenesis. Torpedoed in vitro embryogenesis in plant. Kluwer Academic Publishers, Dordrecht, pp 155–203
Murashige T (1977) Plant cell and organ cultures as horticultural practices. Acta Hortic 78:17
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Noumi E, Amvam ZPH, Lontsi D (1998) Aphrodisiac plants used in Cameroon. Fitoterapia 69:125–134
Oketch-Rabah HA (2012) Mondia whitei, a medicinal plant from Africa with aphrodisiac and antidepressant properties: a review. J Diet Suppl 9:272–284
Pattnaik S, Chand PK (2000) Morphogenic response of the alginate encapsulated axillary buds from in vitro shoot cultures of six mulberries. Plant Cell Tissue Organ Cult 60:177–185
Rai MK, Asthana P, Singh SK, Jaiswal VS, Jaiswal U (2009) The encapsulation technology in fruit plants—a review. Biotechnol Adv 27:671–679
Redenbaugh K (1993) Synseeds: application of synthetic seeds for crop improvement. CRC Press, Boca Raton
Redenbaugh K, Fujii JA, Slade D, Viss P, Kossler M (1991) Artificial seeds-encapsulated embryos. In: Bajaj YPS (ed) High technology and micropropagation I. Biotechnology in agriculture and forestry, vol 17. Springer, Berlin, pp 395–416
Redenbaugh K, Paasch BD, Nichol JW, Kossler ME, Viss PR, Walker KA (1986) Somatic seeds: encapsulation of asexual plant embryos. Bio Technol 4:797–801
SANBI (2013) Statistics: red list of South African Plants version 2013.1. http://redlist.sanbi.org/stats.php
Siddiqui ZH, Mujib A, Maqsood M (2011) Liquid overlaying improves somatic embryogenesis in Catharanthus roseus. Plant Cell Tissue Organ Cult 104:247–256
Sudha CG, Seeni S (2006) Spontaneous somatic embryogenesis on in vitro root segment cultures of Rauvolfia micrantha hook. f.—a rare medicinal plant. In Vitro Cell Dev Biol Plant 42:119–123
Utomo HS, Wenefrida I, Meche MM, Nash JL (2008) Synthetic seed as a potential direct delivery system of mass produced somatic embryos in the coastal marsh plant smooth cordgrass (Spartina alterniflora). Plant Cell Tissue Organ Cult 92:281–291
Watcho P, Kamtchouing P, Sokeng SD, Moundipa PF, Tantchou J, Essame JL, Koueta N (2004) Androgenic effect of Mondia whitei roots in male rats. Asian J Androl 6:269–272
Wilde HD, Meagher RB, Merkle SA (1992) Expression of foreign genes in transgenic yellow-poplar plants. Plant Physiol 98:114–120
Yuan F, Wang Q, Pan Q, Wang G, Zhao J, Tian Y, Tang K (2011) An efficient somatic embryogenesis based plant regeneration from the hypocotyl of Catharanthus roseus. Afr J Biotechnol 10:14786–14795
Acknowledgments
The financial support by the National Research Foundation (NRF), Pretoria and the University of KwaZulu-Natal, Pietermaritzburg is gratefully acknowledged. The authors are grateful to the Microscopy and Microanalysis Unit (MMU), UKZN, Pietermaritzburg for microscopic assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baskaran, P., Kumari, A. & Van Staden, J. Embryogenesis and synthetic seed production in Mondia whitei . Plant Cell Tiss Organ Cult 121, 205–214 (2015). https://doi.org/10.1007/s11240-014-0695-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0695-x