Abstract
A suspension culture capable of producing a high quantity of proembryogenic masses (PEMs) was evaluated to provide adequate support for synthetic seed production in saltmarsh species Spartina alterniflora. Using immature inflorescences, callus was induced on MS medium supplemented with 2 mg 1−1 2,4-dichlorophenoxyacetic acid (2,4-D). Prior to initiation of suspension culture, calli were proliferated on a modified R4 medium (R4+) for 2–4 weeks. Suspension culture was carried out in a liquid General Medium modified by adding FeSO4 · 7H20, myo-inositol, thiamine · HCl, pyridoxine · HCl, and casein acid hydrolysate. Suspension cells were subcultured weekly by replacing the spent medium with fresh medium. The amount of PEMs tripled in 1 week. Cells from suspension culture had an average regeneration rate of 90% for 6 months of culture. About 40 g of PEMs can be harvested from 1 l of suspension culture in a weekly basis. Plated on R4+ medium, these PEMs produced somatic embryos (SEs) that gave rise to 2,000 plantlets. Encapsulation of SEs or microplantlets (MPs) derived from suspension cells may facilitate direct delivery of micro-propagated S. alterniflora to field planting. Evaluated under in vitro conditions, both SEs and MP-derived synthetic seeds had high conversion rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Smooth cordgrass (Spartina alterniflora Loisel.) can be found in the estuarine around Europe, America, Australia, New Zealand, and Asia. In the US, it is the dominant native plant in salt marshes along the eastern seaboard and the Gulf Coast (Bruno 2000). However, S. alterniflora is considered an invasive plant in Gray’s Harbor, Puget Sound, and Willapa Bay, Washington; the Suislaw River estuary, Oregon; and San Francisco Bay, California (Thompson 1991). S. alterniflora has high adaptation to many soil types from sandy to silt and clay, with a pH range of 3.7–7.9. It is capable of tolerating fluctuating water levels and continuous inundations with 0–35 parts per thousand salinity. Its vegetation can provide an effective buffer that disperses wave energy, reduces shoreline scouring, and entraps floating sediments and other solids. Because of that, S. alterniflora has been used extensively in coastal erosion control and habitat restoration in Lousiana, which suffers from a tremendous coastal marsh loss at an estimated rate of 4,300 ha per year (Craig et al. 1979; Baustian 2006).
Ecotypes of S. alterniflora that grow along the Gulf Coast are generally sterile with a little or no viable seed production (Seneca 1974). Up to the present date, S. alterniflora materials that are used for erosion control and habitat restoration are produced through vegetative propagation using sprigs, plugs, or potted plants. Current planting practices in restoration projects are both labor-intensive and costly (Jerome 1979). Labor requirements for planting sprigs, plugs or potted S. alterniflora range from 25 to 125 h per acre. Mechanized digging, such as using adapted small agricultural tractors for separating and planting, requires about half the time. Labor requirements for collecting, cultivation and planting container plants range from $6 to $8 per container and can easily approach $2,700 to $3,500 per acre, depending on planting location (Jerome 1979; Broome et al. 1988). Synthetic seed can be used as an alternative to overcome the low seed set problem.
Synthetic seed can serve as a rapid delivery system of somatic embryo (SE). Cell culture and micro-propagation technology have made significant progress in the last two decades encompassing a wide variety of plant species. As an automation system becomes a more common feature in many laboratories, mass micro-propagation potentially can be automated to produce virtually unlimited quantities of SEs in a mechanized, controlled environment. The cycle of embryogenic cell production through suspension culture is relatively short and proembryogenic mass (PEM) can be proliferated quickly. This system potentially can be used to rival a production of natural seed that typically has a much longer cycle to reproduce.
An in vitro culture system used in the synthetic seed production allows for disease eradication to produce a disease-free population, which in some crops, such as sugarcane, is important for achieving maximum yield potential and profitability. Though significant breakthrough has not been achieved, technology to create synthetic seed and mass produce is in progress. The effort to produce synthetic seeds is expanding for a variety of purposes and a larger range of species, including asparagus, Asparagus officinalis (Mamiya and Sakamoto 2001); apple, Malus pumila (Brischia et al. 2002); papaya, Carica papaya (Castillo et al. 1998; Saha et al. 2004); rice, Oryza sativa (Kumar et al. 2005); sugarcane, Saccharum officinarum (Nieves et al. 2003); hibiscus, Hibiscus moscheutos (West et al. 2006); pistachio, Pistacia vera L. (Onay et al. 1996); and banana, Musa SPP (Ganapathi 2001).
Micro-propagation technique provides an alternative to produce vegetatively propagated S. alterniflora quickly and efficiently. Synthetic seed may facilitate direct seeding of micro-propagated materials. Though tissue culture and plant regeneration of S. alterniflora have been reported (Wang et al. 2003), efficient mass production of SEs has not been established. The objectives of this research were to (1) develop suspension culture to mass produce SEs, (2) characterize the growth and regeneration rate of the suspension cells, (3) evaluate factors affecting an optimum production of SEs, and (4) encapsulation of SEs and MPs to produce S. alterniflora synthetic seed.
Materials and methods
Tissue culture procedure
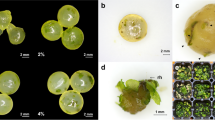
Spartina alterniflora cv. ‘Vermilion’ together with Black Lake and New Orleans accessions were grown in 1-gallon containers containing Crowley silt loam soil (48 pots in 2 × 4 m plastic bench) in a greenhouse with an average temperature set to 25°C, 16:8 cycle of light/dark, and under continuous flooding (2 cm above soil line). The plants were fertilized every 2 weeks with 200 g of Triple 13 (N:P:K, 13:13:13) per plastic bench until flowering. Insecticide Lambda cyhalothrin (Karate, Syngenta) was used to control insect as needed. The top parts of the plants containing immature inflorescence, 1–2 cm long, were harvested and surface sterilized by dipping in a 20% bleach solution for 20 min and rinsed with sterile dH2O three times. Vermilion was used throughout the experiments, while Black Lake and New Orleans accessions were used to verify micro-propagation protocols developed. Callus was induced by plating the immature inflorescences on semi-solid medium in 10 × 150 Petri dishes containing MS basal salts (Murashige and Skoog 1962; supplied by Sigma-Aldrich Co., St. Louis, MO) supplemented with 2 mg 1−1 2,4-dichlorophenoxyacetic acid (2,4-D), 30 g 1−1 sucrose, and 7 g 1−1 Sigma agar (Sigma Co., St. Louis, MO) with the pH adjusted to 5.8 by adding NaOH before autoclaving. The culture was carried out under dark conditions at 25°C for 2–10 weeks without subculture (Fig. 1a). Friable calli formed (Fig. 1b) were used to develop suspension culture.
Suspension cultures were initiated by placing friable embryogenic callus weighing 1.5 g in a 250-ml Erlenmeyer flask containing 50 ml liquid Gm+. Gm+ was a modified version of standard General Medium of Chen (1986). Gm+ was initially used to optimize the growth of rice suspension cells by adding 40 mg 1−1 FeSO4 · 7H20, 100 mg 1−1 myo-inositol, 10 mg 1−1 thiamine · HCl, 1 mg 1−1 pyridoxine · HCl, and 3 g 1−1 casein acid hydrolysate to a standard General Medium (Utomo et al. 1995). Before autoclaving, the pH of Gm+ was adjusted to 5.8 using NaOH. Suspension cells (Fig. 1d) were subcultured weekly by decanting the spent medium and replacing it with fresh Gm+ liquid medium. The suspension culture was maintained at 25°C on a gyratory shaker at 100 rpm under cool-white fluorescent lamps with light intensity of 9 μE m−2 s−1.
Proembryogenic masses (PEMs) from suspension culture were plated on regeneration medium R4+ to produce SEs and MPs through indirect somatic embryogenesis. The R4+ medium was developed from R4 (Chaleff and Stolarz 1981) and supplemented by adding 0.5 g 1−1 casein acid hydrolysate, 100 mg 1−1 myo-inositol, 1 mg biotin, 0.4 mg 1−1 thiamine · HCI, 64 g 1−1 maltose, and 6 g 1−1 DNA grade agarose (Bio-rad Co., Hercules, CA) with the pH adjusted to 5.8. Calli were subcultured weekly and maintained at 25°C under cool-white fluorescent lamps with a daily cycle of 16:8 light/dark and light intensity of 15 μE m−2 s−1. The resulting SEs and MPs 3–5 mm in length were used for synthetic seed experiments.
Growth characteristic, regeneration rate, and PEM composition of S. alterniflora suspension cells
Growth of PEMs was measured daily from day 1 to 7 when suspension cells entered the 5th, 6th, and 7th subculture based on their fresh packed cell volume (PCV). Starting with suspension cells entering the 5th subculture, the PCV was measured from an average of three randomly selected flasks, replicated three times. The same measurement was conducted in the 6th and 7th subculture. Data collected were used to construct the growth curve of S. alterniflora suspension cells. Cell size fractionation was also conducted during this period. At the end of each subculture (day 7), suspension cells used in the cell growth study were size fractionated into three classes (>100, 50–100, and <50 μm).
Regeneration rates of suspension cells were monitored monthly for 9 months. Each month, five flasks containing suspension cells were randomly selected. Four hundred PEMs >100 μm in diameter were collected from each flask and plated onto the R4+ medium (25 PEMs per dish). Subculture was conducted every 2 weeks by transferring PEMs to the R4+ medium. After 8 weeks of culture, regeneration data were collected. A percentage of PEMs giving rise to plantlets was used to generate a regeneration curve.
Productivity of suspension liquid medium (Gm+) was compared with a standard Gm medium. Suspension cells that had undergone seven subcultures were used for comparison. During the 8th subculture period, initial and final PCV of suspension cells from five randomly selected flasks were measured. A sample of 400 PEMs (>100 μm in diameter) was also collected from each flask at the end of the 8th subculture and plated onto the R4+ medium as previously described to determine their regeneration rates.
The frequency of MP formation on R4+ medium was monitored weekly for 8 weeks using 400 PEMs (>100 μm in diameter) from five randomly selected suspension flasks. The PEMs were plated onto R4+ medium, 25 PEMs per Petri dish. Regular subcultures were carried out every 2 weeks by transferring them to a fresh R4+ medium. The experiment was repeated three times. All data collected were subjected to an ANOVA and Duncan’s multiple-range test using SAS ver 9.1 (Gary, NC).
Synthetic seed
Synthetic seed was produced by encapsulating SEs or MPs with sodium alginate concentrations as specified in the treatment. For SE encapsulation, SEs were mixed with the alginate solution and pipetted and dropped into a stirred 100 mM CaCl2 solution for 30 min to form firm gel beads. Encapsulation of PEMs was done in a similar way. To encapsulate MPs, each MP was inserted into the pipetted alginate solution before dropped into the stirred CaCl2 solution. After complexation, the harden gel beads were rinsed twice with sterile water.
Factors affecting conversion rates of SEs and MPs
The effects of three carbon sources (sucrose (30 g 1−1), sorbitol (36.5 g 1−1), and maltose (64 g 1−1)) in four regeneration media (R4+, MS+, GM+, and N6) on the conversion rate of encapsulated and non-encapsulated PEMs, SEs, and MPs were studied in a factorial treatment arrangement with five replications. For conversion of PEM to SE, 0.5 g PEM was used. The 0.5 g PEM typically produced an average of 50 SEs on R4+ medium. For germination of SE to MP1, 400 SEs were used. The same number was used to study the success rates of MP3, 4, and 5 to grow to full size plantlets approximately 10 cm in length. Crucial data on the number of SEs developed from PEMs, % SE conversion, and % success rate of MP3, 4, and 5 to grow to full size plantlets were collected and subjected to ANOVA and Duncan’s multiple-range test (SAS ver 9.1, Gary, NC).
Effects of three gelling agents (DNA grade Agarose, Gelrite (Kelco-Merck Co., San Diego, CA), and Sigma agar) in two regeneration media (R4+ and MS+) on a conversion rate of encapsulated and non-encapsulated PEMs, SEs, and MPs were also studied in the factorial treatment arrangement with five replications. The amount of PEMs and the number of SEs and MPs used in this study were the same as that used in the carbon source study. Data on number of SEs developed from PEMs, % SE conversion, and % success rate of MP3, 4, and 5 to grow to full size plantlets collected were subjected to ANOVA and Duncan’s multiple-range test (SAS ver 9.1, Gary, NC).
To evaluate synthetic seed hardness, four alginate concentrations, 1, 2, 3, and 4% (w/v), were used to encapsulate 200 each of SE, and MP3, 4, and 5. The R4+ salt bases and additives without CaCl2 were incorporated into alginate solution during encapsulation of PEMs, SEs, MP3, and MP4, while MP5 was encapsulated free of R4+ salt bases and additives. As a control, PEMs and SEs were also encapsulated with alginate solution without R4+ salt bases and additives. Encapsulation was carried out as previously described. Following sodium alginate encapsulation, the synthetic seed was germinated on the ½ strength solidified MS medium containing 30 g 1−1 sucrose (Fig. 1 e, f). Percent seedling establishment on this medium were accessed 1 month later. The experiment was repeated three times. ANOVA and Duncan’s multiple-range test were used for statistical analyses (SAS ver 9.1, Gary, NC).
To evaluate synthetic seed hardness, four alginate concentrations (1, 2, 3, and 4% (w/v)) were used to encapsulate 200 each of SE, MP3, MP4, and MP5. The R4+ salt bases and additives without CaCl2 were incorporated into the alginate solution during encapsulation of PEMs, SEs, MP3, and MP4, while MP5 was encapsulated free of R4+ salt bases and additives. As a control, PEMs and SEs were also encapsulated with alginate solution without R4+ salt bases and additives. Encapsulation was carried out as previously described. Following sodium alginate encapsulation, the synthetic seed was germinated on ½ strength solidified MS medium containing 30 g 1−1 sucrose (Fig. 1 e, f). Percent seedling establishment on this medium was accessed 1 month later. The experiment was repeated three times. ANOVA and Duncan’s multiple-range tests were used for statistical analyses (SAS ver 9.1, Gary, NC).
Results
Suspension culture
The growth of S. alterniflora suspension cells started with a period of a lag phase for 1 day followed by exponential growth that reached its peak at day 6 after subculture (Fig. 2a). At the end of subculture period (day 7), the amount of suspension cells tripled. At the peak of suspension cell growth, 62% of PEMs consisted of tissue mass with the size of more than 100 μm (Figs. 2b and 1d). The clump size of >100 μm was suitable for use in rapid plant regeneration. While one third of the suspension cells would be needed to maintain a cycle of culture, two thirds could be harvested weekly and plated onto solidified medium to produce SEs used in the synthetic seed production. High regeneration rates in the average of 94% were maintained throughout 4 months of culture (Fig. 2c). Though the regeneration rates remained high averaging over 92% after 5 months of culture, the rates started to decline. Sharp decline occurred between 6 and 7 months of culture. After 9 months of culture, the regeneration rate was less than 20%. Modification of standard Gm medium, by adding 100 mg l−1 myo-inositol, 10 mg l−1 thiamine·HCl, 1 mg l−1 pyridoxine·HCl along with 40 mg l−1 FeSO4 · 7H20, and 3 g l−1 casein acid hydrolysate casein acid hydrolysate has resulted in the production of PEMs twice as much as the ones from the standard Gm medium (Fig. 2b). In one cycle of culture, S. alterniflora suspension cells in a standard Gm medium produced only 1.5 times of their initial cell mass. When cultured in modified (Gm+) medium, the amount of cells was tripled. The Gm+ suspension medium also gave 10% improvement in regeneration rate per weight basis, 94% when cultured in Gm+ and 84% in Gm (Fig. 2d).
(a) Growth of S. alterniflora suspension cells in one cycle of subculture; (b) proportion of suspension cells based on their size at day 7 in a cycle of subculture; (c) regeneration ability of suspension cells in 9 months of culture; and (d) left: Growth of suspension cells in General Medium (Gm) and modified General Medium (Gm+) at day 7 after seven cycles of subculture, and Right: Regeneration rate of suspension cells grown in Gm and Gm+ medium measured 2 months after suspension culture initiation
Production of SEs and development of MPs
A majority of PEMs from suspension cells developed into SEs after two subcultures followed by a maturation process when they were plated onto a semi-solid R4+ regeneration medium. Two major factors, type of salt base and carbon source, were critical for successful formation of S. alterniflora SEs. Among four salt-base compositions (MS, GM, N6, and R4), R4 was the most suitable salt base for developing S. alterniflora SEs (Table 1). An average of 52 SEs was produced from 0.5 g PEMs when maltose was supplemented in the R4+ medium. No SE was obtained when maltose, sucrose, or sorbitol was used in a combination with other salt bases tested, N6, Gm+, and MS+. An average conversion rate of 71% was observed for non-encapsulated SEs and 70% for encapsulated SEs on R4+ supplemented with maltose. A combination of R4+ and maltose continued to show a positive impact on the viability of MP3. A 98% success rate was obtained in this medium compared with 27% in R4+ and sucrose, 29% in MS+ and sucrose, 30% in Gm+ and sucrose, and 29% in sucrose and N6 (Table 1).
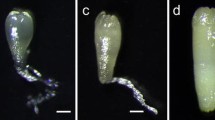
As embryos matured within two to three subcultures, the SEs of S. alterniflora entered a germination phase that involved elongation of hypocotyl-root axis and emergence of the radicle into MP. The term of MP3, 4, and 5 refers to microplantlets that are 3, 5, and 5 mm in length, respectively. As SEs grew into MPs, nutritional growth requirement changed (Table 1). MP3 and 4 showed a transitional shift. While the highest successful rates (96% for non-encapsulated and 97% for encapsulated) were obtained in R4+ and maltose, combination of sorbitol or sucrose with other salt bases showed a fair support for the growth of MP4 into plantlets. The success rates range from 0 in R4+, MS+, GM+, and N6 with sorbitol to 55% in MS+ and sucrose (Table 1). These better effects have not been observed at the MP3 stage. MP5s showed a definite change in nutritional growth requirement. The highest success rate was no longer obtained from a combination of maltose and R4+, but it was provided by a combination of sucrose and MS+ (96%). A 95% success rate was also obtained on an MS with no sucrose. It was obvious that sugar was no longer necessary for the growth of MP5. Therefore, MP5 is a fully autotrophic MP.
Agarose played a significant role in the development of PEM to SE, SE conversion, and growth of MP3. The production of SEs was the highest when agarose was used in R4+ medium (52 SEs/0.5 g PEMs; Table 2). Only eight and seven SEs were produced from 0.5 g PEMs when the PEMs were plated on the R4+ with Gelrite and Sigma agar, respectively. A 71% conversion rate was obtained on the R4+ with agarose, while 27% was on the R4+ with Sigma agar. No conversion was obtained on the R4+ with Gelrite. The R4+ with agarose also gave the highest success rate (98%) for MP3 to grow into plants. While agarose still maintained a high success rate in MP4 (94% for non-encapsulated and 98% for encapsulated MP4), a combination of Gelrite and MS+ also provided success rates of 91% for both encapsulated and non-encapsulated MP4. The effect of agarose, however, was different in MP5. Only a 86% success rate was obtained when a combination of agarose and the R4+ was used. At this stage, the three gelling agents (agarose, Sigma agar, and Gelrite) produced high success rates, ranged from 92 to 98%, when used in combination with MS+.
In general S. alterniflora PEMs developed into MPs within a 1- to 8-week period with bi-weekly subcultures. The peak formation of MP5s occurred at week 2, with an average formation of 38% (Fig. 3a). Within each subculture, the formation of MP5 in the first week was higher than that in the second week. For example, in the first subculture, MP5 formation was 4% in the first week and 38% in the second week; in the second subculture, MP5 formation was 14% in the first week and 19% in the second week. The formation of MP5 was very low and highly sporadic after 8 weeks in culture.
Gel hardness and germination of synthetic seeds
Encapsulation of PEMs with 1% (w/v) alginate gave an average of 6.5% seedling rate, while encapsulation with 4% alginate hindered the growth (Fig. 3b). Softer gel provided a more favorable condition for SE growth; 70, 64, 60, and 54% seedling establishment rates were obtained when encapsulated with 1, 2, 3, and 4% alginate, respectively. While encapsulation with 4% alginate provided the hardest gel breaking point, in most cases, it reduced seedling establishment rates. Encapsulation with 1% alginate provides the most suitable gel pressure for the growth of PEMs, SEs, and MP3s. However, it was too soft for handling purposes. Encapsulation with 2% produced bead hardness of 0.5–2.0 kg breaking pressure per bead and therefore it might be of sufficient gel integrity against mechanical handling and planting without breakage.
Discussion
Suspension cell culture provides a rapid proliferation of S. alterniflora PEMs. The potential of suspension culture to generate a large number of embryonic cells has been recognized earlier in other plant species (Sharp et al. 1984). It provides a constant supply of embryogenic cells and helps reduce time needed for plant regeneration. It has also been used in mutant selection and mass propagation (Naill and Roberts 2005; Kishinami and Widholm 1986). The amount of S. alterniflora PEMs was tripled in 1 week of culture. While one third of the PEMs was needed to maintain the culture, two-thirds of the masses could be used in SE and MP production. Modification of General Medium (Chen 1986) that was originally used for suspension culture of rice (Utomo et al. 1995) was suitable for use in producing high quantities of embryogenic PEMs. In 1 l of suspension culture, 40 g of PEMs can be harvested weekly. Since an average of 53 SEs can be produced from 0.5 g PEMs when plated on the regeneration medium R4+, PEMs obtained from 1 l of suspension culture will gave rise to 4,000 SEs from which over 2,000 viable microplantlets can be selected. These resulting microplantlets can be encapsulated to produce 2,000 synthetic seeds. Wang et al. (2003) reported plant regeneration of S. alterniflora through callus culture using MS medium supplemented with thidiazuron, 6-benzylaminopurine in combination with indole-3-acetic acid, or thidiazuron in combination with indole-3-acetic acid. Effective mass production of PEMs, SEs, and MPs, however, has not been previously reported. The suspension culture and plant regeneration system described can facilitate a mass production of clonally propagated S. alterniflora through indirect somatic embryogenesis. Tens of liters of suspension cell culture can be maintained on several orbital shakers in a small room. If required, the suspension cell cultures of S. alterniflora can easily be scaled up and the system can potentially be automated to fit industrial settings. Using the procedure and media described, a production of smooth cordgrass embryogenic cells will not be a limiting factor in synthetic seed production.
Maltose provided a high SE formation (53 SEs/0.5 g PEMs) and conversion rate of >75%. Even though sucrose is the most common carbon source in micro-propagation of many plants, maltose was a better carbon source to support S. alterniflora SE formations. A carbon source has been known to play important roles in various biological functions, including a growth promoting factor, induction of secondary somatic embryogenesis, tuber formation, and embryogenic callus formation by providing osmotic pressure and inhibiting chlorophyll formation (Tonon et al. 2001; Ticha et al. 1998; Agarwal et al. 2004). Many cultures, especially embryo and some shoot cultures, require a medium with a specific osmotic potential. Sucrose readily breaks down into glucose and fructose in the medium causing an increase of osmolarity (Iraqi and Tremblay 2001). Unlike sucrose, maltose remains static in the medium producing a constant osmotic level. A high level of S. alterniflora embryo development observed when maltose was used could be attributed to the role of maltose in facilitating the creation of a constant and more natural osmotic environment. Data showed that in the absence of maltose, S. alterniflora PEMs could not develop into SEs and the maturation of SEs did not occur. Maltose improved embryo maturation (Li et al. 1998) and increased production of superior SEs (Asano et al. 1994). Maltose, together with lactose, has also been reported to promote embryo maturation more effectively than sucrose in Abies nordmannia (Norgaard and Krogstrup 1991).
The use of DNA grade agarose has improved S. alterniflora SE production. Agarose has been reported to improve regeneration rates of protoplasts, rejuvenate long-term cell suspension, and improve plant regeneration from callus culture (Tang et al. 2000). The higher cost associated with the use of DNA grade agarose could be the downside in SE production via indirect somatic embryogenesis. However, high success rates, six to seven times higher when agarose was used in the media, provides a significant advantage over two other gelling agents. With high success rates, the time and cost of labor required to generate the same number of SEs for synthetic seed production can significantly be reduced.
Encapsulation of SEs or MPs inside the gel matrix provides a physical protection during seed handling and planting. For S. alterniflora, 2% (w/v) alginate provided an optimum encapsulating material because it gave gel hardness that could tolerate mechanical handling with no visible adverse effects associated with the growth of SEs and MPs. Since the growth of SEs, when encapsulated with 2% alginate, was not affected, hollow encapsulations to encourage better growth, as suggested by Patel et al. (2000), may not be necessary. High germination rates were obtained from synthetic seed developed from SEs, MP3s, MP4s, and MP5s. Sodium alginate has been widely used in synthetic seed in various plant species. It has also been used to encapsulate plant organs for various purposes, such as improving a success rate of nodal segment propagation (West et al. 2006), low temperature storage (Lisek and Olikowska 2004), desiccation (Janick et al. 1989), bud propagation (Pattnaik and Chand 2000), and shoot tip propagation (Singh et al. 2006). This study shows that alginate solution could be used to develop synthetic seeds by encapsulating S. alterniflora SEs or MPs derived from suspension cells. The successful establishment of suspension culture protocol for S. alterniflora that has high regeneration rates for up to 6 months of culture can be used as a basis to produce a significant number of MPs for synthetic seed production.
Genetic diversity has been an issue related to the use of plants for coastal erosion control and wetlands reclamation. Indirect somatic embryogenesis often produce a genetic variation known as somaclonal variation (Larkin and Scowcroft 1981). While this laboratory induced somaclonal variation could be beneficial to improving genetic diversity (Seliskar and Gallagher 2000), components of genetic diversity existing in the natural populations can directly be incorporated during synthetic seed production. Parental lines to be used in a large-scale synthetic seed production can be systematically selected based on DNA marker data and phenotypic observation to represent the genetic diversity of the original populations in the target areas. The protocols developed for S. alterniflora cv. Vermilion for callus induction, suspension culture, and plant regeneration could be used to micro-propagate accessions from Black Lake and New Orleans, which represent the eastern and western Louisiana ecotypes. Since micro-propagation protocols developed were successfully applied for these two ecotypes, they could be expanded to other Louisiana S. alterniflora ecotypes. The number of parental lines, as many as required, can potentially be included in the synthetic seed production to keep the genetic diversity in the target regions. The composition of parental lines and proportion of synthetic seeds derived from each parental line can be adjusted so that resulting synthetic seeds are tailored to provide the desirable level of genetic diversity.
Abbreviations
- SE:
-
Somatic embryo
- MP:
-
Microplantlet
- PEM:
-
Proembryogenic mass
References
Agarwal S, Kanwar K, Sharma DR (2004) Factors affecting secondary somatic embryogenesis and embryo maturation in Morus alba L. Sci Hortic 102:359–368
Asano Y, Ito Y, Ohara M, Sugiura K, Fujiie A (1994) Improved regeneration response of creeping bentgrass and japonica rice by maltose and lactose. Plant Cell Tiss Org 39:101–103
Baustian JJ (2006) Restoration success of backfilling canals in coastal louisiana marshes. Restor Ecol 14:636–644
Brischia R, Piccioni E, Standardi A (2002) Micropropagation and synthetic seed in M.26 apple rootstock (II): a new protocol for production of encapsulated differentiating propagules. Plant Cell Tiss Org 68:137–141
Broome SW, Seneca ED, Woodhouse Jr. WW (1988) Tidal salt marsh restoration. Aquat Bot 32:1–22
Bruno JF (2000) Facilitation of cobble beach plant communities through habitat modification by Spartina alterniflora. Ecology 81:1179–1192
Castillo B, Smith MAL, Yadava UL (1998) Plant regeneration from encapsulated somatic embryos of Carica papaya L. Plant Cell Rep 17:172–176
Chaleff RS, Stolarz A (1981) Factors influencing the frequency of callus among cultured Oryza sativa anthers. Physiol Plant 51:201–206
Chen Y (1986) Anther and pollen culture in rice. In: Hu H, yang HY (eds) Haploid of higher plants in vitro. China Acad Pub and Springer-Verlag, Berlin, pp 1–25
Craig NJ, Turner RE, Day Jr. JW (1979) Land loss in coastal Louisiana (USA). Environ Manage 3:133–144
Ganapathi TR (2001) Regeneration of plants from alginate-encapsulated somatic embryos of banana cv. Rasthali (Musa spp. AAB group). In Vitro Cell Devel Biol Plant 37(2):178–181
Iraqi D, Tremblay FM (2001) The role of sucrose during maturation of black spruce (Picea mariana) and white spruce (Picea glauca) somatic embryos. Physiol Plant 111:381–388
Janick J, Kitto SL, Kim Y (1989) Production of synthetic seed by desiccation and encapsulation. In Vitro Cell Devel Biol Plant 25:1167–1172
Jerome LE (1979) Marsh restoration: economic rewards of a healthy salt marsh. Ocean 12:57–61
Kishinami I, Widholm JM (1986) Selection of copper and zinc resistant Nicotiana plumbaginifolia cell suspension cultures. Plant Cell Physiol 27:1263–1268
Kumar MBA, Vakeswaran V, Krishnasamy V (2005) Enhancement of synthetic seed conversion to seedlings in hybrid rice. Plant Cell Tiss Organ 81:97–100
Larkin P, Scowcroft W (1981) Somaclonal variation a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Li XY, Huang H, Murphy BJ, Gbur EE Jr (1998) Polyethylene glycol and maltose enhance somatic embryo maturation in loblolly pine (Pinus taeda L.). In Vitro Cell Dev Biol Plant 34:22–26
Lisek A, Olikowska T (2004) In vitro storage of strawberry and raspberry in calcium-alginate beads at 4°C. Plant Cell Tiss Org 78:167–172
Mamiya K, Sakamoto Y (2001) A method to produce encapsulatable units for synthetic seeds in Asparagus oficinalis. Plant Cell Tiss Org 64:27–32
Murashige T, Skoog FA (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Naill MC, Roberts SC (2005) Cell cycle analysis of Taxus suspension cultures at the single cell level as an indicator of culture heterogeneity. Biotechnol Bioeng 90:491–500
Nieves N, Zambrano Y, Tapia R, Cid M, Pina D, Castillo R (2003) Field performance of artificial seed-derived sugarcane plants. Plant Cell Tiss Org 75:279–282
Norgaard JV, Krogstrup P (1991) Cytokinin-induced somatic embryogenesis from immature embryos of Abies nordmanniana Lk. Plant Cell Rep 9:509–513
Onay A, Jeffree CE, Yeoman MM (1996) Plant regeneration from encapsulated embryoids and an embryogenic mass of pistachio, Pistacia vera L. Plant Cell Rep 15:723–726
Patel A, Pusch L, Mix-Wagner G, Vorlop KD (2000) A novel encapsulation technique for the production of artificial seeds. Plant Cell Rep 19:868–874
Pattnaik S, Chand PK (2000) Morphogenic response of the alginate-encapslated axillary buds from in vitro shoot cultures of six mulberries. Plant Cell Tiss Org 60:177–185
Saha M, Phatak A, Chandra N (2004) Generation of synthetic seeds from different varieties of Carica papaya L. J Tiss Res 4:207–209
Seliskar DM, Gallagher JL (2000) Exploiting wild population diversity and somaclonal variation in the salt marsh grass Distichlis spicata (Poaceae) for marsh creation and restoration. Am J Bot 87:141–146
Seneca ED (1974) Germination and seedling response of Atlantic and Gulf coasts populations of Spartina alterniflora. Am J Bot 61:947–956
Sharp WR, Evans A, Ammirato PV, Yamada Y (1984) Handbook of plant cell culture, vol. 2, Crop Species. Macmillan, New York
Singh AK, Varshney R, Sharma M, Agarwal SS, Bansal KC (2006) Regeneration of plants from alginate-encapsulated shoot tips of Withania somnifera (L.) dunal, a medicinally important plant species. J Plant Physiol 163:220–223
Tang K, Sun X, An D, Power JB, Cocking EC, Davey MR (2000) Plant regeneration from protoplasts of a commercial Asian long-grain javanica rice. Plant Cell Tiss Org 60:79–82
Thompson J.D. 1991. The biology of an invasive plant. Bioscience 41:393–401
Ticha I, Cap F, Pacovska D, Hofman P, Haisel D, Capkova V, Schafer C (1998) Culture on sugar medium enhances photosynthetic capacity and light resistance of plantlets grown in vitro. Plant Physiol 102:155–162
Tonon G, Capuana M, Rossi C (2001) Somatic embryogenesis and embryo encapsulation in Fraxinus angustifolia Vhal. J Hort Sci Biotechnol 76:753–757
Utomo HS, Croughan SS, Croughan TP (1995) Suspension and protoplast culture of US rice cultivars. Plant Cell Rep 15:34–37
Wang J, Seliskar DM, Gallagher J (2003) Tissue culture and plant regeneration of Spatina alterniflora: implications for wetland restoration. Wetlands 23:386–393
West TP, Ravindra MB, Preece JE (2006) Encapsulation, cold storage, and growth of Hibiscus moscheutos nodal segments. Plant Cell Tiss Org 81:97–100
Author information
Authors and Affiliations
Corresponding author
Additional information
Approved for publication by the Director of the Louisiana Agricultural Experiment Station as manuscript number 07-61-0353.
Rights and permissions
About this article
Cite this article
Utomo, H.S., Wenefrida, I., Meche, M.M. et al. Synthetic seed as a potential direct delivery system of mass produced somatic embryos in the coastal marsh plant smooth cordgrass (Spartina alterniflora). Plant Cell Tiss Organ Cult 92, 281–291 (2008). https://doi.org/10.1007/s11240-007-9334-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-007-9334-0