Abstract
Thirteen tetranitro-diazinodiazines have been designed and investigated to find the importance of diazinodiazine fused-heterocyclic backbone for energetic materials. The positional influence of four nitrogen atoms in the diazinodiazine backbone on the heat of formation, performance, and stability has been investigated by density functional theory. It is observed that diazinodiazine is an efficient backbone to improve energy content in the designed derivatives. The energy contribution from the diazinodiazine backbone varies from 352–558 kJ/mol. Introducing four –NO2 groups into the diazinodiazine backbone resulted in new energetic molecules with acceptable detonation performance and stability. Their densities, detonation velocities, and detonation pressures were 1.87–1.89 g/cm3, 8.36–8.68 km/s, and 31.72–34.42 GPa, respectively. The bond dissociation energies for the longer C–NO2 bonds fall in the range of 207–233 kJ/mol, reveals the stable nature of tetranitro-diazinodiazines. Thus, diazinodiazine isomers may be regarded as promising aromatic heterocyclic backbone, and tetranitro-diazinodiazines appear to be potential candidates for energetic materials.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
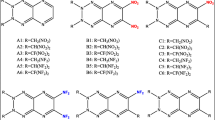
The search and development of new energetic materials that fulfill explicit requirements of high heat of formation (HOF), positive oxygen balance (OB), high crystal density, high detonation properties, and less sensitivity have attracted immense attention worldwide and a stubborn challenge for researchers. Considering the stringent requirement of performance and safety of explosives and propellants, in the past few decades, researchers’ attention has drifted from benchmark nitramines, nitro esters, and nitroaromatic materials to nitrogen-rich heterocyclic compounds [1,2,3,4,5,6,7,8,9,10]. The introduction of nitrogen-rich heterocyclic backbone in energetic materials is proven to be an excellent approach to improve the HOF, density, OB, and performance than their carbocyclic analogs [1, 2]. Furthermore, the high volume of nitrogen gas released in decomposition products exhibits the material’s eco-friendly nature. Aromatic fused-heterocyclic rings are of great interest as energetic backbone owing to their high HOF and good thermal stability [2]. The current strategies to improve detonation performance involve introducing several nitro groups in the fused-heterocyclic rings, which would combine the advantages of each of the components in molecular structure. Here we scrutinize the diazinodiazines having fused six-membered diazine rings [11,12,13,14]. Replacement of the four methines (–CH=) in naphthalene by nitrogen atoms gives diazinodiazine framework without altering the resulting rings aromatic behavior (see Fig. 1). The aromatic structure of diazinodiazines with four nitrogens can be a potential precursor and substrate for developing novel energetic materials. The nitro derivatives of diazinodiazines are less studied and less explored for their potential application in explosives. This work reports a computational study that reveals the significance of fused heterocyclic diazinodiazine backbone in designing energetic materials. We have introduced four –NO2 groups in the diazinodiazine backbone to improve the oxygen balance, density, and performance and understanding the structure-performance-stability characteristics (see Fig. 2). We envisioned that the arrangement of four nitrogens in the diazinodiazine framework and incorporation of –NO2 groups offers opportunities for finding insensitive energetic materials with admirable performance properties.
Computational method
Gaussian 09 program [15] was used to optimize the structures of tetranitro-diazinodiazines (Fig. 2) at B3PW91/6-31G(d,p) level. Optimized structures were confirmed as local energy minima on the potential energy surface without imaginary frequencies. The molecular surface analysis was carried out using the Multiwfn program [16]. The methods and equations adopted for the estimation of the energetic properties of the tetranitro-diazinodiazines are identical to our previous reports [17,18,19] and summarized in the Supporting Information.
Results and discussion
Heat of formation
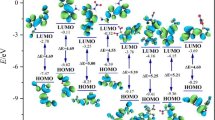
In energetic materials, HOF indicates the energy stored in the molecule. Compared to the single ring pyridazine, pyrimidine, and pyrazine frameworks, the fusion of these rings as diazinodiazine isomers possesses high positive HOFs due to a large number of C–N and N–N bonds (see Fig. 1). The HOF of diazinodiazine isomers was predicted using the Gaussian-4 (G4) theory [20, 21] and isodesmic reaction approach [22, 23] (see Figure S14). The HOFGas obtained from the G4 method are close to those computed from the isodesmic reaction approach. Prior studies also showed that the HOF values calculated with the G4 method are reliable and comparable to experimental values [23,24,25]. Tables 1 and 2 summarize the calculated thermochemistry values and energy content for the diazinodiazine isomers and tetranitro-diazinodiazines, respectively. We studied the effect of isomerism in the diazinodiazine backbone on the HOFs of the designed compounds. In diazine isomers, pyridazine (278.5 kJ/mol) exhibits higher HOF than pyrimidine (185.4 kJ/mol) and pyrazine (203.9 kJ/mol). Hence, it is evident that the presence of pyridazine in fused rings will help to gain better HOF over pyrimidine and pyrazine. The fusion of pyrimidine-pyrazine (B3), pyrazine-pyrazine (B4), and pyrimidine-pyrimidine (B8, B9) rings shows HOF between 352 and 379 kJ/mol, while pyridazine-pyrazine (B1, B2), pyridazine-pyrimidine (B5-7), and pyridazine-pyridazine (B10–13) backbone show HOF between 449 and 558 kJ/mol (see Fig. 1). The HOFGas of tetranitro-diazinodiazines was computed by using the isodesmic reaction method (see Figure S15). With the incorporation of four –NO2 groups in the diazinodiazine backbone, their HOFGas increases by 50–105 kJ/mol, indicating that –NO2 group improves the energy content. It is noteworthy that all the diazinodiazine isomers possess four –NO2 groups, and hence, their HOF values depend on energy contribution from the backbone and position of –NO2 groups on the ring. The four diazinodiazine isomers (P10–13) with fused pyridazine rings exhibit high HOFSolid among the designed compounds. The highest HOFSolid is for P10 (556 kJ/mol), which has the fused pyridazine rings, and four –NO2 groups kept close to each other on the backbone. Overall, all the tetranitro-diazinodiazines show endothermic HOFSolid ranging from 296 (P9) to 556 (P10) kJ/mol caused by the high energy contribution from the diazinodiazine backbone (see Fig. 1) and –NO2 groups.
Detonation performance
Oxygen balance (OB) and density are the crucial properties contributing to the detonation performance of an energetic molecule. All of the tetranitro-diazinodiazines possess a negative OB (–20.5 %), indicating the oxygen deficiency in a molecule that is necessary to transform all hydrogen into H2O and all carbon into CO2. The OB values of tetranitro-diazinodiazines are comparable to RDX and HMX (–21.6%). The densities of tetranitro-diazinodiazines were predicted by using the Politzer method [26] based on electrostatic interaction index and molecular surface properties (see Table S2). The predicted densities of the tetranitro-diazinodiazines range from 1.87–1.89 g/cm3, which are more significant than RDX (1.80 g/cm3) and close to HMX (1.90 g/cm3) [27]. The higher densities of these compounds may be owed to the coplanar fused heterocyclic backbone and four –NO2 groups. The detonation properties were predicted by the Kamlet-Jacobs equation [28]. The computed detonation velocities and pressures of the tetranitro-diazinodiazines are in the range from 8.36 (P9) to 8.68 (P13) km/s and from 31.72 (P9) to 34.42 (P13) GPa, respectively (see Table 3). The higher detonation properties originate from the high energy content, density, and OB, benefited from the nitrogen-rich fused backbone and –NO2 groups. The fusion of pyrimidine and pyrazine rings (P3, P4, P8, and P9) reveals slightly lower detonation performance than the pyridazine ring in the fused backbone. P10–13 displays the best performance, having calculated detonation velocities exceeding 8.65 km/s and detonation pressures above 34.09 GPa, mainly due to their high HOFSolid. These values are comparable to those of RDX (8.60 km/s, 33.92 GPa) [27]. All the tetranitro-diazinodiazines show detonation properties better than TNT (6.94 km/s, 22 GPa) and TATB (8.11 km/s, 31.1 GPa) [27]. According to earlier report [28], 4N2(g), 4CO2(g), and 2C(s) are expected to be the detonation products of P1–P13 (C6N8O8).
Sensitivity correlation
A suitable balance between superior detonation performance and lower sensitivity for practical application and safety is crucial in designing new energetic materials. In this work, bond dissociation energies (BDE), detonation heat release per gram (Q), free space in the crystal lattice (ΔV), and charge on –NO2 group (QNO2) are used for sensitivity comparison with RDX and HMX (see Tables 3 and 4) [29,30,31]. BDE indicates the stability of a chemical bond, where the higher value of BDE specifies the stronger bond and difficulty to rupture. Generally, a BDE more than 120 kJ/mol reveals the stability chemical bond [32, 33]. The bond lengths of the longest C−NO2 range from 1.4835 to 1.4945 Å. All of the tetranitro-diazinodiazines have BDEs above 200 kJ/mol and are expected to exhibit good stability owing to their aromatic fused backbone. The computed BDEs for the longest N–NO2 bond in RDX and HMX are 141 and 152 kJ/mol, respectively. Politzer and Murray [34] demonstrated that from the perspective of sensitivity, large Q values are not preferable. Higher Q values indicate greater sensitivity. The predicted Q values for tetranitro-diazinodiazines range from 1432 (P9) to 1631 (P10) cal/g (see Table 3). Computed Q values of compounds P1, P2, P5–7, and P10–13 are higher than RDX and HMX (1500 cal/g), and likely to be more sensitive. Politzer et al. [35,36,37] showed a correlation between free space (ΔV) available crystal lattice of energetic compound and impact sensitivity, where the greater ΔV value indicates more sensitivity of the compound. The ΔV values of tetranitro-diazinodiazines fall in the range of 68–70 Å3 (see Table 4). This would make tetranitro-diazinodiazines more sensitive than RDX (46 Å3) and HMX (49 Å3). Zhang et al. [38,39,40] suggested the correlation between net –NO2 group charge and molecular stability. The more negative –NO2 charge (–QNO2) stands for the more stable nitro compounds. The computed –QNO2 charges of tetranitro-diazinodiazines range between 0.250 (P2) and 0.301 (P10) e, which are higher than RDX (0.105 e) and HMX (0.112 e), can be regarded as stable. The sensitivity predictions with BDE and QNO2 approach show contradictory results to Q and ΔV method and do not correlate well. These results can be viewed as general trends and provide a crude evaluation of sensitivity for tetranitro-diazinodiazines. Application of uniform and specific approach/equation to estimate the sensitivity of newly designed compounds is very challenging as sensitivity is governed by many molecular, physical, and environmental factors and prone to significant uncertainties.
Conclusions
Diazinodiazine and its tetranitro derivatives are theoretically studied at B3PW91/6-31G(d,p) level. The results exemplify the importance of planar, aromatic, and nitrogen-rich diazinodiazine fused backbone as valuable scaffolds for building potential, stable, and less sensitive energetic materials. The addition of nitro groups to the diazinodiazine backbone subsequently improves their energetic performance and leads to a combination of features that enhance HOF, oxygen balance, density, and performance. The HOFs relate to pyridazine, pyrimidine, and pyrazine rings in the fused backbone and the position of nitro groups. In terms of HOF, fused pyridazine rings have a significant advantage over both pyrimidine and pyrazine rings. The tetranitro-diazinodiazines show HOF > 295 kJ/mol, OB ≈ –20.5 %, density > 1.87 g/cm3, D > 8.36 km/s, and P > 31.72 GPa. Our observations indicate that a combination of diazinodiazine isomers with suitable explosophoric groups could obtain potential energetic compounds while preserving molecular stability.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material.
Code availability
All computations were performed with Gaussian 09 program and Multiwfn program. Gaussian 09 program can be obtained under academic or commercial license from Gaussian, Inc. (https://gaussian.com/). Multiwfn is available free of charge and open-source for both academic and commercial usages (http://sobereva.com/multiwfn/).
References
Gao H, Shreeve JM (2011) Azole-based energetic salts. Chem Rev 111:7377–7436
Gao H, Zhang Q, Shreeve JM (2020) Fused heterocycle-based energetic materials (2012–2019). J Mater Chem A 8:4193–4216
Huynh MHV, Hiskey MA, Hartline EL, Montoya DP, Gilardi R (2004) Polyazido high-nitrogen compounds: hydrazo- and azo-1,3,5-triazine. Angew Chem Int Ed 43:4924–4928
Qu Y, Babailov SP (2018) Azo-linked high-nitrogen energetic materials. J Mater Chem A 6:1915
Fershtat LL, Makhova NN (2020) 1,2,5-Oxadiazole-based high-energy-density materials: synthesis and performance. ChemPlusChem 85:13–42
Koch EC (2016) Insensitive high explosives II: 3,3′-diamino-4,4′-azoxyfurazan (DAAF). Propellants Explos Pyrotech 41:526–538
Gospodinov I, Singer J, Klapötke TM, Stierstorfer J (2019) The pyridazine scaffold as a building block for energetic materials: synthesis, characterization, and properties. Z Anorg Allg Chem 645:1247–1254
Pagoria P (2016) A comparison of the structure, synthesis, and properties of insensitive energetic compounds. Propellants Explos Pyrotech 41:452–469
Zhang S, Gao Z, Lan D, Jia Q, Liu N, Zhang J, Kou K (2020) Recent advances in synthesis and properties of nitrated-pyrazoles based energetic compounds. Molecules 25(15):3475
Politzer P, Murray JS (2017) Nitro groups vs. n-oxide linkages: effects upon some key determinants of detonation performance. Cent Eur J Energ Mater 14(1):3–25
Hurst JK, Wormell P, Krausz E, Lacey AR (1999) The electronic spectrum of 1, 4, 5, 8-tetraazanaphthalene. Chem Phys 246:229–246
Molchanova MS, Pivina TS, Arnautova EA, Zefirov NS (1999) Computer-aided search for high-density energetic compounds among hydrogen-free heterocycles. J Mol Struct (THEOCHEM) 465:11–24
Ishikawa T (2004) Product Class 22: Other Diazinodiazines. Sci Synth 16:1337–1397
Marin-Yaseli MR, Mompean C, Ruiz-Bermejo M (2015) A Prebiotic synthesis of pterins. Chem Eur J 21:13531–13534
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Kurant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salwador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, Revision E.01. Gaussian Inc., Wallingford
Lu T, Chen F (2012) Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Ghule VD (2012) Computational studies on energetic properties of trinitro-substituted imidazole−triazole and pyrazole−triazole derivatives. J Phys Chem A 116:9391–9397
Maan A, Mathpati RS, Ghule VD (2020) Substituted triazolo-triazine derivatives as energetic materials: a computational investigation and assessment. J Mol Model 26:184
Maan A, Mathpati RS, Ghule VD (2020) Energetic triazolo-triazolo-furazano-pyrazines: a promising fused tetracycle building block with diversified functionalities and properties. ChemistrySelect 5:8557–8561
Curtiss LA, Redfern PC, Raghavachari K (2007) Gaussian-4 theory using reduced order perturbation theory. J Chem Phys 127(12):124105
Curtiss LA, Redfern PC, Raghavachari K (2007) Gaussian-4 theory. J Chem Phys 126:084108
Sivaramakrishnan R, Tranter RS, Brezinsky K (2005) Ring conserved isodesmic reactions: a new method for estimating the heats of formation of aromatics and PAHs. J Phys Chem A 109:1621–1628
Dorofeeva OV, Ryzhova ON, Suntsova MA (2013) Accurate prediction of enthalpies of formation of organic azides by combining G4 theory calculations with an isodesmic reaction scheme. J Phys Chem A 117:6835–6845
Dorofeeva OV, Ryzhova ON, Suchkova TA (2017) Enthalpies of formation of hydrazine and its derivatives. J Phys Chem A 121:5361–5370
Suntsova MA, Dorofeeva OV (2014) Use of G4 theory for the assessment of inaccuracies in experimental enthalpies of formation of aliphatic nitro compounds and nitramines. J Chem Eng Data 59(9):2813–2826
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbé A (2009) An electrostatic interaction correction for improved crystal density prediction. Mol Phys 107:2095–2101
Badgujar DM, Talawar MB, Asthana SN, Mahulikar PP (2008) Advances in science and technology of modern energetic materials: An overview. J Hazard Mater 151:289–305
Kamlet MJ, Jacobs SJ (1968) Chemistry of detonations. I. A simple method for calculating detonation properties of C−H−N−O explosives. J Chem Phys 48:23–36
Wang F, Wang G, Du H, Zhang J, Gong X (2011) Theoretical studies on the heats of formation, detonation properties, and pyrolysis mechanisms of energetic cyclic nitramines. J Phys Chem A 115:13858–13864
Yan QL, Zeman S (2013) Theoretical evaluation of sensitivity and thermal stability for high explosives based on quantum chemistry methods: a brief review. Int J Quantum Chem 113:1049–1061
Zeman S, Jungova M (2016) Sensitivity and performance of energetic materials. Propellants Explos Pyrotech 41:426–451
Chung G, Schmidt MW, Gordon MS (2000) An ab initio study of potential energy surfaces for N8isomers. J Phys Chem A 104:5647–5650
Zhao GZ, Lu M (2013) Comparative theoretical studies of energetic pyrazole-pyridine derivatives. J Mol Model 19:3403–3410
Politzer P, Murray JS (2015) Impact sensitivity and the maximum heat of detonation. J Mol Model 21:262
Politzer P, Murray JS (2014) Impact sensitivity and crystal lattice compressibility/free space. J Mol Model 20:2223
Pospíšil M, Vávra P, Concha MC, Murray JS, Politzer P (2010) A possible crystal volume factor in the impact sensitivities of some energetic compounds. J Mol Model 16:895–901
Politzer P, Lane P, Murray JS (2013) Computational characterization of two di-1,2,3,4-tetrazine tetraoxides, DTTO and iso-DTTO, as potential energetic compounds. Cent Eur J Energ Mater 10(1):37–52
Zhang C, Shu Y, Wang X, Zhao X, Tan B, Peng R (2005) A new method to evaluate the stability of the covalent compound: by the charges on the common atom or group. J Phys Chem A 109:6592–6596
Zhang C, Shu Y, Huang Y, Zhao X, Dong H (2005) Investigation of correlation between impact sensitivities and nitro group charges in nitro compounds. J Phys Chem B 109:8978–8982
Zhang C (2009) Review of the establishment of nitro group charge method and its applications. J Hazard Mater 161:21–28
Acknowledgements
Anjali thank UGC-CSIR, Ministry of Human Resource Development, Government of India for the Junior Research Fellowship. VDG thank Armament Research Board, Defence R&D Organization, DRDO for a research grant no. ARMREB/CDSW/2019/211. SD grateful to the financial support from the Start-up Research Grant (No. SRG/2020/000023) and EMEQ scheme (No. EEQ/2020/000025), Science and Engineering Research Board, Department of Science and Technology, Government of India.
Author information
Authors and Affiliations
Contributions
Anjali Maan: conception and design, performed the computations and the numerical calculations, and acquisition of data.
Vikas Dasharath Ghule: designed the model and the computational framework, analysis and interpretation of data, drafting the article, and critical revision of the article
Srinivas Dharavath: analysis and interpretation of data and drafting the article
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supporting information
Computational details, isodesmic reactions, molecular surface properties, selective structural parameters, and optimized coordinates of designed compounds are given in the Supporting Information.
ESM 1
(DOCX 926 kb)
Rights and permissions
About this article
Cite this article
Maan, A., Ghule, V.D. & Dharavath, S. Tetranitro-diazinodiazines as high energy materials: computational investigation of structural aspects of fused heterocyclic backbone and isomerism. Struct Chem 32, 2175–2181 (2021). https://doi.org/10.1007/s11224-021-01791-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-021-01791-1