Abstract
Snake venom contains a myriad of classes of enzyme which have been investigated for medicinal and toxinological purposes, including phospholipase A2 (PLA2), which is responsible for anticoagulant, myotoxic and neurotoxic effects. Given the importance of PLA2, the purposes of the present investigation were to characterize the coagulation kinetic behavior of a PLA2 purified from Crotalus adamanteus venom (Ca-PLA2) in human plasma with thrombelastography and determine if carbon monoxide could inhibit its activity. Coagulation kinetics were determined in human plasma with a range of Ca-PLA2 activity (0–2 U/ml) via thrombelastography. Then, using carbon monoxide releasing molecule-2 or its inactivated molecule (0 or 100 µM), the vulnerability of Ca-PLA2 activity to carbon monoxide mediated inhibition was assessed. Lastly, the inhibitory response of Ca-PLA2 activity to exposure to carbon monoxide releasing molecule-2 (0–100 µM) was determined. Ca-PLA2 activity degraded the velocity of clot growth and clot strength in an activity dependent, exponential manner. Carbon monoxide inhibited Ca-PLA2 activity in a concentration dependent fashion, with loss of detectable activity at 100 µM of carbon monoxide releasing molecule-2. These findings, while preliminary, open the possibility that other PLA2 contained in snake venom with multiple toxicities (e.g., myotoxin, neurotoxin) may be heme bearing and CO-inhibitable, which have profound potential basic and clinical science implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Snake venom phospholipase A2 enzymes (PLA2) play key roles in inflammation and toxicity.
-
PLA2 are involved in processes such as local tissue destruction and neuromuscular blockade causing respiratory arrest.
-
Using thrombelastography, it was demonstrated that carbon monoxide inhibited a PLA2 derived from the Eastern diamondback rattlesnake.
-
Vulnerability to carbon monoxide inhibition is the sine qua non of heme bearing enzymes.
-
If these preliminary observations are broadly applicable, then they have profound potential implications concerning the investigation and management of PLA2 toxicity.
Introduction
Snake venom contains a myriad of hemostatically active enzymes and proteins which have been investigated for medicinal and toxinological purposes [1]. These proteins may be procoagulant in nature, directly or indirectly activating thrombin or via thrombin-like activity; conversely, venom can contain proteins that inflict an anticoagulant effect by inhibiting platelet activity, destroying coagulation proteins, or degrading lipids critical to thrombin generation [1]. One particularly important type of snake venom enzyme is phospholipase A2 (PLA2), which is responsible for anticoagulant, myotoxic and neurotoxic effects [2]. PLA2 are calcium-dependent and hydrolyze glycerophospholipids at the sn-2 position of the glycerol backbone, freeing fatty acids and lysophospholipids [2], such as those found in plasma phospholipids that are critical to coagulation [3, 4]. Taken as a whole, while PLA2 are typically site-directed in mediating their whole organism toxicity, they can affect plasmatic coagulation by destroying circulating phospholipids in vitro and in vivo.
As an example of such snake venom PLA2 activity effects, an isolated, purified protein from Naja sputatrix was demonstrated to be anticoagulant in nature in platelet poor rabbit plasma four decades ago [5]. However, the anticoagulant activity was demonstrated with a crude kaolin-cephalin clotting time method that provided little insight into PLA2 mediated effects on coagulation [5]. Of interest, our laboratory has demonstrated with thrombelastographic methods that four diverse Naja species venoms possessed activity that is anticoagulant and carbon monoxide (CO) inhibitable [6, 7]. During the conduct of these investigations [6, 7] it was posited that the Naja species’ venoms exerted their anticoagulant effects via snake venom metalloproteinase (SVMP) or serine protease (SVSP) activity as the thrombelastographic pattern was similar to that seen after exposure of plasma to venom derived from vipers with fibrinogenolytic SVMP/SVSP [8, 9]. However, proteomic analyses of the Naja species’ venoms we assessed via thrombelastography [6, 7] have been demonstrated to have up to ten-fold more PLA2 activity than SVMP/SVSP activity [10,11,12]. Given that it has been demonstrated that a purified, isolated SVMP that was fibrinogenolytic was further determined to be heme bearing and CO inhibitable [13], it may also be possible that PLA2 activity may have been inhibited by CO in Naja species venom by a similar mechanism given the degree of whole venom activity inhibition [6, 7]. In summary, another class of snake venom enzyme, PLA2, may be inhibited by CO.
The goals of the present investigation were to characterize the coagulation kinetic behavior of a purified snake venom PLA2 in human plasma with thrombelastography and determine if CO could inhibit its activity. As a secondary goal, the inhibitory effects of the PLA2 activity inhibitor, quinacrine [14,15,16], was assessed in this system to potentially assist in the isolation of activity from SVMP/SVSP mediated effects in future investigations with venom containing multiple enzyme classes [10,11,12]. To achieve these goals a commercially available, well-characterized PLA2 derived from the venom of the Eastern diamondback rattlesnake (Crotalus adamanteus) that inflicts tissue edema was selected [17,18,19].
Materials and methods
Human plasma and chemicals
Pooled normal human plasma anticoagulated with sodium citrate (nine parts blood to one part 0.105M sodium citrate) was obtained from George King Bio-Medical, Overland Park, KS, USA and stored at − 80 °C. Calcium-free phosphate buffered saline (PBS), tricarbonyldichlororuthenium (II) dimer (CORM-2, a CO releasing molecule), dimethyl sulfoxide (DMSO) and quinacrine dihydrochloride, were obtained from Sigma-Aldrich, St. Louis, MO, USA. Calcium chloride was obtained from Haemonetics Inc., Braintree, MA, USA. One milligram of purified, dialyzed, lyophilized PLA2 derived from C. adamanteus venom (hereafter referred to as Ca-PLA2) was obtained form from Worthington Biochemical Corporation, Lakewood, NJ, USA. The specific activity of this preparation was 510 U/mg protein, with a unit liberating 1 μmole of acid from soybean lecithin per minute at 25 °C and pH 8.9. Ca-PLA2 was dissolved into PBS for a final activity of 0.5 U/µl, aliquoted and stored at − 80 °C.
Thrombelastographic analyses of human plasma coagulation kinetics
Plasma was rapidly thawed in a water bath at 37 °C just prior to experimentation. One milliliter samples of plasma had a 1% addition of subsequently described Ca-PLA2 mixtures prior to analysis. The sample was immediately placed into three disposable cups (320 µl per cup) with addition of 20 µl PBS in a computer-controlled thrombelastograph® hemostasis system (Model 5000, Haemonetics Inc., Braintree, MA, USA) and then 20 µl of CaCl2 was added to commence contact activation of coagulation (from the plastic surfaces), with the sample mixed by raising the cup over the plastic pin five times. As it was anticipated that Ca-PLA2 was an anticoagulant enzyme, data was collected for 30 min at 37 °C. The following elastic modulus-based parameters previously described [6,7,8,9] were determined: time to maximum rate of thrombus generation (TMRTG): this is the time interval (minutes) observed prior to maximum speed of clot growth; maximum rate of thrombus generation (MRTG): this is the maximum velocity of clot growth observed (dynes/cm2/s); and total thrombus generation (TTG, dynes/cm2), the final viscoelastic resistance observed after clot formation.
Activity-response association of Ca-PLA2 activity and plasma coagulation kinetics
Ca-PLA2 was diluted in PBS so that the addition of 10 µl of the mixture to 1 ml of plasma would result in a final concentration of 0, 0.25, 0.5, 1.0 or 2.0 U/ml. Each Ca-PLA2 activity was tested in triplicate (1 ml preparation previously described divided into three individual thrombelastographic channels).
Effects of CORM-2 on Ca-PLA2 activity in plasma
Based on the results of the aforementioned experiments, an activity of 0.5 U/ml was chosen for experimentation involving exposure of Ca-PLA2 to CO via CORM-2. Solutions were made with the ratio of 1 µl of Ca-PLA2 (0.5 U) being diluted in 9 µl of PBS containing 1% DMSO, CORM-2 or inactivated CORM-2 (iRM). CORM-2 was inactivated as previously presented [6,7,8,9]. These solutions were then incubated for 5 min at room temperature prior to being placed in plasma. Therefore, the experimental conditions were: (1) PBS without Ca-PLA2; (2) PBS with Ca-PLA2 (0.5 U); (3) PBS with Ca-PLA2 (0.5 U) and 100 µM CORM-2; (4) PBS with Ca-PLA2 (0.5 U) and 100 µM iRM. Each condition was represented with six replicates composed of individual samples with all constituents placed sequentially into the thrombelastographic cup.
Activity-response association of Ca-PLA2 activity over a range of CORM-2 concentrations
Solutions were made with the ratio of 1 µl of Ca-PLA2 (0.5 U) being diluted in 9 µl of PBS containing 1% DMSO/CORM-2 with a final concentration of 0, 25, 50, 75 or 100 µM CORM-2. This mixture was incubated at room temperature for 5 min prior to placement into plasma and subsequent thrombelastographic analysis. Each concentration of CORM-2 was represented in triplicate as separate plasma samples within the thrombelastographic cup with Ca-PLA2 solutions added.
Effects of quinacrine on Ca-PLA2 activity in plasma
Quinacrine has been demonstrated to inhibit PLA2 from various venoms in a range of 10 µM–2 mM [14,15,16] in in vitro and in vivo settings. Thus, a middle range concentration was chosen so the final concentration of quinacrine in plasma with a 1% (v/v) addition would be 100 µM for experimentation. Solutions were made with the ratio of 1 µl of Ca-PLA2 (0.5 U) being diluted in 9 µl of PBS containing 0–10 mM quinacrine and incubated for 5 min at room temperature prior to placement in plasma. Given the aforementioned, the experimental conditions were: 1) PBS without Ca-PLA2; 2) PBS with Ca-PLA2 (0.5 U); 3) PBS with quinacrine 10 mM; 4) PBS with Ca-PLA2 (0.5 U) and quinacrine 10 mM. Each condition was represented with six replicates composed of individual samples with all constituents placed sequentially into the thrombelastographic cup.
Statistical analyses
Data are presented as raw data or mean ± SD. Graphics depicting raw coagulation kinetic data were generated with commercially available programs (OrigenPro 2017, OrigenLab Corporation, Northampton, MA, USA; CorelDRAW X8, Corel Corporation, Mountain View, CA, USA). Modeling of the association between coagulation kinetic parameter values and copper concentration was performed with one of these programs as well (OrigenPro 2017). A commercially available statistical program was used for one-way analysis of variance followed by Holm-Sidak post hoc analysis to assess the effects of CORM-2 or quinacrine on Ca-PLA2 activity (SigmaStat 3.1, Systat Software, Inc., San Jose, CA, USA). P < 0.05 was considered significant.
Results
Activity-response association of Ca-PLA2 activity and plasma coagulation kinetics
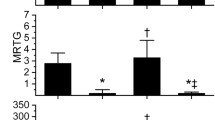
The data generated by these experiments are depicted in Fig. 1. With regard to TMRTG, there was a biphasic response as Ca-PLA2 activity increased with 0.25 and 0.5 U/ml activities associated with an increase in TMRTG values followed by a rapid decrease in plasma exposed to 1.0 and 2.0 U/ml. In contrast, both MRTG and TTG values decreased in exponential decay patterns with increasing Ca-PLA2 activity. Given these activity-coagulation kinetic results, all subsequent experimentation was performed with a maximum Ca-PLA2 activity of 0.5 U/ml.
Effects of Ca-PLA2 activity (0–2 U/ml) on plasmatic coagulation kinetics. Each concentration of Ca-PLA2 was represented by n = 3 replicates. TMRTG time to maximum rate of thrombus generation (min); MRTG maximum rate of thrombus generation (dynes/cm2/s); TTG total thrombus generation (dynes/cm2). The equations for the association of Ca-PLA2 activity on each coagulation kinetic parameter was modeled and depicted as a dashed line, with the coefficient of determination (R2) and level of significance indicated. There was no equation generated for TMRTG secondary to its activity dependent, biphasic response. The equations for the associations of the other two parameters are as follows: MRTG (dynes/cm2/s) = 2.93e(−Ca−PLA2U/ml/0.066) + 0.233; TTG (dynes/cm2) = 176e(−Ca−PLA2U/ml/0.126) + 22
Effects of CORM-2 on Ca-PLA2 activity in plasma
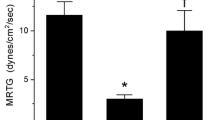
These results are displayed in Fig. 2. Compared to plasma without active additives, plasma with Ca-PLA2 activity demonstrated significantly greater TMRTG values and significantly smaller MRTG and TTG values. Exposure of Ca-PLA2 activity to CORM-2 restored coagulation kinetic parameter values to those observed in plasma without active additives and significantly different from samples with Ca-PLA2 activity without CORM-2 exposure. Lastly, in plasma with addition of Ca-PLA2 activity exposed to iRM, coagulation kinetic parameters were compromised to the same extent as samples only exposed to Ca-PLA2 activity and significantly different from the other two conditions.
Effects of exposure to CORM-2 and iRM on Ca-PLA2 activity mediated changes in plasmatic coagulation kinetics. Data are displayed as mean ± SD. Each experimental condition was represented by n = 6 replicates. TMRTG time to maximum rate of thrombus generation (min); MRTG maximum rate of thrombus generation (dynes/cm2/s); TTG total thrombus generation (dynes/cm2). Control = plasma without active additives; P = 0.5 U/ml Ca-PLA2 activity; P + CO = 0.5 U/ml Ca-PLA2 activity exposed to 100 µM CORM-2; P + iRM = 0.5 U/ml Ca-PLA2 activity exposed to 100 µM inactivated CORM-2. *P < 0.05 versus control; †P < 0.05 versus P; ‡P < 0.05 versus P + CO
Activity-response association of Ca-PLA2 activity over a range of CORM-2 concentrations
Figure 3 contains the results of these experiments. Exposure of Ca-PLA2 activity to CORM-2 resulted in a concentration-dependent decrease in the effects of Ca-PLA2 activity on plasmatic coagulation kinetics. There was a linear decrease in TMRTG values with increasing concentrations of CORM-2; an exponential increase in MRTG values with increasing concentrations of CORM-2; and, an exponential increase in TTG values with increasing concentrations of CORM-2.
Effects of increasing CORM-2 concentration (0-100 µM) exposure on Ca-PLA2 activity (0.5 U/ml) on plasmatic coagulation kinetics. Each concentration of CORM-2 was represented by n = 3 replicates. TMRTG time to maximum rate of thrombus generation (min); MRTG maximum rate of thrombus generation (dynes/cm2/s); TTG total thrombus generation (dynes/cm2). The equations for the association of CORM-2 concentration on Ca-PLA2 activity mediated changes of each coagulation parameter was modeled and depicted as a dashed line, with the coefficient of determination (R2) and level of significance indicated. The equations for the associations of are as follows: TMRTG (min) = 30.6 − [0.200 (Ca-PLA2 U/ml)]; MRTG (dynes/cm2/s) = 0.126e(Ca−PLA2U/ml/36.0) + 0.189; TTG (dynes/cm2) = 116e(Ca−PLA2U/ml/122) − 80
Effects of Quinacrine on Ca-PLA2 activity in plasma
The data generated by these experiments can be found in Fig. 4, and the experiments performed in this series utilized a lot of pooled normal plasma different from the preceding experiments. Exposure of Ca-PLA2 activity to quinacrine had no significant effect on the degradation of plasmatic coagulation kinetics. Critically, plasma exposed to quinacrine alone demonstrated significantly increased TMRTG values, decreased MRTG values and decreased TTG values compared to plasma samples without any active additives.
Effects of exposure to quinacrine (100 µM) on Ca-PLA2 activity mediated changes in plasmatic coagulation kinetics. Data are displayed as mean ± SD. Each experimental condition was represented by n = 6 replicates. TMRTG time to maximum rate of thrombus generation (min); MRTG maximum rate of thrombus generation (dynes/cm2/s); TTG total thrombus generation (dynes/cm2). Control = plasma without active additives; P = 0.5 U/ml Ca-PLA2 activity; Q = 100 µM quinacrine; P + Q = 0.5 U/ml Ca-PLA2 activity with 100 µM quinacrine. *P < 0.05 versus control; †P < 0.05 versus P; ‡P < 0.05 versus Q
Discussion
The primary findings of this study were that Ca-PLA2 acted as an anticoagulant in human plasma in this thrombelastographic system, the anticoagulant effect was Ca-PLA2 activity-dependent, and Ca-PLA2 activity was inhibitable in a CO concentration-dependent manner. This apparent in vitro anticoagulant effect of Ca-PLA2 in human plasma is distinctly different from its known in vivo properties of inflicting paw swelling in mice [19]. C. adamanteus venom is a known defibrinogenating agent with a complex proteome [20], and our laboratory confirmed with thrombelastography that the predominant coagulation kinetic profile of this venom in human plasma was consistent with a previously characterized SVSP with thrombin-like activity that was inhibited by CO [21]. Considered as a whole, these data support the concept that thrombelastographic assessments of snake venom PLA2 activity can be determined independent of the typical in vivo site of action (e.g., local tissue destruction, neuromuscular junction inhibition) via PLA2 mediated catalysis of plasmatic phospholipases critical to coagulation kinetics. If this paradigm is supported by similar results with diverse PLA2 activities, it may be possible to assess the effects of inhibitors, such as CO, on myotoxin and neurotoxin activities (as lipases) via in vitro thrombelastographic assessments, complementing in vivo assessments of tissue injury and neurotoxicity in animal models.
Quinacrine exposure had no important effect on Ca-PLA2 activity at concentrations of quinacrine that significantly diminished normal plasmatic coagulation kinetics (Fig. 4). The reason for such failure to inhibit Ca-PLA2 activity may be that this particular enzyme is not sensitive to quinacrine inhibition in contrast to the snake venom enzymes demonstrated to [14,15,16] be inhibited. Another possibility is that the concentration of quinacrine required to inhibit Ca-PLA2 activity may be far greater than 100 µM; however, the concentration of quinacrine required to inhibit Ca-PLA2 activity may inhibit plasmatic coagulation to the point that assessments with thrombelastography are not possible. In sum, while quinacrine inhibition may be worthwhile as a diagnostic method to detect PLA2 activity in snake venom or modulate other PLA2 activity in other settings, detection of inhibition of Ca-PLA2 activity with human plasmatic coagulation kinetics is likely not possible.
The biphasic effect of Ca-PLA2 activity on TMRTG (Fig. 1) is difficult to explain, where in at the greatest Ca-PLA2 activities TMRTG values decreased far below values obtained from plasma without any active additives. The thrombi formed in the presence of large Ca-PLA2 activities commenced coagulation very quickly, but were still very slow growing and very weak. The vast majority of citations demonstrate anticoagulant, not procoagulant properties of PLA2 activity in plasmatic milieus with only one except that I could identify. A unique PLA2 isolated from the venom of Gloydius ussuriensis demonstrated thrombin-like activity [22], initiating coagulation rapidly in an activity-dependent manner without engagement of factor XIII, which would be predicted to affect TMRTG values in a fashion similar to that seen with 1–2 U/ml Ca-PLA2 activity displayed in Fig. 1. Therefore, based on known PLA2 effects, it may be possible that Ca-PLA2 may have latent thrombin-like activity resulting in fibrinogen polymerization that is independent of plasma phospholipid concentrations in addition to its known phospholipase activity. While interesting, this particular finding and its potential implications are beyond the scope of the present work and should be a subject of future investigation.
In conclusion, the present work documents the first in vitro characterization of a PLA2 activity in human plasma with thrombelastography. Further, this PLA2 was demonstrated to be CO-inhibitable, strongly supporting that it is heme bearing and heme modulated as seen with a purified SVMP [13]. These findings, while preliminary, open the possibility that other PLA2 contained in snake venom with multiple toxicities (e.g., myotoxin, neurotoxin) may be heme bearing and CO-inhibitable, which have profound potential basic and clinical science implications. It has already been demonstrated in vivo with a rabbit model of envenomation that CO inhibits the anticoagulant effects of the SVMP-rich, fibrinogenolytic venom of Crotalus atrox [23]; inhibition of neurotoxic venom by the same mechanism could be lifesaving. Further, I have posited in several works that CO delivered by various releasing molecules into the bite site may serve as a way to delay coagulopathy prior to antivenom administration [6,7,8,9, 21]. In conclusion, the present work may serve as the rational basis for future in vitro and in vivo investigation to determine if PLA2 with various effects are CO-inhibitable.
References
Sajevic T, Leonardi A, Križaj I (2011) Haemostatically active proteins in snake venoms. Toxicon 57:627–645
Xiao H, Pan H, Liao K, Yang M, Huang C (2017) Snake venom PLA2, a promising target for broad-spectrum antivenom drug development. Biomed Res Int 2017:6592820
Krawczyk W, Dmoszynska A, Ledwozyw A, Marczewski K (1996) Human erythropoietin improves blood plasma phospholipids concentration in chronically hemodialyzed patients. Nephron 72:109–110
Hanahan DJ, Nelson DR (1984) Phospholipids as dynamic participants in biological processes. J Lipid Res 25:1528–1535
Tan NH, Arunmozhiarasi A (1989) The anticoagulant activity of Malayan cobra (Naja naja sputatrix) venom and venom phospholipase A2 enzymes. Biochem Int 19:803–810
Nielsen VG, Frank N, Matika RW (2018) Carbon monoxide inhibits hemotoxic activity of Elapidae venoms: potential role of heme. Biometals 31:51–59
Nielsen VG, Cerruti MA, Valencia OM, Amos Q (2016) Decreased snake venom metalloproteinase effects via inhibition of enzyme and modification of fibrinogen. Biometals 29:913–919
Nielsen VG, Boyer LV (2016) Iron and carbon monoxide attenuate degradation of plasmatic coagulation by Crotalus atrox venom. Blood Coagul Fibrinolysis 27:506–510
Nielsen VG, Bazzell CM (2016) Carbon monoxide attenuates the effects of snake venoms containing metalloproteinases with fibrinogenase or thrombin-like activity on plasmatic coagulation. MedChemComm 7:1973–1979
Dutta S, Chanda A, Kalita B, Islam T, Patra A, Mukherjee AK (2017) Proteomic analysis to unravel the complex venom proteome of eastern India Naja naja: correlation of venom composition with its biochemical and pharmacological properties. J Proteom 156:29–39
Lauridsen LP, Laustsen AH, Lomonte B, Gutiérrez JM (2017) Exploring the venom of the forest cobra snake: toxicovenomics and antivenom profiling of Naja melanoleuca. J Proteom 150:98–108
Petras D, Sanz L, Segura A, Herrera M, Villalta M, Solano D, Vargas M, León G, Warrell DA, Theakston RD, Harrison RA, Durfa N, Nasidi A, Gutiérrez JM, Calvete JJ (2011) Snake venomics of African spitting cobras: toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. J Proteome Res 10:1266–1280
Suntravat M, Langlais PR, Sánchez EE, Nielsen VG (2018) CatroxMP-II: a heme-modulated fibrinogenolytic metalloproteinase isolated from Crotalus atrox venom. Biometals 31:585–593
Fujimoto Y, Akamatsu N, Hattori A, Fujita T (1984) Stimulation of prostaglandin E2 synthesis by exogenous phospholipase A2 and in rabbit kidney medulla slices. Biochem J 218:69–74
Trebien HA, Calixto JB (1989) Pharmacological evaluation of rat paw oedema induced by Bothrops jararaca venom. Agents Actions 26:292–300
Judge RK, Henry PJ, d’Aprile AC, Lynch D, Jelinek GA, Wilce MC, Wilce JA (2002) Identification of PLA(2) and alpha-neurotoxin proteins in the venom of Pseudonaja affinis (dugite). Toxicol Appl Pharmacol 181:184–191
Tsao FH, Keim PS, Henrikson RL (1975) Crotalus adamanteus phospholipase A2-alpha: subunit structure. NH2-terminal sequence, and homology with other phospholipases. Arch Biochem Biophys 167:706–717
Smith CM, Wells MA (1981) A further examination of the active form of Crotalus adamanteus phospholipase A2. Biochim Biophys Acta 663:687–694
da Silva SL, Calgarotto AK, Maso V, Damico DC, Baldasso P, Veber CL, Villar JA, Oliveira AR, Comar M Jr, Oliveira KM, Marangoni S (2009) Molecular modeling and inhibition of phospholipase A2 by polyhydroxy phenolic compounds. Eur J Med Chem 44:312–321
Margres MJ, McGivern JJ, Wray KP, Seavy M, Calvin K, Rokyta DR (2014) Linking the transcriptome and proteome to characterize the venom of the eastern diamondback rattlesnake (Crotalus adamanteus). J Proteomics 96:145–158
Nielsen VG, Bazzell CM (2017) Carbon monoxide releasing molecule-2 inhibition of snake venom thrombin-like activity: novel biochemical “brake”? J Thromb Thrombolysis 43:203–208
Zhang Q, Wang J, Han Y, Xie Q, An L, Bao Y (2007) Identification of a novel thrombin-like phospholipase A2 from Gloydius ussuriensis snake venom. Blood Coagul Fibrinolysis 18:723–729
Nielsen VG (2018) Crotalus atrox venom exposed to carbon monoxide has decreased fibrinogenolytic activity in vivo in rabbits. Basic Clin Pharmacol Toxicol 122:82–86
Funding
This investigation was supported by the Department of Anesthesiology, College of Medicine, at the University of Arizona.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
This was an in vitro investigation and did not involve any living subjects.
Rights and permissions
About this article
Cite this article
Nielsen, V.G. Carbon monoxide inhibits the anticoagulant activity of phospholipase A2 purified from Crotalus adamanteus venom. J Thromb Thrombolysis 47, 73–79 (2019). https://doi.org/10.1007/s11239-018-1763-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-018-1763-6