Abstract
Envenomation by hemotoxic enzymes continues to be a major cause of morbidity and mortality throughout the world. With regard to treatment, the gold standard to abrogate coagulopathy caused by these venoms is still the administration of antivenom; however, despite antivenom therapy, coagulopathy still occurs and recurs. Of interest, this laboratory has demonstrated in vitro and in vivo that coagulopathy inducing venom derived from snakes of the family Viperidae exposed to carbon monoxide (CO) is inhibited, potentially by an attached heme. The present investigation sought to determine if venoms derived from snakes of the Elapidae family (taipans and cobras) could also be inhibited with CO or with the metheme inducing agent, O-phenylhydroxylamine (PHA). Assessing changes in coagulation kinetics of human plasma with thrombelastography, venoms from Elapidae snakes were exposed in isolation to CO (five species) or PHA (one specie) and placed in human plasma to assess changes in procoagulant or anticoagulant activity. The procoagulant activity of two taipan venoms and anticoagulant activity of three cobra venoms were significantly inhibited by CO. The venom of the inland taipan was also inhibited by PHA. In sum, these data demonstrate indirectly that the biometal heme is likely bound to these disparate venoms as an intermediary modulatory molecule. In conclusion, CO may not just be a potential therapeutic agent to treat envenomation but also may be a potential modulator of heme as a protective mechanism for venomous snakes against injury from their own proteolytic venoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Envenomation by hemotoxic enzymes continues to be a major cause of morbidity and mortality throughout the world (Berling and Isbister 2015). With regard to treatment, the gold standard to abrogate coagulopathy caused by these venoms is still the administration of antivenom (Bush et al. 2015); however, despite antivenom therapy, coagulopathy still occurs and recurs. With the objective of developing a therapy complimentary to antivenom administration, our laboratory has been investigating the phenomena of direct inhibition of hemotoxic enzymes by exposing the venom to carbon monoxide (CO) with in vitro experiments in human and animal plasmas (Nielsen and Bazzell 2016, 2017; Nielsen et al. 2016, 2017; Nielsen and Losada 2017) and in vivo in a sedated rabbit model (Nielsen 2017). Specifically, both snake venom metalloproteinases (SVMP) and snake venom serine proteases (SVSP) obtained from venom from snakes in the family Viperidae exposed to CO in vitro demonstrated a marked reduction fibrinogenolytic, thrombin-like, or prothrombin activating activity in human plasma (Nielsen and Bazzell 2016, 2017; Nielsen et al. 2016; Nielsen and Losada 2017). Of interest, only one member of the family Elapidae (Naja naja) has had its venom assessed in our laboratory, which was found to be fibrinogenolytic and susceptible to CO mediated inhibition (Nielsen et al. 2016). In sum, substantial in vitro and preliminary in vivo investigations had demonstrated the inhibitory effect of CO on hemotoxic venom activity.
Despite this robust beginning, it is of interest to continue to determine if CO mediated inhibition has diverse application; specifically, assessing hemotoxic venom from snakes in the family Elapidae (e.g., taipans, coral snakes and cobras) that are responsible for considerable human morbidity and mortality. Elapidae venom tends to include important neurotoxins (Clarke et al. 2006; Davidson et al. 1995; Hodgson et al. 2007; Kularatne et al. 2009; Lalloo et al. 1995) as well as hemotoxins (Chester and Crawford 1982; Lalloo et al. 1995; MacKay et al. 1969; Petras et al. 2011; Speijer et al. 1986), with death secondary to respiratory paralysis an important threat. The rationale to hypothesize the CO could inhibit hemotoxic venom from this family of snakes is based on the CO mediated inhibition observed with Naja naja venom (Nielsen et al. 2016), and the inhibition of taipan venom prothrombotic activity by sodium cyanide (Speijer et al. 1986), a molecule that would be expected to bind to a biometal such as heme bound to venom enzymes just as CO has been posited to do (Nielsen and Bazzell 2016). In sum, it would be of great interest to determine if CO could directly inhibit hemotoxic Elapidae venom activity via intermediary biometal (heme) interactions.
Considering the aforementioned, the purposes of the present investigation were to: (1) thrombelastographically define in human plasma the effects on coagulation kinetics of both procoagulant (taipan) and anticoagulant (cobra) hemotoxic venom activity; (2) determine if CO directly inhibits these hemotoxic venom effects; and, (3) determine if placing a hemotoxic venom into an environment favoring metheme formation would affect venom activity to further indirectly support the concept that SVMP/SVSP may be heme modulated proteins.
Materials and methods
Venoms, chemicals, and human plasma
Lyophilized venoms depicted in Table 1 were obtained from Mtoxins (Oshkosh, WI, USA) or the National Natural Toxins Research Center at Texas A&M University (Kingsville, TX, USA). The venom was reconstituted in calcium-free phosphate buffered saline (PBS, Sigma-Aldrich, Saint Louis, MO, USA) at a concentration of 50 mg/ml, aliquoted, and stored at − 80 °C until experimentation. CORM-2 (tricarbonyldichlororuthenium (II) dimer, a CO releasing molecule), dimethyl sulfoxide (DMSO) and O-phenylhydroxylamine were also obtained from Sigma-Aldrich. Lastly, pooled normal human plasma (George King Bio-Medical, Overland Park, KS, USA) anticoagulated with sodium citrate (nine parts blood to one part 0.105 M sodium citrate) stored at − 80 °C was utilized in all subsequently described experiments.
Thrombelastographic analyses
Specific volumes of subsequently described whole blood and chemical additives varied by condition but summated to 360 µl. Sample composition consisted of 320 µl of plasma; 16.4 µl of PBS, 20 µl of 200 mM CaCl2, and 3.6 µl of PBS or venom (final concentration dependent on species of snake), which were placed into a disposable cup in a computer-controlled thrombelastograph® hemostasis system (Model 5000, Haemonetics Inc., Braintree, MA, USA) at 37 °C, and then rapidly mixed by moving the cup up against and then away from the plastic pin five times before leaving the mixture between the cup and pin. The following elastic modulus-based parameters previously described (Nielsen 2017; Nielsen and Bazzell 2016, 2017; Nielsen et al. 2016, 2017; Nielsen and Losada 2017) were determined: time to maximum rate of thrombus generation (TMRTG): this is the time interval (minutes) observed prior to maximum speed of clot growth; maximum rate of thrombus generation (MRTG): this is the maximum velocity of clot growth observed (dynes/cm2/sec); and total thrombus generation (TTG, dynes/cm2), the final viscoelastic resistance observed after clot formation. Data were collected for 15 min with venoms that were procoagulant, whereas venoms with anticoagulant properties had data collection for 30 min.
Determination of concentration of venom for experimentation and effects of isolated venom exposures to CORM-2 or PHA
This in vitro, plasma based model allows differentiation between procoagulant and anticoagulant venom effects on coagulation assessed by thrombelastography. The initial concentration assessed for all venoms was 1 µg/ml; if the onset and velocity of coagulation was procoagulant with commencement of coagulation beginning in half the time or less and/or the velocity of clot formation proceeded at twice or greater than plasma without venom addition, this concentration of venom was used in subsequent experimentation. If this was not the case, then the concentration of procoagulant venom was progressively increased until these conditions were met. With regard to anticoagulant venom, the onset of coagulation had to be double and/or the velocity of clot formation half of plasma without venom addition to be acceptable for further investigate with that concentration of venom. However, as was the case here, if coagulation was not detectable, then the concentration of anticoagulant venom was progressively decreased until at least detectable coagulation occurred; then this concentration was subsequently used in experimentation. In sum, this approach allowed comparison of relative potencies of the venoms in addition to determination of the overall effect of each venom on plasmatic coagulation.
With regard to exposure to CORM-2 to assess the effects of CO on venom activity, the following four experimental conditions were created: (1) control condition—no venom, DMSO 1% addition (v/v) in PBS; (2) venom condition—venom, DMSO 1% addition (v/v) in PBS; (3) CO condition—venom, CORM-2 1% addition in DMSO (100 µM final concentration; (4) inactive releasing molecule (iRM) condition—venom, inactivated CORM-2 1% addition in DMSO (100 µM final concentration). CORM-2 was inactivated as previously described (Nielsen and Garza 2014). Venom was placed in PBS with the aforementioned additions, and after 5 min of incubation at room temperature, 3.6 µl of one these four solutions were added to the aforementioned plasma mixture in a thrombelastographic cup.
As for experiments conducted to expose venom to PHA, this was done to determine if activity would change when the conditions would favor the formation of metheme (Fe+3). This approach altered the heme state during investigation of fibrinogen in vitro (Nielsen et al. 2011) and ex vivo in human plasma (Nielsen et al. 2013), decreasing the function of fibrinogen as a substrate for thrombin. Venom was placed in PBS with addition of PHA 1% addition (v/v, 30 mM final concentration) for 5 min prior to addition to the aforementioned plasma mixture in a thrombelastographic cup. Results obtained from this condition were compared to the results obtained from condition 2 in the immediately preceding series of experiments.
Statistical analyses
Data are presented as mean ± SD. Experimental conditions were represented by n = 6 replicates per condition as this provides a statistical power > 0.8 with P < 0.05 using this thrombelastographic methodology (Nielsen and Bazzell 2016, 2017; Nielsen et al. 2016, 2017; Nielsen and Losada 2017). A commercially available statistical program was used for one way analyses of variance (ANOVA) comparisons between conditions, followed by Holm-Sidak post hoc analysis or unpaired, two-tailed Student’s t-tests as appropriate (SigmaStat 3.1, Systat Software, Inc., San Jose, CA, USA). Graphics depicting coagulation kinetic data were generated with commercially available programs (OrigenPro 2017, OrigenLab Corporation, Northampton, MA, USA; CorelDRAW X8, Corel Corporation, Mountain View, CA, USA). P < 0.05 was considered significant.
Results
Effects of CO exposure on venom mediated changes in human plasmatic coagulation
The data generated by these experiments are depicted in Figs. 1, 2, 3, 4 and 5. The results concerning plasma coagulation kinetics are presented in Figs. 3, 4 and 5. The procoagulant kinetics of taipan venoms were so remarkably accelerated compared to control plasma that there was no need for statistical comparison. For clarity, each individual venom data set will subsequently be presented.
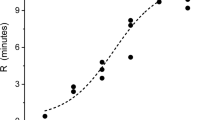
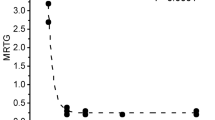
Effects of Oxyuranus microlepidotus venom exposure to CO on coagulation kinetics in human plasma. Data are presented as mean ± SD. TMRTG time to maximum rate of thrombus generation (min), MRTG maximum rate of thrombus generation (dynes/cm2/sec), TTG total thrombus generation (dynes/cm2). Venom/CO venom exposed to CO, Venom/iRM venom exposed to iRM. *P < 0.05 versus Venom; †P < 0.05 versus Venom/CO
Effects of Oxyuranus scutellatus canni venom exposure to CO on coagulation kinetics in human plasma. Data are presented as mean ± SD. TMRTG time to maximum rate of thrombus generation (min), MRTG maximum rate of thrombus generation (dynes/cm2/sec), TTG total thrombus generation (dynes/cm2). Venom/CO venom exposed to CO, Venom/iRM venom exposed to iRM. *P < 0.05 versus Venom; †P < 0.05 versus Venom/CO
Effects of Naja melanoleuca venom exposure to CO on coagulation kinetics in human plasma. Data are presented as mean ± SD. TMRTG time to maximum rate of thrombus generation (min), MRTG maximum rate of thrombus generation (dynes/cm2/sec), TTG total thrombus generation (dynes/cm2). V/CO venom exposed to CO, V/iRM venom exposed to iRM. *P < 0.05 versus Control; †P < 0.05 versus Venom, ‡P < 0.05 versus V/CO
Effects of Naja nigricollis venom exposure to CO on coagulation kinetics in human plasma. Data are presented as mean ± SD. TMRTG time to maximum rate of thrombus generation (min), MRTG maximum rate of thrombus generation (dynes/cm2/sec), TTG total thrombus generation (dynes/cm2). V/CO venom exposed to CO, V/iRM venom exposed to iRM. *P < 0.05 versus Control; †P < 0.05 versus Venom, ‡P < 0.05 vs. V/CO
Effects of Naja nubiae venom exposure to CO on coagulation kinetics in human plasma. Data are presented as mean ± SD. TMRTG time to maximum rate of thrombus generation (min), MRTG maximum rate of thrombus generation (dynes/cm2/sec), TTG total thrombus generation (dynes/cm2). V/CO venom exposed to CO, V/iRM venom exposed to iRM. *P < 0.05 versus Control; †P < 0.05 versus Venom, ‡P < 0.05 versus V/CO
Oxyuranus microlepidotus data (Fig. 1)
The concentration used for experimentation was 1 µg/ml. The venom caused the onset of coagulation more than tenfold sooner and at three times the velocity of growth of control plasma values. Exposure of the venom to CO resulted in a significant increase in TMRTG, decrease in MRTG, and decrease in TTG values compared to venom without CO addition. Lastly, exposure of the venom to the iRM resulted in TMRTG, MRTG and TTG values not different from venom without CO addition condition values but significantly different from venom samples exposed to CO.
Oxyuranus scutellatus canni data (Fig. 2)
The concentration used for experimentation was 1 µg/ml. The venom caused the onset of coagulation about eightfold sooner and at twice the velocity of growth of control plasma values. Exposure of the venom to CO resulted in a significant increase in TMRTG, decrease in MRTG, and decrease in TTG values compared to venom without CO addition. Lastly, exposure of the venom to the iRM resulted in TMRTG, MRTG and TTG values not different from venom without CO addition condition values but significantly different from venom samples exposed to CO.
Naja melanoleuca data (Fig. 3)
The concentration used for experimentation was 2.5 µg/ml. Compared to control sample conditions, venom addition to plasma resulted in significantly greater TMRTG values, smaller MRTG values, but not significantly different TTG values. Venom exposed to CO caused plasma coagulation kinetics to have TMRTG values significantly smaller, MRTG values significantly greater, and TTG values significantly greater than in plasma exposed to CO naïve venom. Of interest, venom exposed to CO did not have coagulation kinetic values significantly different from control conditions. Lastly, exposure of venom to iRM placed into plasma resulted in TMRTG and MRTG values significantly different from control condition and venom exposed to CO condition values but not significantly different from CO naïve venom conditions; however, TTG values of plasma with iRM exposed venom added were only different from plasma with addition of CO exposed venom.
Naja nigricollis data (Fig. 4)
The concentration used for experimentation was 50 ng/ml. Compared to control sample conditions, venom addition to plasma resulted in significantly greater TMRTG values, smaller MRTG values, and smaller TTG values. Venom exposed to CO caused plasma coagulation kinetics to have TMRTG values significantly smaller, MRTG values significantly greater, and TTG values significantly greater than in plasma exposed to CO naïve venom. Venom exposed to CO did not have TMRTG or TTG values significantly different from control conditions but did have MRTG values significantly less than control condition values. Exposure of venom to iRM placed into plasma resulted in TMRTG, MRTG and TTG values significantly different from control condition and venom exposed to CO condition values but not significantly different from CO naïve venom conditions.
Naja nubiae data (Fig. 5)
The concentration used for experimentation was 0.5 µg/ml. Compared to control sample conditions, venom addition to plasma resulted in significantly greater TMRTG values, smaller MRTG values, and smaller TTG values. Venom exposed to CO caused plasma coagulation kinetics to have TMRTG values significantly smaller, MRTG values significantly greater, and TTG values significantly greater than in plasma exposed to CO naïve venom. However, venom exposed to CO placed in plasma resulted in TMRTG values significantly greater, MRTG values significantly smaller, and TTG values significantly smaller than that observed under control conditions. In the condition with exposure of venom to iRM placed into plasma, TMRTG, MRTG and TTG values were significantly different from control condition and venom exposed to CO condition values but not significantly different from CO naïve venom conditions.
Effects of PHA exposure on Oxyuranus microlepidotus venom mediated changes in human plasmatic coagulation
The data obtained during these experiments are displayed in Fig. 6. Exposure of the venom to PHA resulted in significantly greater TMRTG values, smaller MRTG values and smaller TTG values when compared to kinetic values obtained with PHA naïve venom.
Effects of Oxyuranus microlepidotus venom exposure to PHA on coagulation kinetics in human plasma. Data are presented as mean ± SD. TMRTG time to maximum rate of thrombus generation (min), MRTG maximum rate of thrombus generation (dynes/cm2/sec); TTG total thrombus generation (dynes/cm2). *P < 0.05 versus Venom
Discussion
The primary findings of the present study include that both procoagulant and anticoagulant venoms obtained from five different species from the Elapidae family were inhibited by CO, with the one species tested with the metheme forming compound PHA also demonstrating inhibition of procoagulant activity. These findings are of importance to us for two reasons. First, CO inhibition all but abrogated the fibrinogenolytic effects of Crotalus atrox venom in an in vivo rabbit model (Nielsen 2017) and also inhibits the remarkably deadly venoms tested in this investigation, offering the possibility that CO inhibition may be a therapy to complement antivenom therapy worldwide. Second, when the data of the present investigation is coupled with the works documenting numerous venoms being inhibited by CO derived from species of the family Viperidae (Nielsen and Bazzell 2016, 2017; Nielsen et al. 2016; Nielsen and Losada 2017), the notion that an intermediate and modulating molecule, heme, may be at the center of this CO dependent mechanism of inhibition becomes more plausible. In sum, the aggregate of the data of the present investigation and our previous works supports the concept that the biometal, heme, may be key to understanding how some venom enzymes may be inhibited therapeutically and modulated in the snake venom gland.
This investigation does have some important limitations. First, we tested unfractionated venom, so individual identification of specific SVMP or SVSP was not performed. Instead, we identified for the first time the thrombelastographic signatures of the coagulopathic effect of each venom in human plasma, demonstrating the nature (procoagulant vs. anticoagulant) and vulnerability to CO of the specific snake venom. This hopefully will be a roadmap for future investigation of fractionated venom of such species to further define which enzymes are potentially heme modulated. Further investigation of such proteins with modalities such as mass spectroscopy are also needed to determine definitively if SVMP and SVSP are heme bound. While beyond the scope of the present investigation, such studies will be needed to directly implicate heme as a central mechanism of enzymatic inhibition by CO to complement our indirect findings of inhibition of these enzymes with CO and now PHA.
With regard to known mechanisms of endogenous venom gland inhibitors of SVMP and SVSP to prevent damage to the snake, there has been identification of low molecular weight peptide compounds in a variety of species (Storz et al. 2015). Of interest, as previously noted in this discussion, the sum of our data lays the ground work to consider the potential use of endogenous CO production in the snake venom gland as an endogenous inhibitor of SVMP and SVSP activity. Such CO production would be the result of venom gland tissue heme oxygenase activity; however, this investigative line has not been pursued. Indeed, aside from knowing that snake blood contains heme (Wagstaff et al. 2008), there has been no determination of heme oxygenase activity of any snake tissue. In sum, our data serve as a rationale to pursue future study to determine if endogenous CO production in venom glands may be a mechanism to inhibit SVMP and SVSP activity to protect snakes from venom mediated injury.
In conclusion, this investigation successfully demonstrated CO mediated inhibition of procoagulant and anticoagulant activity derived from five different species of snake from the family Elapidae, similar to that demonstrated with various venoms obtained from the family Viperidae. Further, inducing conditions favorable for metheme formation with inland taipan venom also resulted in inhibition of its procoagulant activity. When metheme mediated inhibition of such a venom is coupled with the knowledge that the heme binding molecules cyanide (Speijer et al. 1986) and CO also inhibit taipan venom activity, the hypothesis that SVMP and SVSP may be heme modulated proteins is strongly, albeit indirectly, supported. In sum, this investigation serves as a rationale for ongoing in vitro and in vivo investigation to determine the role played by CO as not just a potential therapeutic approach to treating envenomation but also as a potential modulator of the biometal heme as a protective mechanism for venomous snakes.
References
Berling I, Isbister GK (2015) Hematologic effects and complications of snake envenoming. Transfus Med Rev 29:82–89
Bush SP, Ruha AM, Seifert SA, Morgan DL, Lewis BJ, Arnold TC, Clark RF, Meggs WJ, Toschlog EA, Borron SW, Figge GR, Sollee DR, Shirazi FM, Wolk R, de Chazal I, Quan D, García-Ubbelohde W, Alagón A, Gerkin RD, Boyer LV (2015) Comparison of F(ab’)2 versus Fab antivenom for pit viper envenomation: a prospective, blinded, multicenter, randomized clinical trial. Clin Toxicol 53:37–45
Chester A, Crawford GPM (1982) In vitro coagulant properties of venoms from Australian snakes. Toxicon 20:501–504
Clarke C, Kuruppu S, Reeve S, Ian Smith A, Hodgson WC (2006) Oxylepitoxin-1, a reversible neurotoxin from the venom of the inland taipan (Oxyuranus microlepidotus). Peptides 27:2655–2660
Davidson TM, Schafer S, Killfoil J (1995) Cobras. Wilderness Environ Med 6:203–219
Hodgson WC, Dal Belo CA, Rowan EG (2007) The neuromuscular activity of paradoxin: a presynaptic neurotoxin from the venom of the inland Taipan (Oxyuranus microlepidotus). Neuropharmacology 52:1229–1236
Kularatne SA, Budagoda BD, Gawarammana IB, Kularatne WK (2009) Epidemiology, clinical profile and management issues of cobra (Naja naja) bites in Sri Lanka: first authenticated case series. Trans R Soc Trop Med Hyg 103:924–930
Lalloo DG, Trevett AJ, Korinhona A, Nwokolo N, Laurenson IF, Paul M, Black J, Naraqi S, Mavo B, Saweri A, Hutton RA, Theakston RDG, Warrell DA (1995) Snake bites by the Papuan taipan (Oxyuranus scutellatus canni): paralysis, hemostatic and electrocardiographic abnormalities, and effects of antivenom. Am J Trop Med Hyg 52:525–531
MacKay N, Ferguson JC, McNicol GP (1969) Effects of three cobra venoms on blood coagulation, platelet aggregation, and fibrinolysis. J Clin Pathol 22:304–311
Nielsen VG (2017) Crotalus atrox venom exposed to carbon monoxide has decreased fibrinogenolytic activity in vivo in rabbits. Basic Clin Pharmacol Toxicol. https://doi.org/10.1111/bcpt.12846
Nielsen VG, Bazzell CM (2016) Carbon monoxide attenuates the effects of snake venoms containing metalloproteinases with fibrinogenase or thrombin-like activity on plasmatic coagulation. MedChemComm 7:1973–1979
Nielsen VG, Bazzell CM (2017) Carbon monoxide releasing molecule-2 inhibition of snake venom thrombin-like activity: novel biochemical “brake”? J Thromb Thrombolysis 43:203–208
Nielsen VG, Garza JI (2014) Comparison of the effects of CORM-2, CORM-3 and CORM-A1 on coagulation in human plasma. Blood Coagul Fibrinolysis 25:801–805
Nielsen VG, Losada PA (2017) Direct inhibitory effects of carbon monoxide on six venoms containing fibrinogenolytic metalloproteinases. Basic Clin Pharmacol Toxicol 120:207–212
Nielsen VG, Arkebauer MR, Vosseller K (2011) Redox-based thrombelastographic method to detect carboxyhemefibrinogen-mediated hypercoagulability. Blood Coagul Fibrinolysis 22:657–661
Nielsen VG, Hafner DT, Steinbrenner EB (2013) Tobacco smoke-induced hypercoagulation in human plasma: role of carbon monoxide. Blood Coagul Fibrinolysis 24:405–410
Nielsen VG, Cerruti MA, Valencia OM, Amos Q (2016) Decreased snake venom metalloproteinase effects via inhibition of enzyme and modification of fibrinogen. Biometals 29:913–919
Nielsen VG, Sánchez EE, Redford DT (2017) Characterization of the rabbit as an in vitro and in vivo model to assess the effects of fibrinogenolytic activity of snake venom on coagulation. Basic Clin Pharmacol Toxicol. https://doi.org/10.1111/bcpt.12848
Petras D, Sanz L, Segura A, Herrera M, Villalta M, Solano D, Vargas M, León G, Warrell DA, Theakston RD, Harrison RA, Durfa N, Nasidi A, Gutiérrez JM, Calvete JJ (2011) Snake venomics of African spitting cobras: toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. J Proteome Res 10:1266–1280
Speijer H, Govers-Riemslag JW, Zwaal RF, Rosing J (1986) Prothrombin activation by an activator from the venom of Oxyuranus scutellatus (Taipan snake). J Biol Chem 261:13258–13267
Storz JF, Natarajan C, Moriyama H, Hoffmann FG, Wang T, Fago A, Malte H, Overgaard J, Weber RE (2015) Oxygenation properties and isoform diversity of snake hemoglobins. Am J Physiol Regul Integr Comp Physiol 309:R1178–R1191
Wagstaff SC, Favreau P, Cheneval O, Laing GD, Wilkinson MC, Miller RL, Stöcklin R, Harrison RA (2008) Molecular characterisation of endogenous snake venom metalloproteinase inhibitors. Biochem Biophys Res Commun 365:650–656
Funding
This investigation was supported by the Department of Anesthesiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose except that Mr. Frank is the owner of Mtoxins.
Rights and permissions
About this article
Cite this article
Nielsen, V.G., Frank, N. & Matika, R.W. Carbon monoxide inhibits hemotoxic activity of Elapidae venoms: potential role of heme. Biometals 31, 51–59 (2018). https://doi.org/10.1007/s10534-017-0066-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-017-0066-2