Abstract
Embryogenic callus was initiated by culturing in vitro taro corm slices on agar-solidified half-strength MS medium containing 2.0 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D) for 20 days followed by transfer to 1.0 mg/L thidiazuron (TDZ). Callus was subsequently proliferated on solid medium containing 1.0 mg/L TDZ, 0.5 mg/L 2,4-D and 800 mg/L glutamine before transfer to liquid medium containing the same components but with reduced glutamine (100 mg/L). After 3 months in liquid culture on an orbital shaker, cytoplasmically dense cell aggregates began to form. Somatic embryogenesis was induced by plating suspension cells onto solid media containing reduced levels of hormones (0.1 mg/L TDZ, 0.05 mg/L 2,4-D), high concentrations of sucrose (40–50 g/L) and biotin (1.0 mg/L). Embryo maturation and germination was then induced on media containing 0.05 mg/L benzyladenine (BA) and 0.1 mg/L indole-3-acetic acid (IAA). Histological studies of the developing embryos revealed the presence of typical shoot and root poles suggesting that these structures were true somatic embryos. The rate of somatic embryos formation was 500–3,000 per mL settled cell volume while approximately 60% of the embryos regenerated into plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taro (Colocasia esculenta var. esculenta) is an important food crop grown throughout many Pacific Island countries. In addition to contributing to sustained food security in the domestic market, it also provides a source of export earnings in some countries. Since taro is largely asexually propagated (Strauss et al. 1979; Ivancic 1992), there is little genetic variation within cultivars. Consequently, it is susceptible to numerous pests and diseases which can place serious constraints on production (Ivancic 1992).
The use of conventional breeding to obtain pest and/or disease resistant taro cultivars has been hampered by numerous problems including the unavailability of resistant cultivars, sexual incompatibility between parents, and variable climatic conditions affecting pollination and fertilization rates (Wilson 1990). Molecular breeding is an attractive alternative strategy as a single trait can be added to an already accepted cultivar in a single step without the requirement for further breeding. An essential pre-requisite for molecular breeding, however, is the availability of suitable target tissue from which large numbers of transgenic plants can be generated.
Embryogenic cells from liquid culture represent a suitable candidate target tissue for transformation as (1) the unicellular origin of somatic embryos reduces the likelihood of chimerism and (2) the relatively small size of cell clumps creates a large surface for exposure to the transforming agent and more effective selection of transformants. In addition, the rapid growth of cells in liquid culture in comparison to solid media means embryogenic cell suspensions can be used as an efficient means of producing large numbers of plants with reduced space requirements and labor costs.
Adventitious shoot production from callus has been reported in C. esculenta var. antiquorum using medium containing naphthalene acetic acid (NAA) and kinetin (Abo El-Nil and Zettler 1976) and in C. esculenta var. esculenta using taro corm extract (TE) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) (Yam et al. 1990). Recently, we reported the development of an efficient protocol for initiating embryogenic callus from corm slices of in vitro taro plantlets (Deo et al. 2009). To enhance the utility of this system for generating transgenics and particularly as a method for mass propagation, a protocol for proliferating embryogenic callus was required. In this paper, we examined the effects of 2,4-D, TDZ, glutamine and sucrose concentrations on embryogenic callus proliferation, both on solid media and as suspension cultures, and report a protocol for initiating embryogenic taro cell suspensions from which plants can be easily regenerated.
Materials and methods
Source of plant material
A virus-free accession of taro (Colocasia esculenta var. esculenta) cv. CPUK (originally derived from Cook Islands) was sourced from the Centre for Pacific Crops and Trees (CePaCT)-Secretariat of the Pacific Community (SPC), Fiji.
Callus initiation and proliferation on solid medium
Embryogenic callus was initiated from corm slices of in vitro plantlets as previously described (Deo et al. 2009). The incubation temperature in all experiments was 25°C and unless otherwise stated all culture steps were in the dark. The culture medium for callus proliferation consisted of half-strength MS medium, 30 g/L sucrose, 7 g/L agar with a pH of 5.8 and various combinations of 2,4-D, TDZ and glutamine. Filter sterilized glutamine was added to the medium after autoclaving. Hereafter, callus proliferation medium is referred to as callus maintenance medium (CMM). Callus produced by corm slices (Deo et al. 2009) and deemed to be embryogenic by the presence of translucent globular structures was cut into equal sizes (~2 mm × 2 mm × 1 mm) and placed on CMM. For each combination of 2,4-D, TDZ and glutamine, seven to ten pieces of callus were placed on each of seven CMM plates. The precise combinations of 2,4-D, TDZ and glutamine are described in Tables 1 and 2. Cultures were checked periodically for callus growth and after 2 months without subculture, the fresh weight of each callus piece was recorded as an indicator of proliferation. Following the first 2 months on CMM, embryogenic callus was maintained by monthly subculture onto fresh CMM.
Initiation, maintenance and optimization of suspension cultures
Approximately 0.5 g of embryogenic callus was placed in 10 mL of liquid medium in a 100 mL Erlenmeyer flask and agitated on an orbital shaker at 90 rpm. This medium was the same as CMM except agar was omitted and the concentration of glutamine was reduced to 100 mg/L. After 7 days, 10 mL of fresh medium was added. After an additional 7 days, the cells were allowed to settle to the bottom of the flask and 10 mL of the supernatant was removed and replaced with an equal volume of fresh medium. Half the media was refreshed a second time after which the entire contents of the flask were transferred to 250 mL flasks and the volume was made up to 50 mL. Henceforth, subculture was at 7-day intervals by either replacing 40 mL of old medium or dividing the cells between two flasks. When cells were of sufficient density, they were divided between flasks by allowing cells to settle, removing 30 mL of old medium, re-suspending cells, dividing the remaining 20 mL equally between flasks and adding 40 mL of fresh medium. In general, the volume of cells in 250 mL flasks was maintained as 1–2 mL settled cell volume (SCV) in 50 mL of medium. The effects of higher concentrations of glutamine (400 and 800 mg/L) and sucrose (20 g/L) on proliferation and regeneration of suspensions cells were also investigated.
Plant regeneration from suspension cells
Four embryogenesis media (EM) were examined for their efficacy at inducing embryogenesis from suspension cells. EM had the same components as liquid CMM except the concentrations of growth regulators were altered as follows: EM1 = TDZ (1.0 mg/L) + 2,4-D (0.5 mg/L); EM2 = TDZ (0.1 mg/L) + 2,4-D (0.05 mg/L); EM3 = TDZ (0.01 mg/L) + 2,4-D (0.005 mg/L) and EM4 = Zeatin (0.1 mg/L) + NAA (0.05 mg/L).
Suspension cells were collected 4 days after subculture, passed through a 500 μm stainless steel mesh filter and the filtrate was collected. The cells within the filtrate were allowed to settle in graduated 50 mL Falcon tubes and sufficient supernatant was removed to leave a settled cell volume/liquid medium ratio of approximately 1:5. The cells were then resuspended and 250 μL aliquots were dispensed directly onto sterile 70 mm Whatman filter paper discs overlaid on various EMs in 90 mm × 15 mm Petri dishes. After determining a suitable EM, this media was further refined by varying sucrose concentration (30, 40 and 50 g/L) and incorporating biotin (1.0 mg/L).
After 2 months on EM, the pro-embryogenic masses (PEMs) together with the somatic embryos were removed from the filter paper and transferred to new media for maturation and germination. Two media were examined: (1) hormone-free half-strength MS (designated RM) and (2) half-strength MS containing 0.05 mg/L BA and 0.1 mg/L IAA (designated GM). The cultures were maintained in darkness for 2–3 weeks then incubated under low light intensity (5 μmoles photons m−2 s−1). After 2 weeks at low light intensity, germinating embryos were transferred to higher light (25 μmoles photons m−2 s−1).
Histology of PEMs and somatic embryos
The pro-embryogenic masses (PEMs) and somatic embryos were fixed in formaldehyde: alcohol: acetic acid (FAA) (1:1:8 v/v) for 4 days, dehydrated in a xylene and ethanol series, then infiltrated and embedded with paraplast and wax, respectively. Thin sections (6 μm) were cut using a rotary microtome. The sections were heat fixed to 3-aminopropyltriethoxysilane (APES)-coated glass slides, dewaxed and stained with either Ehrlich’s HX and Eosin or Safranin O-Fast Green then viewed using a compound microscope (Olympus BX41).
Statistical analysis
Data were analyzed by analysis of variance (ANOVA) using a 95% confidence interval. Where P < 0.05, significant differences between individual treatment means were determined using Fisher’s Least Significant Difference (LSD) test. All data were analyzed by SPSS for Windows, version 11.
Results
Callus proliferation and maintenance on solid medium
To investigate the parameters affecting proliferation, embryogenic callus was removed from the original explants and placed on half-strength MS medium containing various concentrations of 2,4-D and TDZ (Table 1). In general, the proliferation of callus increased with increasing hormone concentration up to a TDZ and 2,4-D combination of 1.0 and 0.5 mg/L, respectively. This was both in terms of the percentage of callus pieces which proliferated and the average fresh weight. Increasing the 2,4-D concentration over 0.5 mg/L resulted in a decrease in callus proliferation. Overall, 2,4-D appeared to be more important as in its absence there was little or no callus proliferation even at high TDZ levels.
Although a combination of 1.0 mg/L TDZ and 0.5 mg/L 2,4-D was effective in inducing callus proliferation, much of the callus became watery and non-regenerable within 3–4 weeks. In an attempt to prevent this, the effect of glutamine was examined (Table 2). Although glutamine did not have a significant effect on the percentage of callus pieces proliferating, the use of 800 mg/L glutamine resulted in a significant increase in the mean fresh weight per callus pieces. Further, the callus remained firm (did not become watery) for over a month and, by monthly subculturing, could be maintained in this state for up to 18 months. Glutamine concentrations higher than 800 mg/L did not result in a further increase in fresh weight. The above experiments indicated that the optimal medium for callus proliferation on solid medium was half-strength MS containing 1.0 mg/L TDZ, 0.5 mg/L 2,4-D and 800 mg/L glutamine; this medium is henceforth referred to as solid callus maintenance medium (CMMS).
Initiation and characterization of suspension cultures
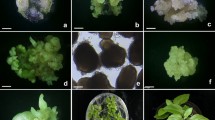
Suspension cultures were initiated by transferring approximately 0.5 g embryogenic callus into liquid CMM containing glutamine (100 mg/L) with continuous agitation at 90 rpm. When callus was taken directly from the original explants (corm slices on callus induction media (CIM; Deo et al. 2009) and placed in liquid CMM, some callus pieces enlarged while others became necrotic after 2 weeks. However, when callus was removed from the original explants and placed on CMMS for 2 months with monthly subcultures prior to transfer into liquid CMM, nearly all inoculated calli formed suspensions. These callus pieces increased in size by two-three fold 2 weeks after inoculation into liquid medium and began to produce single cells and small cell aggregates by the third week. Initially, most of the cells released into the liquid were singular, large and vacuolated (Fig. 1a), however, multicellular aggregates containing cells with dense cytoplasm began to form with subsequent weekly subcultures (Fig. 1b).
Suspension cultures contained two distinct cell types; (1) spherical cytoplasmically dense cells with small vacuoles and numerous starch granules present as small multicellular clumps and (2) elongated cells with large vacuoles, which appeared transparent and contained very few or no starch grains. Moreover, the cultures were heterogeneous since they contained single cells, small multicellular aggregates (0.1–0.5 mm diameter) and larger clumps (0.5–1.0 mm diameter). Three to four months after initiation, most suspension cultures produced cytoplasmically dense cell aggregates suitable for regeneration. However, after 7 months, the cell lines began to vary in their proliferation rate and proportion of different cell types. For example, some cell lines doubled in SCV within 2 weeks and contained a high proportion of small, dense isodiametric cells which were yellow in color. In contrast, other cell lines took 1 month to double in SCV, contained a high proportion of large, vacuolated cells and became pale yellow or white. The latter type of cell line was regarded as having poor regeneration capacity and was discarded.
Concentrations of glutamine greater than 100 mg/L (400 and 800 mg/L) were also trialed in suspension culture media and, although this appeared to increase the proliferation rate, cells cultured in this medium became necrotic 2 weeks after plating on RM. A similar negative effect on regeneration was observed by reducing the concentration of sucrose in liquid culture from 30 g/L to 20 g/L. Since suspension cultures maintained in liquid CMM containing 100 mg/L glutamine and 30 g/L sucrose appeared to cope best with the transfer from liquid to solid media, this medium was used in all subsequent experiments and is referred to as liquid callus maintenance medium (CMML).
Regeneration and plant development
Despite their survival in the short term, suspension cells plated on RM did not form embryos but, instead, proliferated slightly then turned necrotic after 1–2 months (Fig. 2a). Therefore, four embryogenesis media (EM) were examined for their efficacy in maintaining cells in a healthy state and inducing embryogenesis from suspension cells (Table 3). Three weeks after plating cells on various EMs, the large vacuolated cells became necrotic while the yellow cell aggregates, consisting of small cytoplasmically dense cells, proliferated and formed pro-embryogenic masses (PEMs). Cell aggregates which were white formed soft, white, watery callus. Globular structures began to form on the surface of PEMs after 3 weeks and continued to do so for 2 months (Fig. 2b). Histological studies showed somatic embryos contained two meristems, presumably the shoot and root poles (Fig. 3a), and early in development were attached to PEM through a suspensor-like structure (Fig. 3b) indicating they were most likely derived from the surface cells of PEMs.
Formation of PEMs and somatic embryos from embryogenic suspension cells of taro (Colocasia esculenta var. esculenta) cv. CPUK. Suspension cells proliferated and turned necrotic on RM (a) whereas on EM they formed PEMs (black arrow) with globular, translucent embryo-like structures (white arrows) forming on the surface of PEMs (b). Scale bar 5 mm (a), 2 mm (b)
Histology of mature and immature somatic embryos derived from cell suspension cultures of taro (Colocasia esculenta var. esculenta) cv. CPUK. (a) A mature somatic embryo with shoot apical meristem (SAM) between leaf primordia (LP), root apical meristem (RAM); (b) a globular somatic embryo (SE) attached to proembryogenic mass (PEM) via suspensor-like structure (black arrow). Scale bar 100 μm
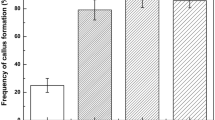
The highest rate of somatic embryo formation was from suspension cells plated on EM2 (25 ± 4.3) (Table 3). On other EMs, the rate of somatic embryo formation was low with callus displaying a range of responses; cells proliferated profusely but with very few embryos formed (EM1), poor cell proliferation and necrosis (EM3) and cell proliferation as soft watery callus (EM4). To further increase the embryo formation rate on EM2, the effect of increased sucrose concentration and the addition of biotin were examined (Table 4). After 2 months on various EM2 media, somatic embryos were transferred to germination medium (GM) and the percentage germination recorded. In general, the frequency of somatic embryo formation and the germination rate increased with increasing sucrose concentration. The addition of biotin to EM2 containing 40 g/L sucrose resulted in a significantly higher frequency of somatic embryos which was comparable to that of 50 g/L sucrose but with a lower germination frequency. Embryo formation was non-synchronous with various stages of embryo development being observed at the same time. When transferred to GM, embryos were closely associated and difficult to separate without damage occurring. After 3 weeks on GM, embryos began to enlarge and turn from translucent to opaque. In the subsequent 2–3 months, they turned green and germinated (Fig. 4a, 4b). At this stage individual plants could be separated (Fig. 5a) and were transferred into 28 mL McCartney bottles containing 10 mL of half-strength MS medium for further development (Fig. 5b). After 1 month in culture, all the plants reached a height of 6–8 cm and appeared phenotypically normal.
Maturation and germination of somatic embryos from suspension cultures of taro (Colocasia esculenta var. esculenta) cv. CPUK. Upon transfer to GM, embryo formation continued while the existing somatic embryos began to turn opaque and then green after 2 months (a). Germination commenced after 2 months (b). Scale bar 2 mm
Discussion
Callus proliferation is an integral part of any efficient regeneration system since it provides a continuous supply of tissue thus reducing the requirement for initiating new cultures. In this study, half-strength MS medium with 1.0 mg/L TDZ, 0.5 mg/L 2,4-D and 800 mg/L glutamine was shown to be a suitable solid medium for callus maintenance as it promoted proliferation whilst maintaining embryogenic capacity. A combination of TDZ and 2,4-D without glutamine induced callus proliferation, however, approximately 40% of callus became soft, watery and non-regenerable. The addition of glutamine at 800 mg/L to the callus maintenance medium increased the proliferation rate while at the same time maintaining the regeneration capacity. Glutamine concentrations higher than 800 mg/L appeared to have a negative effect and reduced both the frequency of proliferation and the mean fresh weight of callus. Glutamine readily increases the amount of available nitrogen which enhances the synthesis of certain macromolecules or metabolites (Ogita et al. 2001) while maintaining inorganic nitrogen at a low concentration. In this study, the effect of glutamine was found to be dependent on whether the media was liquid or solid. For example, embryogenesis was inhibited in cells derived from liquid medium containing 800 mg/L glutamine with all cells turning necrotic after 2–3 weeks following transfer to hormone-free medium. In contrast, the same concentration in solidified callus maintenance medium did not appear to be inhibitory even when transferred to hormone-free medium. The inhibitory effect of glutamine on liquid-cultured cells was avoided by reducing the concentration to 100 mg/L. While glutamine provides nitrogen in an organic form, it is chemically unstable and degrades to release ammonia (Barrett et al. 1997; Gorret et al. 2004). It is possible, therefore, that at concentrations above 100 mg/L, too much glutamine or its degradation products were made available to cells immersed in liquid media.
The inability to initiate suspension cultures using callus taken directly from explants on callus initiation media may have been due to (1) the shock from the physical isolation of callus from the initial explants or alternatively, (2) the characteristics of the callus at this particular stage of development. Transferring callus to CMMS prior to CMML may have provided a transition step for the callus to proliferate and allowed it to adapt to the different media composition. The improved friability of callus cultured on CMMS may also have contributed to the ability of cells to dissociate when agitated in CMML.
In general, highly prolific cultures tend to lose the ability to regenerate more rapidly than slower growing cultures (Ikeda-Iwai et al. 2002). In contrast, rapidly growing taro suspension cells were found to be more regenerable than slower growing cell lines. The highly prolific suspension cultures doubled in cell volume fortnightly and contained a large proportion of cells with embryogenic characteristics, namely cells that were small, cytoplasmically dense, isodiametric in shape and often present in small multicellular clumps. Such cultures were cream/yellow in appearance. When plated on EM, the yellow cell aggregates formed PEMs and SEs and the ability of cells derived from these rapidly growing cell lines to regenerate persisted for over 12 months.
Somatic embryogenesis from callus has been reported in C. esculenta var. esculenta using hormone-free media (Deo et al. 2009). In this present study, regeneration from suspension cells required successive steps. In contrast to callus taken directly from corm slices (Deo et al. 2009), no embryos formed on hormone-free medium using cells from suspension culture. However, when suspension cells were plated on embryogenesis medium (EM) containing 0.1 mg/L TDZ and 0.05 mg/L 2,4-D, they proliferated and formed PEMs with globular somatic embryos forming on their surface. One of the critical events leading to the formation of somatic embryos is the establishment of cell polarity, which can result from an auxin concentration gradient when callus is transferred to medium with low or no auxin (Souter and Lindsey 2000). Such a gradient may be established as a result of endogenous auxin synthesis or by the provision of exogenous auxin (Ribnicky et al. 1996). It would appear that taro suspension cells could not synthesize and accumulate the required level of endogenous IAA and/or other cellular metabolites required for embryo formation. Consequently, the application of very low concentrations of exogenous 2,4-D and TDZ was necessary. Both of these growth regulators have been reported to modulate endogenous auxin (Visser et al. 1992; Ribnicky et al. 1996; Panaia et al. 2004).
An increase in sucrose concentrations (up to 50 g/L) in the embryogenesis medium enhanced the frequency of somatic embryogenesis from suspension cells. A high frequency of embryogenesis at high sucrose concentrations has also been reported in maize (Kamo et al. 1985), cucumber (Lou and Kako 1995), sugar cane (de los Blanco et al. 1999; Gandonou et al. 2005) and melon (Nakagawa et al. 2001). At these concentrations, the action of sucrose is likely to be as an osmoticum or other developmental regulator rather than solely a carbon source. The combination of biotin (1.0 mg/L) with sucrose (40 g/L) increased embryo formation by 2.6-fold when compared with the numbers formed on 40 g/L sucrose alone. Biotin is important in carboxylation reactions and regulating genes involved in synthesis of some fatty acids, and development of plant embryos (Wurtele and Nikolau 1992). The stimulating effect of biotin on embryogenesis has also been reported in date palm (Al-Khayri 2001) and carrot (Wurtele and Nikolau 1992). Although the use of biotin in this present study increased the number of somatic embryos formed, the germination rate was lower than using 50 g/L sucrose alone indicating high sucrose was important for both embryo formation and maturation leading to a higher germination rate.
Following embryo development and early maturation, further maturation and germination was achieved by complete removal of 2,4-D and TDZ from the media while maintaining very low concentrations of BA (0.05 mg/L) and IAA (0.1 mg/L). However, the highest germination rate was 58% indicating that there is further scope for improving regeneration by modifications to EM and/or GM.
In summary, two effective callus maintenance media (CMMS and CMML) for taro were developed and embryogenic callus could be proliferated for over a year without losing regenerability. The highly regenerable and rapidly growing nature of suspension cell cultures represents an ideal target tissue for the genetic transformation and mass propagation of this plant.
References
Abo El-Nil MM, Zettler FW (1976) Callus initiation and organ differentiation from shoot tip cultures of Colocasia esculenta. Plant Sci Lett 6:401–408
Al-Khayri JM (2001) Optimization of biotin and thiamine requirements for somatic embryogenesis of date palm (Phoenix dactylifera L.). In Vitro Cell Dev Biol Plant 37:453–456
Barrett JD, Park YS, Bonga JM (1997) The effectiveness of various nitrogen sources in white spruce [Picea glauca (Moench) Voss] somatic embryogenesis. Plant Cell Rep 16:411–415
de los Blanco MA, Segura-Nieto M, Castillo R, Nieves N (1999) Storage proteins in sugarcane: an interesting exception in monocots. Plant Cell Tissue Organ Cult 59:217–218
Deo PC, Harding RM, Taylor M, Tyagi AP, Becker DK (2009) Somatic embryogenesis, organogenesis and plant regeneration in taro (Colocasia esculenta var. esculenta). Plant Cell Tissue Organ Cult 99:61–71
Gandonou C, Errabii T, Abrini J, Idaomar M, Chibi F, Senhaji NS (2005) Effect of genotype on callus induction and plant regeneration from leaf explants of sugarcane (Saccharum spp.). Afr J Biotechnol 4:1250–1255
Gorret N, bin Rosli SK, Oppenheim SF, Wallis LB, Lessard DA, Rha CK, Sinskey AJ (2004) Bioreacter culture of oil palm (Elaeis guimensis) and effects of nitrogen source, inoculum size and conditioned medium on biomass production. J Biotechnol 108:253–263
Ikeda-Iwai M, Satoh S, Kamada H (2002) Establishment of a reproducible tissue culture system for the induction of Arabidopsis somatic embryos. J Exp Bot 53:1575–1580
Ivancic A (1992) Breeding and genetics of taro (Colocasia esculenta (L.) Schott). Ministry of Agriculture and Lands, Solomon Islands UNDP, Food and Agriculture Organizations of the United Nations, pp 1–97
Kamo K, Becwar MR, Hodges TK (1985) Regeneration of Zea mays L. from embryogenic callus. Bot Gaz 146:327–334
Lou H, Kako S (1995) Role of high sugar concentrations in inducing somatic embryogenesis from cucumber cotyledons. Sci Hortic 64:11–20
Nakagawa H, Saijyo T, Yamauchi N, Shigyo M, Kako S, Ito A (2001) Effects of sugars and abscisic acid on somatic embryogenesis from melon (Cucumis melo L.) expanded cotyledon. Sci Hortic 90:85–92
Ogita S, Sasamoto H, Yeung EC, Thorpe TA (2001) The effects of glutamine on the maintenance of embryogenic cultures of Cryptomeria japonica. In Vitro Cell Dev Biol Plant 37:268–273
Panaia M, Senaratma T, Dixon KW, Sivasithamparam K (2004) The role of cytokinins and thidiazuron in the stimulation of somatic embryogenesis in key members of the Restionaceae. Aust J Bot 52:257–265
Ribnicky DM, Ilić N, Cohen JD, Cooke TJ (1996) The effects of exogenous auxins on endogenous indole-3-acetic acid metabolism. Plant Physiol 112:549–558
Souter M, Lindsey K (2000) Polarity and signaling in plant embryogenesis. J Exp Bot 51:971–983
Strauss MS, Michaud JD, Arditti J (1979) Seed storage and germination and seedling proliferation in taro (Colocasia esculenta (L.) Schott). Ann Bot 43:603–612
Visser C, Qureshi JA, Gill R, Saxena PK (1992) Morphoregulatory role of thidiazuron- substitution of auxin and cytokinin requirement for the induction of somatic embryogenesis in geranium hypocotyl cultures. Plant Physiol 99:1704–1707
Wilson JE (1990) Agro facts, taro breeding. IRETA Publication No: 3/89
Wurtele ES, Nikolau BJ (1992) Differential accumulation of biotin enzymes during carrot somatic embryogenesis. Plant Physiol 99:1699–1703
Yam TW, Young JLP, Fan KPL, Arditti J (1990) Induction of callus from axillary buds of taro (Colocasia esculenta var. esculenta, Araceae) and subsequent plant regeneration. Plant Cell Rep 9:459–462
Acknowledgments
The authors wish to thank New Zealand’s International Aid and Development Agency and The University of the South Pacific for their financial support, and the Centre for Tropical Crops and Biocommodities (Queensland University of Technology) and the Secretariat of the Pacific Community for their provision of facilities and technical support during this project. PCD was a PhD candidate at The University of the South Pacific.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deo, P.C., Taylor, M., Harding, R.M. et al. Initiation of embryogenic cell suspensions of taro (Colocasia esculenta var. esculenta) and plant regeneration. Plant Cell Tiss Organ Cult 100, 283–291 (2010). https://doi.org/10.1007/s11240-009-9648-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9648-1