Abstract
Lizards in the genus Heloderma are the most ancient venomous reptiles, with a traceable lineage nearly 100 million years old. The proteome of the venom of three of the remaining species (Heloderma suspectum, H. exasperatum, H. horridum) are very conserved, with kallikrein-like activity present to cause critical hypotension to immobilize and outright kill prey. Kallikrein-like activity would be expected to activate the contact protein pathway of coagulation, which would be detectable with thrombelastography in human plasma. Thus, it was proposed to determine if kallikrein-like activity could be detected with thrombelastography, and if this activity could be inhibited by carbon monoxide (CO) via a putative heme-based mechanism. Procoagulant activity of each venom was assessed via thrombelastography with normal plasma, and kallikrein-like activity confirmed with FX-depleted plasma. Venom was then exposed to carbon monoxide releasing molecule-2 (CORM-2) or its inactive releasing molecule to assess CO inhibition. All three venoms demonstrated kallikrein-like activity with the same potency and inhibition of activity by CO. In conclusion, the present work documented that procoagulant, kallikrein-like activity containing venoms of the oldest species of venomous reptiles was inhibited by CO, potentially via heme modulation. This is also the first identification and characterization of a kallikrein-like enzyme utilizing coagulation factor-depleted plasma to assess venom that inflicts hypotension. Future investigations will continue to define the vulnerability of venom enzymatic activities to CO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Heloderma species are the most ancient venomous reptiles, with little phenotypical change in nearly 100 million years.
-
Heloderma venoms contain kallikrein-like activities used to cause hypotension in prey that could affect human plasmatic coagulation.
-
Using thrombelastography, it was demonstrated that carbon monoxide inhibited procoagulant, kallikrein-like activity in venoms obtained from Heloderma species.
-
Vulnerability of venom enzymatic activity to carbon monoxide inhibition via likely heme modulation is thus one of the oldest modifications to venom.
Introduction
There are only a few remaining species of venomous lizards of the genus Heloderma, a clade of species with an origin nearly a 100 million years old as recently reviewed [1,2,3,4]. Of these, three particular species occupy overlapping areas in the southwestern United States and Central America: Heloderma suspectum (Gila monster), H. exasperatum (Rio Fuerte beaded lizard) and H. horridum (Mexican beaded lizard). H. suspectum is found in the southwestern United States and Northern Mexico, H. horridum is located from central Mexico to Guatemala, and H. exasperatum is distributed within and between the other species geographically. While in general these lizards are not considered a remarkable human threat secondary to their relatively placid nature, their venom proteomes are capable of inflicting significant morbidity as recently reviewed [5]. Specifically, their venom contains: (1) cysteine rich secretory proteins (CRiSP) that induce hypothermia; (2) exendins that are myocardial depressants; (3) helofensins, a compound that inhibits diaphragm activity and causes respiratory compromise; (4) kallikrein-like enzymes that increase vascular permeability and inflict hypotension; and, (5) phospholipase A2 (Type III) that inhibits platelet activity [5]. When summated, these venom enzymes immobilize and kill their prey as well as serve as a defense against would be predators [5,6,7,8,9]. The proteomes of these three species’ venoms are remarkably conserved despite having several million years of divergent evolution between them as noted [1,2,3, 5], with the venoms containing species-specific proportions of kallikrein-like and phospholipase A2 (PLA2) activities [5, 6]. Critically, it the kallikrein-like and PLA2 activities contained in these venoms that would be expected to affect coagulation in vitro and in vivo. In summary, the venom proteomes of these organisms are the most ancient among reptiles.
Given the previously mentioned information, Heloderma venom contains enzymes that would be expected to affect plasmatic coagulation based on fundamental biochemistry [10, 11]. With regard to kallikrein-like activity, this class of enzyme would be anticipated to cleave factor XII (FXII) to its activated form (FXIIa), which in turn converts factor XI (FXI) to its activated form (FXIa) that subsequently continues to activate the serine protease-dominated contact protein pathway to ultimately form thrombin [10, 11]. Further, a feed-forward activation of human prekallikrein by FXIIa would result in formation of human kallikrein that in turn activates more FXII [10, 11]. Lastly, FXIa also contributes to this feed-forward, thrombin-generating system by activating FXII [10, 11]. Of interest, the thrombelastographic effects of progressive increases in FXII activity in FXII-deficient plasma have been documented [12], characterized by decreased time of onset of coagulation and increased velocity of coagulation as FXII activity increases to normal. Importantly, if plasma is depleted of FXI, FXIIa mediated coagulation is markedly delayed [12]. In contrast to this anticipated procoagulant effect by Heloderma venom, phospholipase A2 activity in human plasma would be posited to act as an anticoagulant by digesting key plasma lipids and decreasing thrombin generation, as was recently demonstrated thrombelastographically with a purified, isolated snake venom phospholipase A2 [13]. Depending on which enzyme class, kallikrein-like or phospholipase A2, has a greater velocity of catalysis, the crude venom may be either procoagulant or anticoagulant in nature in human plasma. As a major focus of our laboratory has been the assessment of potential heme modulation of snake venom enzymes that affect human coagulation [14,15,16,17,18,19,20,21,22,23], we thought it would of great interest to determine if such modulation was present in arguably the most evolutionarily ancient venoms available in Heloderma species.
Considering the aforementioned, the purposes of the present work were to: (1) perform a thrombelastographic analysis to define in human plasma the procoagulant or anticoagulant effects on coagulation kinetics of Heloderma venom activities; (2) determine if carbon monoxide (CO) inhibits these venom mediated effects; and (3) use coagulation factor XI depleted human plasma to separate kallikrein-like activity from other venom mediated effects on coagulation if the venoms were procoagulant in nature.
Materials and methods
Venoms and human plasma
Lyophilized venoms collected from Heloderma species were obtained from Mtoxins (Oshkosh, WI, USA). Venoms were dissolved into calcium-free phosphate buffered saline (PBS, Sigma-Aldrich, Saint Louis, MO, USA) to a final 50 mg/ml concentration, aliquoted, and maintained at − 80 °C. CORM-2 (tricarbonyldichlororuthenium (II) dimer, a CO releasing molecule) and dimethyl sulfoxide (DMSO) were acquired from Sigma-Aldrich. Pooled normal human plasma (George King Bio-Medical, Overland Park, KS, USA) that was sodium citrate anticoagulated and maintained at − 80 °C was used in experiments involving assessment of venom kallikrein-like activity. Factor-XI depleted (< 0.05 IU/ml) sodium citrate anticoagulated human plasma (Affinity Biologicals, Inc., Ancaster, Ontario, Canada) was used to verify that the procoagulant activity of the venom was kallikrein-like and to assess other venom mediated effects on plasmatic coagulation without the interference of kallikrein-like activity effects.
Thrombelastographic analyses
In preliminary experiments it was determined that the venoms from all three lizards were procoagulant, so a variety of different sample compositions in the two different aforementioned plasmas were utilized as subsequently presented.
In the series of experiments assessing kallikrein-like activity, the samples were composed of 320 µl of plasma; 16.4 µl of PBS, 20 µl of 200 mM CaCl2, and 3.6 µl of PBS or venom mixture. These sample mixtures were pipetted into a disposable cup in a thrombelastograph® hemostasis system (Model 5000, Haemonetics Inc., Braintree, MA, USA) at 37 °C, and then rapidly mixed by moving the cup up against and then away from the plastic pin five times. The following viscoelastic parameters described previously [13,14,15,16,17,18,19,20,21,22,23] were measured: time to maximum rate of thrombus generation (TMRTG): this is the time interval (minutes) observed prior to maximum speed of clot growth; maximum rate of thrombus generation (MRTG): this is the maximum velocity of clot growth observed (dynes/cm2/s); and total thrombus generation (TTG, dynes/cm2), the final viscoelastic resistance observed after clot formation. Data were collected for 15 min.
In the various experiments assessing venom mediated effects on coagulation that did not involve kallikrein-like activity, some involved the same plasma sample mixture proportions as in the aforementioned experiments but with a data collection time of 30 min, using both normal plasma and FXI-depleted plasma. In a series to assess the potential effects of venom PLA2 activity on coagulation kinetics, sample mixtures containing 320 µl of FXI-depleted plasma 6.4 µl of PBS, 20 µl of 200 mM CaCl2, and 3.6 µl of PBS or venom mixture were incubated in thrombelastographic cups at 37 for 15 min. This was done as the PLA2 of this venom is calcium-dependent [8]. Then, to initiate coagulation, 10 µl of tissue factor reagent (1:1000 final concentration, Diagnostica Stago S.A.S., Asnieres sur Seine, France) was added to the mixture, with the sample mixed and data collected for 15 min.
The initial concentration for all venoms assessed was 1 µg/ml; if the time to commencement and velocity of coagulation was procoagulant with onset of coagulation beginning in half the time or less and/or the speed of clot formation proceeded at two-times or greater than plasma without venom addition, this concentration of venom was used. If this did not occur, then the concentration of venom was gradually increased until these conditions were met. If, in contrast, the venom was anticoagulant in nature, the onset of coagulation had to be twice as long in duration and/or the velocity of clot formation half of plasma without venom addition to be acceptable for investigation. However, if coagulation was not detectable, then the concentration of anticoagulant venom was progressively diminished until at least detectable coagulation occurred; this concentration was then used. In sum, this approach permitted comparison of relative potencies of the venoms and allowed the determination of the predominant effect of a specific venom on coagulation.
CO exposures
As for exposure to CORM-2 to assess the effects of CO on venom activity, the subsequent four experimental conditions were utilized: (1) control condition—no venom, DMSO 1% addition (v/v) in PBS; (2) venom condition—venom, DMSO 1% addition (v/v) in PBS; (3) CO condition—venom, CORM-2 1% addition in DMSO (100–1000 µM final concentration); (4) inactive releasing molecule (iRM) condition—venom, inactivated CORM-2 1% addition in DMSO (100–1000 µM final concentration). CORM-2 was inactivated as previously described [13,14,15,16]. Venom was added to PBS with the described additions, incubated for 5 min at room temperature, and then 3.6 µl of one of these solutions was added to the plasma sample in the plastic cup.
Statistical analyses
Data are presented as mean ± SD. Experimental conditions were composed of n = 6 replicates per condition as this provides a statistical power > 0.8 with P < 0.05 utilizing these techniques [13,14,15,16,17,18,19,20,21]. Graphics were generated with a commercially available program (Origen Pro 2018, OrigenLab Corporation, Northampton, MA, USA). A statistical program was used for one-way analyses of variance (ANOVA) comparisons between conditions, followed by Holm-Sidak post hoc analysis or unpaired, or two-tailed Student’s t-tests as appropriate (SigmaPlot 14, Systat Software, Inc., San Jose, CA, USA). P < 0.05 was considered significant.
Results
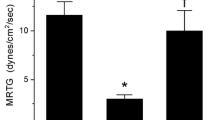
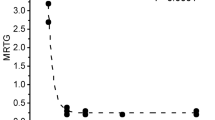
Progressive concentration trials of the three venoms demonstrated a procoagulant nature, with a thrombelastographic coagulation kinetic profile consistent with increasing FXII activation [12]. Of interest, the venoms were also equipotent, with a concentration of 10 µg/ml providing very similar degrees of procoagulation as can be seen in Figs. 1, 2 and 3. The TMRTG values were significantly decreased and MRTG values increased without significant change in TTG compared to control plasma values, which is a pattern diagnostic for thrombin-generating venoms that do not directly interact with fibrinogen alone [15, 16, 19]. Exposure to CORM-2 at a concentration of 100 µM had no effect on the procoagulant activity of any of the three venoms in preliminary work, so an exposure to of 1000 µM CORM-2 was performed, with all three venoms demonstrating significant inhibition of procoagulant activity. Lastly, exposure to 1000 µM iRM had no affect on venom mediated procoagulation, demonstrating that CO inhibited all three venoms’ mediated changes in coagulation.
Effects of Heloderma suspectum venom on plasmatic coagulation kinetics. Data are represented by mean ± SD. The final venom concentration was 10 µg/ml. Control no venom or other additions, V venom addition without exposure to other compounds, V/CO venom addition after exposure to CORM-2, V/iRM venom addition after exposure to inactivated CORM-2. Venom was exposed in isolation to 1000 µM CORM-2 and iRM. All venom mixtures were 1% (v/v) additions to plasma mixtures. TMRTG time to maximum rate of thrombus generation (min), MRTG maximum rate of thrombus generation (dynes/cm2/s), TTG total thrombus generation (dynes/cm2). *P < 0.05 versus control, †P < 0.05 versus V, ‡P < 0.05 versus V/CO
Effects of Heloderma exasperatum venom on plasmatic coagulation kinetics. Data are represented by mean ± SD. The final venom concentration was 10 µg/ml. Control no venom or other additions, V venom addition without exposure to other compounds, V/CO venom addition after exposure to CORM-2, V/iRM venom addition after exposure to inactivated CORM-2. Venom was exposed in isolation to 1000 µM CORM-2 and iRM. All venom mixtures were 1% (v/v) additions to plasma mixtures. TMRTG time to maximum rate of thrombus generation (min), MRTG maximum rate of thrombus generation (dynes/cm2/s), TTG total thrombus generation (dynes/cm2). *P < 0.05 versus control, †P < 0.05 versus V, ‡P < 0.05 versus V/CO
Effects of Heloderma horridum venom on plasmatic coagulation kinetics. Data are represented by mean ± SD. The final venom concentration was 10 µg/ml. Control no venom or other additions, V venom addition without exposure to other compounds, V/CO venom addition after exposure to CORM-2, V/iRM venom addition after exposure to inactivated CORM-2. Venom was exposed in isolation to 1000 µM CORM-2 and iRM. All venom mixtures were 1% (v/v) additions to plasma mixtures. TMRTG time to maximum rate of thrombus generation (min), MRTG maximum rate of thrombus generation (dynes/cm2/s), TTG total thrombus generation (dynes/cm2). *P < 0.05 versus control, †P < 0.05 versus V, ‡P < 0.05 versus V/CO
In order to demonstrate that the control plasma had typical coagulation kinetic values, six more replicate experiments were performed with data collected for 30 min to permit maximum clot strength to occur. The values for this data set were TMRTG = 16.3 ± 1.3, MRTG = 2.6 ± 0.6 dynes/cm2/s, and TTG = 179 ± 15 dynes/cm2.
In order to determine that there was no influence from the presumed kallikrein-like activity within the three venoms on results obtained with FXI-depleted plasma, the following control experiments were performed. First, FXI-depleted plasma that was recalcified demonstrated no thrombelastographic signs of coagulation for 30 min (n = 3 replicates), showing that the amount of FXII activation caused by contact of the plasma with the surfaces of the cup and pin was insufficient to engage the FXI activity remaining to form thrombin. Next, each of the three venoms (n = 2 replicates per venom) at a concentration of 40 µg/ml (the maximum concentration used by our laboratory) were placed into FXI-depleted plasma and data collected for 30 min. Again, there was no sign of coagulation thrombelastographically, demonstrating that venom-contact protein interaction proximate to FXI could not cause thrombin generation. These results provide two conclusions, that the venom will not affect coagulation events remote from FXI, and that the procoagulant effect must be mediated by kallikrein-like activity based on the proteomes of the venoms.
Preliminary work with all three venoms in FXI-depleted plasma demonstrated no anticoagulant effect up to a concentration of 40 µg/ml as could be anticipated from a known proteomic presence of PLA2 activity [5, 6, 8], considering the anticoagulant effect of a snake PLA2 activity recently described [13]. Instead, it appeared that there was a venom mediated enhancement of MRTG in all three venoms compared to control plasma condition. This effect was formally investigated with Heloderma suspectum venom and the results are displayed in Fig. 4. This venom significantly increased MRTG values after TF activation of coagulation, and exposure of the venom to CORM-2 had no effect on this phenomenon. Lastly, TTG values were significantly increased in samples with CORM-2 exposed venom added compared to control plasma values. Since there was no significant decrease in venom mediated effects on MRTG and only small changes in TTG following CORM-2 exposure, it was decided that an iRM condition would not be of any particular use in interpreting these data.
Effects of Heloderma suspectum venom on coagulation kinetics in FXI-depleted plasma. Data are represented by mean ± SD. The final venom concentration was 40 µg/ml. Control no venom or other additions, V venom addition without exposure to other compounds; V/CO venom addition after exposure to CORM-2. Venom was exposed in isolation to 1000 µM CORM-2. All venom mixtures were 1% (v/v) additions to plasma mixtures. TMRTG time to maximum rate of thrombus generation (min), MRTG maximum rate of thrombus generation (dynes/cm2/s), TTG total thrombus generation (dynes/cm2). *P < 0.05 versus control
Discussion
The primary findings of this study were that Heloderma venom effects are procoagulant in nature, are driven by kallikrein-like activity, and are inhibitable by CO. The most significant aspect of these findings is that it is likely that heme modulation of venom enzymes was a very early posttranslational modification in the evolution of venom, considering that this clade of reptiles has had little change phenotypically for nearly 100 million years [1,2,3,4]. To put this in a different perspective, CO mediated inhibition as a potential mechanism of controlling venom activity predates the development of upper jaw fangs and specialized venom glands as seen in the thousands of advanced snake species, as Heloderma species release venom from lower jaw glands which passively travels up external grooves in their teeth to enter the bite wound of their prey [4]. In contrast, we were unable to assess PLA2 activity from this venom with our system, which would have been predicted to be anticoagulant in nature based on our recent work [13]. This may be due to snake venom PLA2 belonging in class II of this enzyme type [24], whereas Heloderma species PLA2, which decrease platelet aggregation, belong to class III [6, 8, 9]. Perhaps there is a difference in affinity for catalysis of phospholipids in human plasma based on PLA2 class that has not been previously noted; while interesting, this question is beyond the scope of the present work. In sum, our data demonstrates that likely heme mediated inhibition via CO exposure was responsible for decreasing the venom activity derived from the most ancient venomous reptiles yet living.
Another matter to consider is that we assessed the activity and inhibition of a toxin (kallikrein-like activity) that is deadly to prey animals via infliction of severe hypotension [7, 9] via bradykinin formation with a coagulation kinetic assessment technology. Heloderma venom kallikrein-like activity also causes hemorrhagic exophthalmia with bleeding behind and into the eye, as well as into other internal organs [7]. We were fortunate that the kallikrein-like activity in Heloderma venom was the only likely procoagulant agent present, and the ability to identify it by knowing the proteomes involved and confirming kallikrein-specific activity with FXI-depleted plasma controls. Many advanced snake venoms contain several thrombin-generating compounds that may include kallikrein-like activity but may also include factor X and factor II activating enzymes [15,16,17], making differentiation of contribution of specific enzyme types via thrombelastography problematic. Thus, by exploiting the biochemical cross talk that kallikrein has between the contact protein pathway of coagulation and the kallikrein-kinin system, we were able to determine that a very significant toxin that inflicts damage outside of changes in coagulation was inhibited by CO.
With regard to the procoagulant effect exerted by Heloderma suspectum venom in FXI-depleted, TF activated plasma, we do not have any molecular “suspects” based on the published proteome of this specie’s venom [5, 6, 9]. There is hyaluronidase toxin, helofensins and exendins in Heloderma species’ venom that adversely affect prey but are not known to affect coagulation [5, 6, 9]. Whatever the procoagulant agent of these venoms are under the aforementioned conditions, it is only measurable in circumstances of large concentrations of venom, and it is not inhibited by CO. Again, while an interesting finding, the biochemical identification of this procoagulant is beyond the scope of the present manuscript.
Given the infrequency of envenomation by these lizards, an antivenom is not available and therapy is supportive in general. However, the nature of this morbid event is very different than that observed with snakes and the circumstances of such a bite and its potential treatment with CORMs worthy of consideration. Using the Gila monster as an example, a species occasionally encountered by the first author, a bite is typically provoked, with the lizard maintaining its grinding bite until it is incapacitated humanly by first responders (by immersing the animal in water causing it to open its mouth). Depending on the duration of the bite and the size of the victim, local and systemic illness will be mild to potentially severe even with rapid medical care. Looking forward, cleaning the wound and applying CO releasing compounds in topical gel form may be anticipated to inhibit at least the kallikrein-like activity locally, and perhaps the inactivated enzyme could be cleared from the circulation as it is released without inflicting hypotension [7, 9]. In sum, such an approach may be of therapeutic utilization in Heloderma bites, and bites from related species such as Lanthantus and Varanus lizards [25].
In conclusion, the present work documented that procoagulant, kallikrein-like activity containing venoms of the oldest species of venomous reptiles was likely heme modulated as it was inhibited by CO. As we have posited in the past, endogenous CO production may be used by vipers (and now lizards) to protect themselves from the effects of their own venom [15, 16, 19]. Heme modulation may also be a mechanism to attenuate the effects of envenomation, or to modulate venom derived enzymes used medicinally [18, 23]. Future investigations will continue to define the endogenous protective functions and potentially therapeutic aspects of heme modulated venom enzymatic activities.
References
Beaman KR, Beck DD, McGurty BM (2006) The beaded lizard (Heloderma horridum) and gila monster (Heloderma suspectum): a bibliography of the family Helodermatidae. Smithson Herpetol Inf Serv 136:1–66
Fry BG, Vidal N, Norman JA, Vonk FJ, Scheib H, Ramjan SF, Kuruppu S, Fung K, Hedges SB, Richardson MK, Hodgson WC, Ignjatovic V (2006) Early evolution of the venom system in lizards and snakes. Nature 439:584–588
Douglas ME, Douglas MR, Schuett GW, Beck DD, Sullivan BK (2010) Conservation phylogenetics of helodermatid lizards using multiple molecular markers and a supertree approach. Mol Phylogenet Evol 55:153–167
Reiserer RS, Schuett GW, Beck DD (2013) Taxonomic reassessment and conservation status of the beaded lizard, Heloderma horridum (Squamata: Helodermatidae). Amphib Reptile Conserv 7:74–96
Koludarov I, Jackson TN, Sunagar K, Nouwens A, Hendrikx I, Fry BG (2014) Fossilized venom: the unusually conserved venom profiles of Heloderma species (beaded lizards and gila monsters). Toxins 6:3582–3595
Sanggaard KW, Dyrlund TF, Thomsen LR, Nielsen TA, Brøndum L, Wang T, Thøgersen IB, Enghild JJ (2015) Characterization of the gila monster (Heloderma suspectum suspectum) venom proteome. J Proteom 117:1–11
Alagon A, Possani LD, Smart J, Schleuning WD (1986) Helodermatine, a kallikrein-like, hypotensive enzyme from the venom of Heloderma horridum horridum (Mexican beaded lizard). J Exp Med 164:1835–1845
Sosa BP, Alagón AC, Martin BM, Possani LD (1986) Biochemical characterization of the phospholipase A2 purified from the venom of the Mexican beaded lizard (Heloderma horridum horridum Wiegmann). Biochemistry 25:2927–2933
Datta G, Tu AT (1997) Structure and other chemical characterizations of gila toxin, a lethal toxin from lizard venom. J Pept Res 50:443–450
Ivanov I, Matafonov A, Sun MF, Cheng Q, Dickeson SK, Verhamme M, Emsley J, Gailani D (2017) Proteolytic properties of single-chain factor XII: a mechanism for triggering contact activation. Blood 129:1527–1537
Mohammed BM, Matafonov A, Ivanov I, Sun MF, Cheng Q, Dickeson SK, Li C, Sun D, Verhamme IM, Emsley J, Gailani D (2018) An update on factor XI structure and function. Thromb Res 161:94–105
Nielsen VG, Cohen BM, Cohen E (2005) Effects of coagulation factor deficiency on plasma coagulation kinetics determined via thrombelastography: critical roles of fibrinogen and factors II, VII, X and XII. Acta Anaesthesiol Scand 49:222–231
Nielsen VG (2019) Carbon monoxide inhibits the anticoagulant activity of phospholipase A2 purified from Crotalus adamanteus venom. J Thromb Thrombolysis 47:73–79
Nielsen VG, Cerruti MA, Valencia OM, Amos Q (2016) Decreased snake venom metalloproteinase effects via inhibition of enzyme and modification of fibrinogen. Biometals 29:913–919
Nielsen VG, Frank N (2019) Role of heme modulation in inhibition of Atheris, Atractaspis, Causus, Cerastes, Echis, and Macrovipera hemotoxic venom activity. Hum Exp Toxicol 38:216–226
Nielsen VG, Frank N, Matika RW (2018) Carbon monoxide inhibits hemotoxic activity of Elapidae venoms: potential role of heme. Biometals 31:51–59
Nielsen VG, Bazzell CM (2016) Carbon monoxide attenuates the effects of snake venoms containing metalloproteinases with fibrinogenase or thrombin-like activity on plasmatic coagulation. MedChemComm 7:1973–1979
Nielsen VG, Bazzell CM (2017) Carbon monoxide releasing molecule-2 inhibition of snake venom thrombin-like activity: novel biochemical “brake”? J Thromb Thrombolysis 43:203–208
Nielsen VG, Frank N (2018) Differential heme-mediated modulation of Deinagkistrodon, Dispholidus, Protobothrops and Pseudonaja hemotoxic venom activity in human plasma. Biometals 31:951–959
Nielsen VG, Frank N, Matika RW (2018) Effects of heme modulation on Ovophis and Trimeresurus venom activity in human plasma. Toxins (Basel) 10:E322
Nielsen VG, Losada PA (2017) Direct inhibitory effects of carbon monoxide on six venoms containing fibrinogenolytic metalloproteinases. Basic Clin Pharmacol Toxicol 120:207–212
Suntravat M, Langlais PR, Sánchez EE, Nielsen VG (2018) CatroxMP-II: a heme-modulated fibrinogenolytic metalloproteinase isolated from Crotalus atrox venom. Biometals 31:585–593
Nielsen VG (2018) Crotalus atrox venom exposed to carbon monoxide has decreased fibrinogenolytic activity in vivo in rabbits. Basic Clin Pharmacol Toxicol 122:82–86
Murakami M, Taketomi Y, Sato H, Yamamoto K (2011) Secreted phospholipase A2 revisited. J Biochem 150:233–255
Koludarov I, Jackson TN, Brouw BOD, Dobson J, Dashevsky D, Arbuckle K, Clemente CJ, Stockdale EJ, Cochran C, Debono J, Stephens C, Panagides N, Li B, Manchadi MR, Violette A, Fourmy R, Hendrikx I, Nouwens A, Clements J, Martelli P, Kwok HF, Fry BG (2017) Enter the dragon: the dynamic and multifunctional evolution of Anguimorpha lizard venoms. Toxins (Basel) 9:E242
Acknowledgements
We appreciate the technical assistance of Sarah A. Nielsen in the conduct of the thrombelastographic assays.
Funding
This investigation was supported by the Department of Anesthesiology, College of Medicine, at the University of Arizona.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This was an in vitro investigation and did not involve any living subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nielsen, V.G., Frank, N. The kallikrein-like activity of Heloderma venom is inhibited by carbon monoxide. J Thromb Thrombolysis 47, 533–539 (2019). https://doi.org/10.1007/s11239-019-01853-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-019-01853-6