Abstract
Envenomation by vipers with hemotoxic enzymes continues to be a worldwide source of morbidity and mortality. The present work examined the effects of exposure of venom enzymes to carbon monoxide and O-phenylhydroxylamine, agents that modulate the biometal heme, by forming carboxyheme and metheme, respectively. Four venoms obtained from medically important, diverse snake venom found in Africa, Asia and Australia were analyzed. The species that had venom tested in human plasma with thrombelastography and heme modulating agents were Deinagkistrodon acutus, Protobothrops mucrosquamatus, Dispholidus typus and Pseudonaja textilis. These venoms varied four hundred-fold in potency (ng-µg/ml) to exert procoagulant effects on human plasma; further, there was species specific variability in venom inhibition after exposure to carboxyheme or metheme agents. Lastly, using a wide range of carbon monoxide concentrations, it was determined that the factor V component of P. textilis venom was likely inhibited before the factor X component. Further investigation using this thrombelastograph-based, venom “kinetomic” methodology involving heme modulation will demonstrate in time its laboratory and clinical utility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
What began as a testable hypothesis that a few snake venom hemotoxic enzymes could be inhibited by carbon monoxide (CO) only 2 years ago (Nielsen et al. 2016) has developed into a novel theory concerning the modulation of these disparate venom activities. Based on evidence of modulation of venom activities by the heme-binding molecules CO and O-phenylhydroxylamine (PHA, metheme-forming agent) (Nielsen et al. 2016, 2018), and based on prolonged inhibition of venom activity by CO in an in vivo rabbit model (Nielsen 2018), there was considerable indirect documentation of involvement of an attached heme affecting venom enzymes. Most recently, a CO-inhibitable, purified fibrinogenolytic enzyme with an attached heme was identified in the venom of Crotalus atrox, the most direct evidence to date supporting the theory of heme-modulated snake venom activity (Suntravat et al. 2018). In sum, considerable preliminary investigation has been conducted to obtain mechanistic insight and validation of this new paradigm of snake venom activity modulation.
In this vein, a continued effort to assess the generalizability of this theory is needed. One approach, which is used in the present investigation, is to analyze diverse venoms obtained from snakes that differ geographically and phylogenetically from previous specimens. To achieve this goal, the four venoms displayed in Table 1 were chosen for the following reasons. With regard to Deinagkistrodon acutus and Protobothrops mucrosquamatus, they are medically important and are from a region of the world with venoms not previously assessed with our methodology. Pseudonaja textilis is an iconic snake with a rapid procoagulant, prothrombin activating venom that contains an enzyme complex with remarkable similarity to activated coagulation factor V bound to factor X (FVa/FXa) (Viala et al. 2015, Stocker et al. 1994), similar to but more potent than Oxyuranus species. Lastly, Dispholidus typus is a well-studied and potentially lethal representative of the largest family of snakes, Colubridae, of which only a few species are venomous. Thus, analysis of these diverse and important venoms would significantly contribute to the concept that heme-mediated modulation of enzymatic activity is a common characteristic of venomous snakes worldwide.
Taken as a whole, the goals of the present investigation were to: (1) thrombelastographically characterize the hemotoxic activity of each venom in human plasma; (2) determine if modulation of these activities by bound heme(s) with CO and PHA occurs; and, (3) characterize the CO concentration-procoagulant activity relationship of Pseudonaja textilis venom to provide insight into potential differential modulation of FVa- and FXa-like activities. Finally, to assist with clarity, a list of abbreviations are provided in Table 2 for the readership.
Materials and methods
Venoms, chemicals, and human plasma
Lyophilized venoms depicted in Table 1 were obtained from Mtoxins (Oshkosh, WI, USA). The venom was reconstituted in calcium-free phosphate buffered saline (PBS, Sigma-Aldrich, Saint Louis, MO, USA) at a concentration of 50 mg/ml, aliquoted, and stored at − 80 °C until experimentation. CORM-2 (tricarbonyldichlororuthenium (II) dimer, a CO releasing molecule), dimethyl sulfoxide (DMSO) and O-phenylhydroxylamine were also obtained from Sigma-Aldrich. Lastly, pooled normal human plasma (George King Bio-Medical, Overland Park, KS, USA) anticoagulated with sodium citrate (9 parts blood to 1 part 0.105 M sodium citrate) stored at − 80 °C was utilized in all subsequently described experiments.
Thrombelastographic analyses
Specific volumes of subsequently described plasma and chemical additives varied by condition but summated to 360 µl. Sample composition consisted of 320 µl of plasma; 16.4 µl of PBS, 20 µl of 200 mM CaCl2, and 3.6 µl of PBS or venom (final concentration dependent on species of snake), which were placed into a disposable cup in a computer-controlled thrombelastograph® hemostasis system (Model 5000, Haemonetics Inc., Braintree, MA, USA) at 37 °C, and then rapidly mixed by moving the cup up against and then away from the plastic pin five times before leaving the mixture between the cup and pin. The following elastic modulus-based parameters previously described (Nielsen et al. 2016, 2018) were determined: time to maximum rate of thrombus generation (TMRTG): this is the time interval (minutes) observed prior to maximum speed of clot growth; maximum rate of thrombus generation (MRTG): this is the maximum velocity of clot growth observed (dynes/cm2/second); and total thrombus generation (TTG, dynes/cm2), the final viscoelastic resistance observed after clot formation. One other parameter, reaction time (R, min), defined as the time to develop 2 mm of amplitude as a measure of the commencement of coagulation, was determined during the conduct of the CO concentration-procoagulant activity relationship experiments with Pseudonaja textilis venom. Data were collected for 10–25 min, depending on the capacity of the specific venom to establish a stable final clot strength.
Determination of venom concentration and isolated CORM-2 or PHA venom exposures
The initial concentration assessed for these procoagulant venoms was 1 µg/ml; the concentration was left unchanged, increased or decreased to have conditions wherein TMRTG values were half or less than plasma without venom addition, and/or the velocity of clot formation proceeded at twice or greater than plasma without venom addition.
As for exposure to CORM-2 to assess the effects of CO on venom activity, the following four experimental conditions were created: (1) control condition—no venom, DMSO 1% addition (v/v) in PBS; (2) venom condition—venom, DMSO 1% addition (v/v) in PBS; (3) CO condition—venom, CORM-2 1% addition in DMSO (100 µM final concentration; (4) inactive releasing molecule (iRM) condition—venom, inactivated CORM-2 1% addition in DMSO (100 µM final concentration). If the venom activity was not affected with 100 µM CORM-2, it viewed as CO-resistant and subsequently exposed to 1000 µM CORM-2 and iRM, as this concentration was the largest used in previous work (Nielsen 2018). CORM-2 was inactivated as previously described (Nielsen and Garza 2014). Venom was placed in PBS with the aforementioned additions, and after 5 min of incubation at room temperature, 3.6 µl of one these four solutions were added to the aforementioned plasma mixture in a thrombelastographic cup.
As for experiments conducted to expose venom to PHA, this was done to determine if activity would change when the conditions would favor the formation of metheme (Fe3+) as previously presented (Nielsen et al. 2018). Venom was placed in PBS with addition of PHA 1% addition (v/v, 10–30 mM final concentration) for 5 min prior to addition to the aforementioned plasma mixture in a thrombelastographic cup. Results obtained from this condition were compared to the results obtained from condition 2 in the immediately preceding series of experiments.
Determination of CO concentration-procoagulant activity relationship of Pseudonaja textilis venom
Samples of this venom was exposed to several concentrations of CORM-2 (0, 100, 200, 300, 400, and 500 µM final concentration) for 5 min prior to placement into plasma and subjected to thrombelastographic analysis as previously described. Three replicate experiments per concentration of CORM-2 were performed, with data collected until clot strength stabilized as defined by the software declaring a final maximum amplitude. This range of CORM-2 concentrations corresponds to 0, 70, 140, 210, 280, and 350 µM CO as CORM-2 releases 0.7 mol of CO per mole of CORM-2 (Motterlini et al. 2002).
Statistical analyses
Data are presented as mean ± SD or raw data. Experimental conditions were represented by n = 6 replicates per condition in the series concerning exposures to CORM-2 and PHA as this provides a statistical power > 0.8 with P < 0.05 using this thrombelastographic methodology (Nielsen et al. 2016, 2018). A commercially available statistical program was used for one-way analyses of variance (ANOVA) comparisons between conditions, followed by Holm–Sidak post hoc analysis or unpaired, two-tailed Student’s t-tests as appropriate (SigmaStat 3.1, Systat Software, Inc., San Jose, CA, USA). As the venoms were procoagulant and the concentrations chosen resulted in coagulation kinetic parameter values far different from plasma without additives, there was no formal statistical comparison of venom exposed plasma with the normal plasma parameter values, and they are presented simply as a reference. Graphics depicting coagulation kinetic data were generated with commercially available programs (OrigenPro 2017, OrigenLab Corporation, Northampton, MA, USA; CorelDRAW X8, Corel Corporation, Mountain View, CA, USA). P < 0.05 was considered significant.
Results
General results
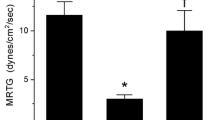
The coagulation kinetic data generated from plasma without any additions observed for 30 min are as follows: TMRTG = 12.8 ± 1.8 min, MRTG = 3.1 ± 0.8 dyne/cm2/s, and TTG = 196 ± 14 dyne/cm2. The results concerning plasma coagulation kinetics following addition of the venoms after exposure to CORM-2, iRM or PHA are presented in Table 3. Lastly, the effects of exposure of P. textilis venom to a range of CO concentrations on R, TMRTG, MRTG and TTG values are displayed in Figs. 1, 2, 3, 4. For clarity, each individual venom data set will subsequently be presented.
Relationship between R and CO after exposure of P. textilis venom to various concentrations of CO. Raw data for six concentrations (n = 3/concentration) of CO released from CORM-2 added to P. textilis venom subsequently added to human plasma for thrombelastographic analyses are depicted. Dashed line represents kinetic modeled relationship, with coefficient of determination (R2) and statistical significance of the relationship displayed. The equation describing this relationship is as follows: \({\text{R}}\,(\hbox{min} ) = \frac{ - 10.8}{{1 + e^{{\left( {[{\text{CO}}] - 168} \right)/56.8}} }} + 11.1\)
Relationship between TMRTG and CO after exposure of P. textilis venom to various concentrations of CO. Raw data for six concentrations (n = 3/concentration) of CO released from CORM-2 added to P. textilis venom subsequently added to human plasma for thrombelastographic analyses are depicted. Dashed line represents kinetic modeled relationship, with coefficient of determination (R2) and statistical significance of the relationship displayed. The equation describing this relationship is as follows: \({\text{TMRTG}}\;\left( {\hbox{min} } \right) = \frac{ - 18.5}{{1 + e^{{\left( {[{\text{CO}}] - 104} \right)/108}} }} + 14.6\)
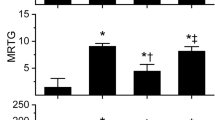
Relationship between MRTG and CO after exposure of P. textilis venom to various concentrations of CO. Raw data for six concentrations (n = 3/concentration) of CO released from CORM-2 added to P. textilis venom subsequently added to human plasma for thrombelastographic analyses are depicted. Dashed line represents kinetic modeled relationship, with coefficient of determination (R2) and statistical significance of the relationship displayed. The equation describing this relationship is as follows: MRTG (dyne/cm2/s) = 3.7 + 6.8e− [CO]/1.3
Relationship between TTG and CO after exposure of P. textilis venom to various concentrations of CO. Raw data for six concentrations (n = 3/concentration) of CO released from CORM-2 added to P. textilis venom subsequently added to human plasma for thrombelastographic analyses are depicted. Dashed line represents kinetic modeled relationship, with coefficient of determination (R2) and statistical significance of the relationship displayed. The equation describing this relationship is as follows: \({\text{TTG}}\;\left( {{\text{dyne}}/{\text{cm}}^{2} } \right) = 189 + \left( {5840} \right) \times \frac{73.3}{{4([{\text{CO}}] - 169)^{2} + 5373}}\)
Deinagkistrodon acutus data (Table 3)
The concentration used for experimentation was 4 µg/ml with a data collection time of 20 min. The additive naïve venom condition displayed classic coagulation kinetics consistent with thrombin-like activity without factor XIII (FXIII) activation, with characteristic small TMRTG, small MRTG and small TTG values. A concentration of 100 µM CORM-2 did not affect venom activity, so all subsequent experiments were performed with 1000 µM CORM-2/iRM. Exposure of the venom to CO resulted in a significant increase in TMRTG, increase in MRTG, and increase in TTG values compared to additive naïve venom conditions. Next, exposure of the venom to the iRM resulted in TMRTG, MRTG and TTG values not different from additive naïve venom condition values but significantly different from venom samples exposed to CO. Lastly, exposure of venom to PHA resulted in samples with significantly greater TTG values, but also significantly smaller TMRTG and TTG values than plasma samples with additive naïve venom exposure. Thus, CO appeared to markedly inhibit the thrombin-like activity of this venom by restoring coagulation kinetic parameter values towards additive-free plasma sample values. In contrast, PHA (30 mM) exposure appeared to only partially inhibit this thrombin-like activity, significantly delaying onset, decreasing the velocity of clot formation and decreasing clot strength compared to samples with additive naïve venom.
Dispholidus typus data (Table 3)
The concentration used for experimentation was 5 µg/ml with a data collection time of 15 min. The additive naïve venom condition displayed coagulation kinetics consistent with a weak prothrombin activator with FXIII activation, with characteristic reduced TMRTG, a third normal MRTG and half normal TTG values. Again, a concentration of 100 µM CORM-2 did not affect venom activity, so all subsequent experiments were performed with 1000 µM CORM-2/iRM. Exposure of the venom to CO resulted in a significant increase in TMRTG, decrease in MRTG, and decrease in TTG values compared to additive naïve venom conditions. Further, exposure of the venom to the iRM resulted in TMRTG values significantly greater than in samples with additive naïve venom but not different from samples with CO-exposed venom. Samples with iRM samples had MRTG and TTG values significantly different from additive naïve or CO-exposed venom. PHA-exposed venom demonstrated no discernable coagulation at 30 mM, and an additional six replicate series of experiments with venom exposed to 10 mM PHA also resulted in no detectable coagulation. Considered as a whole, these data demonstrate CO, iRM and PHA mediated inhibition of a prothrombin activator. However, the deterioration of coagulation kinetics to the point of loss of coagulation with these additives suggests that the venom contains an anticoagulant activity not modulated by heme that is detectable when the procoagulant activity is extinguished. Loss of prothrombin activation should have resulted in coagulation kinetics approximating that of plasma without additives; instead, a loss of coagulation is pathognomonic of the presence of another, anticoagulant activity.
Protobothrops mucrosquamatus data (Table 3)
The concentration used for experimentation was 40 µg/ml with a data collection time of 15 min. The additive naïve venom condition displayed coagulation kinetics consistent with a moderate prothrombin activator with FXIII activation, with reduced TMRTG, a double normal MRTG and normal TTG values. Subsequently, a concentration of 100 µM CORM-2 did not affect venom activity, so all subsequent experiments were performed with 1000 µM CORM-2/iRM. Plasma samples with CO-exposed venom demonstrated no significant change in TMRTG, MRTG or TTG values compared to additive naïve venom conditions. In contrast, plasma with iRM-exposed venom had TMRTG values significantly greater than samples with additive naïve venom; further, plasma with iRM-exposed venom had MRTG and TTG values significantly less than samples containing additive naïve venom or CO-exposed venom. Of interest, plasma with 30 mM PHA-exposed venom had significantly greater TMRTG, significantly smaller MRTG and significantly smaller TTG values than samples with additive naïve venom. Thus, this procoagulant venom activity was not CO sensitive, but instead was inhibited by CO-independent properties of iRM and metheme induction by PHA.
Pseudonaja textilis data (Table 3, Figs. 1, 2, 3, 4)
The concentration of venom used for all experiments was 100 ng/ml, and this first series had a data collection time of 10 min. The additive naïve venom condition displayed vigorous procoagulant coagulation kinetics consistent with a prothrombin activating-like activity with full activation of factor XIII, with characteristic very brief TMRTG, very large MRTG and normal TTG values. This pattern was consistent with our previous reports of taipan venom (Nielsen et al. 2018), with the exception that P. textilis venom in this series of experiments was ten-fold more potent than either taipan venom. A concentration of 100 µM CORM-2 did inhibit this venom, so all experiments were performed with 100 µM CORM-2/iRM. Exposure of the venom to CO resulted in a significant increase in TMRTG, decrease in MRTG, and decrease in TTG values compared to additive naïve venom conditions. Further, exposure of the venom to the iRM resulted in TMRTG, MRTG and TTG values not significantly different compared to values obtained from samples with additive naïve venom; however, iRM-exposed venom containing plasma had TMRTG, MRTG and TTG values significantly different from plasma with CO-exposed venom. Lastly, plasma containing PHA-exposed venom had significantly greater TMRTG values, smaller MRTG values, but not different TTG values compared to samples with additive naïve venom. Thus, this venom had prothrombin activator-like activity that was CO-inhibitable and to a lesser extent metheme-inhibitable.
The data collection time of the second series of experiments varied with the time taken to reach maximum amplitude, which took up nearly 30 min in some cases. As noted in Fig. 1, the time to onset of coagulation, R, increased in a CO-dependent manner, approximating a sigmoidal relationship between R and CO concentration. Similarly, as seen in Fig. 2, TMRTG values increased in a CO-dependent manner, approximating a sigmoidal relationship. The last two largest concentrations of CO had TMRTG values very similar to that of plasma not exposed to venom. As for the relationship between MRTG and CO concentration seen in Fig. 3, MRTG values rapidly decreased with CO exposure in a pattern of exponential decay. The changes in TTG values also were modulated by CO concentration, but in a Lorentz-like manner with a decrease in TTG values with increasing CO concentration followed by a return to TTG values similar to those samples with venom not exposed to CO.
Discussion
The present study documented remarkable diversity among these venoms. There were important differences in potency (µg/ml), with the procoagulant effect of P. mucrosquamatus the weakest ever measured in this laboratory and P. textilis the most potent. Second, when modulating putative attached heme, two of these vipers’ venoms were resistant to CO and one was unaffected; however, establishing a metheme state with PHA inhibited the activity of all four venoms varying from mild inhibition to complete loss of procoagulant activity. Lastly, the thrombelastographic parameter-CO concentration relationships (Figs. 1, 2, 3, 4) provided insight into which component of the FVa/FXa prothrombinase was inhibited first, as will be presented in more detail subsequently.
FVa is a critical cofactor for FXa, enhancing FXa activity several orders of magnitude (Steen 2002). Indeed, it is the juxtaposition of FVa with FXa that is posited to make P. textilis venom such a rapid a catalytic agent (Stocker et al. 1994). The associations of R and TMRTG with increasing CO concentrations (Figs. 1 and 2) are indicative of a heme modulated system, but it is not clear if one or both enzymes of the FVa/FXa venom prothrombin activator is being inhibited. However, the association of change in MRTG and TTG provide insight into this matter. The exponential decay of MRTG values with increasing CO concentrations is very suggestive of a rapid loss of the majority of the thrombin-generating capacity of the venom. This pattern would favor CO mediated inhibition of FVa first, with a gradual decrease in FXa activity. The persistent, stable MRTG values seen at the larger CO concentrations is indicative of the expected thrombin generation from contact protein activation in the plasma by exposure to the thrombelastographic cup and pin surfaces. This transition from venom mediated thrombin generation to cup and pin mediated thrombin generation is best seen in Fig. 4. As venom mediated thrombin generation decreases with progressive inhibition by increasing CO concentrations, there is less engagement of factor XIII as well as decreased fibrin polymer formation, resulting in a decrease in clot strength. However, as thrombin generation increases by contact activation from the cup and pin surfaces, factor XIII activation and fibrin polymer formation again increase with consequent restoration of clot strength. Taken as a whole, the procoagulant enzymes of P. textilis venom are rapidly inhibited by CO and the FVa component of the prothrombin activating complex of enzymes is the first to become inhibited by CO.
In conclusion, the present work demonstrated with four important and diverse venomous species that the biometal, heme, is the most likely modulator of the hemotoxic enzymes contained in each venom. Further, remarkable variability in vulnerability to carboxyheme or metheme inducing agents support the concept that different enzyme systems may be utilized by different vipers to potentially control venom activity in situ in their venom glands. Specifically, either the heme oxygenase system that produces CO or the nitric oxide synthase system that generates nitric oxide (a metheme forming agent) could be involved to greater or lesser degrees in these species. Given the insight provided by the thrombelastographic analyses involving heme modulation, it may be of utility to similarly kinetically profile venoms such as these to provide a functional assessment of the relative importance of any particular enzyme class (e.g., prothrombin activator, thrombin-like activity, fibrinogenolytic activity) in effecting coagulopathy. This approach would complement proteomic analyses of such venoms and could perhaps be termed “venom kinetomics”. Put another way, while more than one coagulation modifying activity can be identified in a venom (e.g., fibrinogenolytic-like, thrombin-like activity) with proteomics, venom kinetomics can identify which of the enzymes predominate and inflict clinical coagulopathy, and which ones can be modulated by the biometal heme. This venom kinetomic approach could also potentially determine the utility of antivenoms across genetically related (same genus) but geographically separated species of venomous snake. Future investigation using this venom kinetomic methodology involving heme modulation will demonstrate in time its laboratory and clinical utility.
References
Debono J, Dobson J, Casewell NR, Romilio A, Li B, Kurniawan N, Mardon K, Weisbecker V, Nouwens A, Kwok HF, Fry BG (2017) Coagulating colubrids: evolutionary, pathophysiological and biodiscovery implications of venom variations between Boomslang (Dispholidus typus) and Twig Snake (Thelotornis mossambicanus). Toxins (Basel). https://doi.org/10.3390/toxins9050171
Hiestand PC, Hiestand RR (1979) Dispholidus typus (boomslang) snake venom: purification and properties of the coagulant principle. Toxicon 17:489–498
Li A, Zhang C, Wang J, Wang J, Jiang H, Li J, Ma X, Zhang W, Lu Y (2018) Cloning, expression, purification and bioactivity evaluation of a thrombin-like enzyme from Deinagkistrodon acutus venom gland library. Biotechnol Lett 40:93–102
Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ (2002) Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res 90:E17–E24
Nielsen VG (2018) Crotalus atrox venom exposed to carbon monoxide has decreased fibrinogenolytic activity in vivo in rabbits. Basic Clin Pharmacol Toxicol 122:82–86
Nielsen VG, Garza JI (2014) Comparison of the effects of CORM-2, CORM-3 and CORM-A1 on coagulation in human plasma. Blood Coagul Fibrinolysis 25:801–805
Nielsen VG, Cerruti MA, Valencia OM, Amos Q (2016) Decreased snake venom metalloproteinase effects via inhibition of enzyme and modification of fibrinogen. Biometals 29:913–919
Nielsen VG, Frank N, Matika RW (2018) Carbon monoxide inhibits hemotoxic activity of Elapidae venoms: potential role of heme. Biometals 31:51–59
Steen M (2002) Factor Va-factor Xa interactions: molecular sites involved in enzyme:cofactor assembly. Scand J Clin Lab Invest Suppl 237:5–11
Stocker K, Hauer H, Müller C, Triplett DA (1994) Isolation and characterization of Textarin, a prothrombin activator from eastern brown snake (Pseudonaja textilis) venom. Toxicon 32:1227–1236
Suntravat M, Langlais PR, Sánchez EE, Nielsen VG (2018) CatroxMP-II: a heme-modulated fibrinogenolytic metalloproteinase isolated from Crotalus atrox venom. Biometals, in press. https://doi.org/10.1007/s10534-018-0107-5
Viala VL, Hildebrand D, Trusch M, Fucase TM, Sciani JM, Pimenta DC, Arni RK, Schlüter H, Betzel C, Mirtschin P, Dunstan N, Spencer PJ (2015) Venomics of the Australian eastern brown snake (Pseudonaja textilis): Detection of new venom proteins and splicing variants. Toxicon 107(Pt B):252–265
Villalta M, Pla D, Yang SL, Sanz L, Segura A, Vargas M, Chen PY, Herrera M, Estrada R, Cheng YF, Lee CD, Cerdas M, Chiang JR, Angulo Y, León G, Calvete JJ, Gutiérrez JM (2012) Snake venomics and antivenomics of Protobothrops mucrosquamatus and Viridovipera stejnegeri from Taiwan: keys to understand the variable immune response in horses. J Proteomics 22(75):5628–5645
Wang S, Xu X, Gao S, Zhu S, Rong R, Li B (2014) Purification and partial characterization of a novel fibrinogenase from the venom of Deinagkistrodon acutus: inhibition of platelet aggregation. Protein Expr Purif 99:99–105
Acknowledgements
This investigation was supported by the Department of Anesthesiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose except that Mr. Frank is the owner of Mtoxins.
Rights and permissions
About this article
Cite this article
Nielsen, V.G., Frank, N. Differential heme-mediated modulation of Deinagkistrodon, Dispholidus, Protobothrops and Pseudonaja hemotoxic venom activity in human plasma. Biometals 31, 951–959 (2018). https://doi.org/10.1007/s10534-018-0137-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-018-0137-z