Abstract
A straightforward, rapid, green, and efficient synthetic procedure has been introduced for easy access to the synthetically highly substituted arylideneisoxazol-5-(4H)-one products. A wide scope of the heterocyclic products was obtained via the three-component cyclocondensation of various aryl aldehydes, NH2OH·HCl, and β-dicarbonyls by zinc oxide nanoparticles (ZnO NPs) as the catalyst. Optimizing the reaction conditions indicated that a relatively small amount of catalyst was required and arylideneisoxazol-5-(4H)-ones were obtained in very short reaction times. Purification of synthesized heterocyclic molecules was carried out with the help of simple filtration and crystallization from ethanol. In this method, exhausting and expensive chromatography methods were not used to purify the products, and the use of organic solvents was also avoided. In addition, this method is important from the point of view of green chemistry. The simplicity of the method, cost-effective catalyst, less time-consuming, mild reaction conditions, conducting experiments at room temperature, as well as recyclability and reusability of ZnO nano-catalyst are among other advantages of this powerful process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
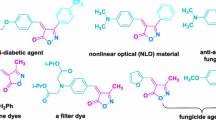
Isoxazoles are the important class of heterocycles that are abundantly found in natural products as well as agrochemicals [1, 2]. The isoxazole ring is present in various bioactive organic compounds with many biological properties, including antibacterial, anticancer, anti-inflammatory, antidiabetic, antidepressant, antifungal, antituberculosis, antirheumatic, antiepileptic, osteoarthritis, analgesic, and anti-Alzheimer [3,4,5,6,7,8,9]. The structure of some commercially available drugs with some aforementioned biologically properties is depicted in Fig. 1. Isoxazoles also show agrochemical applications such as fungicidal, pesticide, insecticide, and herbicide activities [10].
In particular, α,β-unsaturated isoxazole-5(4H)-ones are the type of isoxazole, which have two adjacent hydrogen and oxygen atoms in their ring and are found in many nonlinear optical crystals, donor–acceptor molecules [11, 12], photochromic Stenhouse compounds [13], synthetic agricultural fungicides [14, 15], and bioactive organic molecules [16,17,18,19,20,21,22,23,24]. The structure of a series of heterocycles with isoxazol-5(4H)-one moiety, which have attractive biological properties such as antifungal, anti-Alzheimer, antioxidant, anti-androgen, antitubercular, antileprotic, antimicrobial, anti-HIV, and tyrosinase inhibitor, can be seen in Fig. 2. In addition, α,β-unsaturated isoxazol-5(4H)-ones have been applied as suitable precursors in many organic reactions to synthesize a wide range of diverse densely functionalized active organic compounds, including heterocyclic and acyclic compounds [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Moreover, the isoxazol-5(4H)-one core has become a platform for the development of drug candidates [42]. Therefore, their synthesis is of interest to organic chemistry enthusiasts and they are attractive targets for organic synthesis.
Generally, the best method to synthesize various derivatives of arylideneisoxazole-5(4H)-ones involves the multi-component cyclization of suitable β-keto esters, a variety of aldehydes, and hydroxylamine under catalytic conditions. Some newer catalytic systems including, 1,4-diazabicyclo[2.2.2]octane (DABCO) [43, 44], vitamin B1 [45], supported solid acid [46], Brönsted acidic organic salt [47], lipase [48], amine functionalized dendronized polymer [49], Fe3O4@chitosan-SO3H [50], guanidine hydrochloride [51], ZSM-5 [52], CeO2/TiO2 [53], ionic liquid [HNMP][HSO4] [54], piperazine [55], 2-aminopyridine [56], nano-SiO2–H2SO4 [57], Steglich’s base [58], potassium 2,5-dioxoimidazolidin-1-ide [59], fruit juices [60, 61], silica TLC grade [62], KBr [63], nano-MnO2@zeolite-Y [64], nano-GO@Fe(ClO4)3 [65], Ag-CeO2 [66], Cu(I) in metformin-functionalized β-cyclodextrin (Cu@Met-β‐CD) [67], acid functionalized Fe3O4 nanoparticles [68], urea [69], malic acid [70], citrazinic acid [71], sodium malonate [72], azolium chloride [73], synzyme [74], CuI/Fe3O4NPs@CS-SB [75], and deep eutectic solvents [76, 77].
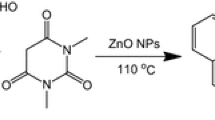
Zinc oxide nanoparticles (ZnO NPs) are one of the most important metal oxide nanoparticles, which, because of their unique physical as well as chemical properties, have many applications as an additive in the rubber, paper, plastic industries, cosmetics, sunscreens, textile industry, optoelectronics industry, semiconductors, agriculture, and nano-dentistry [78,79,80]. The ZnO NPs are nontoxic and biocompatible and show biomedical activities, including anticancer, anti-inflammatory, antioxidant, antidiabetic, antimicrobial, wound healing, bioimaging, as well as in targeted drug delivery [81,82,83,84,85]. These nano-compounds have also been applied as the catalyst in various organic reactions to synthesize various organic compounds due to their advantages such as heterogenous, mild, cheap, minimum execution time, recyclability, commercially available, good chemical stability under ambient temperature, easy workup procedure, and easy preparation [86,87,88,89,90,91,92,93,94]. Considering the unique properties and applications of arylideneisoxazol-5(4H)-ones, herein, we present a new catalyst for the rapid and efficient synthesis of various derivatives of these heterocycles through the three-component cyclocondensation employing hydroxylamine hydrochloride, aryl aldehydes, and β-keto esters (Scheme 1). To the best of our knowledge, ZnO NPs catalyzed synthesis of arylideneisoxazole-5(4H)-ones has not been reported yet.
Experimental
Preparation of ZnO nanoparticles
ZnO NPs were prepared according to reported procedures with modification [95, 96]. At room temperature, zinc chloride (2 mmol) was dissolved in distilled water (20 mL), and then hydrazine hydrate (2 mmol) was added to it, and well stirred for 30 min. The reaction temperature was then increased to 85 °C, and the pH was adjusted to 9 using a sodium hydroxide solution. In this step, gas bubbles were formed on the surface of the solution. After the reaction for 50 min, a white solid was formed. The solid was filtered off, washed with distilled water and ethanol, and dried at 100 °C for 1 h. Lastly, solid was annealed at 350 °C for 3 h.
General procedure for the synthesis of compounds 4a-w using ZnO NPs
In a round-bottom flask, β-keto ester (1a–c, 1 mmol), aryl aldehyde (2, 1 mmol), NH2OH·HCl (3, 1 mmol), and ZnO NPs (5 mol%) were taken. H2O (10 mL) was added to the flask and the reaction mixture was well stirred at room temperature for the duration specified in Table 2. The reaction process was checked by thin-layer chromatography (TLC) analysis. After the reaction was completed, the contents of the reaction vessel were filtered off, washed with water and then cold ethanol, and dried. After that, the remaining precipitate was dissolved in hot EtOH and the catalyst was quickly filtered off. Pure products were obtained after crystallization from the ethanolic filtrate.
4-(4-Hydroxybenzylidene)-3-methylisoxazol-5(4H)-one (4a) 1H NMR (500 MHz, CDC13) δ 2.24 (s, 3H, CH3) 6.94 (d, J = 8.8 Hz, 2H, ArH), 7.79 (s, 1H, H-vinyl), 8.44 (d, J = 8.8 Hz, 2H), 11.1 (s, 1H); 13C NMR (125 MHz, CDC13) δ 11.9 (CH3), 114.6, 124.5, 125.1,136.2, 151.2, 162.6, 164.5 (C=N), 169.4 (C=O).
3-Methyl-4-(4-methylbenzylidene)isoxazol-5(4H)-one (4b) 1H NMR (400 MHz, CDCl3) δ 2.32 (s, 3H, CH3), 2.51 (s, 3H, CH3 of phenyl ring), 7.57 (s, 1H, H-vinyl), 7.27 (d, J = 8.2 Hz, 2H, Ar–H), 8.31 (d, J = 8.2 Hz, 2H, Ar–H); 13C NMR (100 MHz, CDCl3) δ 11.7 (CH3), 22.2 (CH3 of phenyl ring), 118.3, 129.7, 129.8, 134.1, 145.9, 150.1, 161.3 (C=N), 168.4 (C=O).
4-(4-Methoxybenzylidene)-3-methylisoxazol-5(4H)-one (4c) 1H NMR (400 MHz, CDCl3) δ 2.28 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 6.99–7.02 (m, 2H, Ar–H), 7.34 (s, 1H, H-vinyl), 8.45 (d, J = 8.9 Hz, 2H, Ar–H); 13C NMR (100 MHz, CDCl3) δ 11.6 (CH3), 55.7 (OCH3), 114.7, 116.4, 125.8, 136.9, 149.3, 161.3, 164.6 (C=N), 168.8 (C=O).
4-(4-Hydroxy-3-methoxybenzylidene)-3-methylisoxazol-5(4H)-one (4d) 1H NMR (300 MHz, DMSO-d6) δ 2.28 (s, 3H, CH3), 3.91 (s, 3H, OCH3), 7.14 (d, J = 8.6, 1H, Ar–H), 7.76 (s, 1H, H-vinyl), 7.92 (d, J = 8.6, 1H, Ar–H), 8.22 (s, 1H, Ar–H), 9.63 (s, 1 H, OH); 13C NMR (75 MHz, DMSO-d6) δ 11.6 (CH3), 56.2 (OCH3), 111.8, 114.9, 127.2, 128.5, 129.3, 146.4, 151.8, 153.6, 168.7 (C=N), 170.1 (C=O).
4-(4-(Dimethylamino)benzylidene)-3-methylisoxazol-5(4H)-one (4e) 1H NMR (500 MHz, CDCl3) δ 2.29 (s, 3H, CH3), 3.20 (s, 6H, NMe2), 6.76 (dd, J = 1.3, 8.0 Hz, 2H, ArH), 7.26 (s, 1H, H-vinyl), 8.45 (d, J = 8.5 Hz, 2H, ArH); 13C NMR (125 MHz, CDCl3) δ 12.1 (CH3), 40.5 (NMe2), 111.5, 111.9, 121.9, 138.1, 149.7, 154.7, 162.0 (C=N), 170.6 (C=O).
4-(3-Hydroxybenzylidene)-3-methylisoxazol-5(4H)-one (4f) 1H NMR (300 MHz, DMSO-d6) δ 2.28 (s, 3H, CH3), 7.08–7.11 (m, 1H, Ar–H), 7.37 (t, J = 8.0 Hz, 1H, Ar–H), 7.79 (d, J = 7.6 Hz, 1H, Ar–H), 7.86 (s, 1H, H-vinyl), 7.93 (t, 3.0 Hz, 1H, Ar–H), 9.95 (s, 1H, OH); 13C NMR (75 MHz, DMSO-d6) δ 11.8 (CH3), 118.8, 119.8, 121.8, 125.7, 130.2, 134.2, 152.3, 157.8, 162.8 (C=N), 168.3 (C=O).
4-(2-Hydroxybenzylidene)-3-methylisoxazol-5(4H)-one (4g) 1H NMR (300 MHz, DMSO-d6) δ 2.27 (s, 3H, CH3), 6.95 (t, J = 10.0 Hz, 1H, Ar–H), 7.01 (d, J = 9.0 Hz, 1H, Ar–H), 7.47–7.53 (m, 1H, Ar–H), 8.08 (s, 1H, H-vinyl), 8.74 (dd, J = 2.0, 9.5 Hz, 1H, Ar–H), 11.02 (s, 1H, OH); 13C NMR (75 MHz, DMSO-d6) δ 11.8 (CH3), 116.5, 116.8, 119.5, 119.8, 132.9, 137.1, 145.4, 160.2, 162.7 (C=N), 168.9 (C=O).
4-(4-Hydroxy-3-nitrobenzylidene)-3-methylisoxazol-5(4H)-one (4h) 1H NMR (400 MHz, DMSO-d6) δ 2.27 (s, 3H, CH3), 7.27 (d, J = 8.8 Hz, 1H, ArH), 7.92 (s, 1H, H-vinyl), 8.20 (s, 1H, ArH), 8.62 (d, J = 9.2 Hz, 1H, ArH), 9.21 (s, 1H, OH); 13C NMR (100 MHz, CDCl3 and DMSO-d6) δ 11.7 (CH3), 117.8, 119.9, 124.5, 132.3, 137.2, 140.5, 149.6, 157.0, 162.3 (C=N), 168.7 (C=O).
3-Methyl-4-((E)-3-phenylallylidene)isoxazol-5(4H)-one (4i) 1H NMR (500 MHz, CDCl3) δ 2.28 (s, 3H,), 7.47 (m, 2H, ArH), 7.70 (dd, J = 2.04, 5.62 Hz, 2H, ArH), 8.36–8.55 (m, 4H, ArH, H-Ninyl); 13C NMR (125 MHz, CDCl3) δ 17.1 (CH3), 118.3, 122.8, 129.4, 129.6, 131.9, 135.4, 148.4, 151.9, 160.3 (C=N), 170 (C=O).
4-Benzylidene-3-methylisoxazol-5(4H)-one (4j) 1H NMR (300 MHz, DMSO-d6) δ 2.27 (s, 3H, CH3), 7.56 (t, J = 8.0 Hz, 2H, Ar–H), 7.63 (t, J = 8.0 Hz, 1H, Ar–H), 7.97 (s. 1H, H-vinyl), 8.41 (d, J = 8.0 Hz, 2H, Ar–H); 13C NMR (75 MHz, DMSO-d6) δ 11.6 (CH3), 119.3, 129.2, 132.8, 133.9, 134.4, 152.1, 162.7 (C=N), 168.4 (C=O).
3-(Chloromethyl)-4-(4-hydroxybenzylidene)isoxazol-5(4H)-one (4k) 1H NMR (400 MHz, DMSO-d6) δ 4.91 (s, 2H, CH2Cl), 7.03 (d, J = 8.4 Hz, 2H, Ar–H), 8.03 (s, 1H, H-vinyl), 8.47 (d, J = 8.8 Hz, 2H, Ar–H), 11.27 (s, 1H, OH); 13C NMR (100 MHz, DMSO-d6) δ 35.5 (CH2Cl), 110.7, 116.8, 125.1, 138.5, 153.2, 162.1, 164.8 (C=N), 168.8 (C=O).
3-(Chloromethyl)-4-(4-methylbenzylidene)isoxazol-5(4H)-one (4l) 1H NMR (400 MHz, DMSO-d6) δ 2.15 (s, 3H, CH3), 4.88 (s, 2H, CH2Cl), 7.43 (d, J = 8.0 Hz, 2H, Ar–H), 8.11 (s, 1H, H-vinyl), 8.33 (d, J = 8.0 Hz, 2H, Ar–H), 3C NMR (100 MHz, DMSO-d6) δ 21.4 (CH3), 34.8 (CH2Cl), 114.1, 129.6, 134.2, 146.1, 152.9, 161.6 (C=N), 167.6 (C=O).
3-(Chloromethyl)-4-(4-methoxybenzylidene)isoxazol-5(4H)-one (4m) 1H NMR (300 MHz, DMSO-d6) δ 3.94 (s, 3H, OCH3), 4.59 (s, 2H, CH2Cl), 7.04 (dd, J = 2.0, 7.2 Hz, 2H, Ar–H), 7.72 (s, 1H, H-vinyl), 8.53 (dd, J = 2.0, 7.2 Hz, 2H, Ar–H); 13C NMR (75 MHz, DMSO-d6) δ 35.5 (CH2Cl), 55.9 (OCH3), 112.4, 114.7, 125.5, 137.6, 151.1, 160.3, 165.2 (C=N), 168.4 (C=O).
3-(Chloromethyl)-4-(4-hydroxy-3-methoxybenzylidene)isoxazol-5(4H)-one (4n) 1H NMR (300 MHz, DMSO-d6) δ 3.86 (s, 3H, OCH3), 4.87 (s, 2H, CH2Cl), 6.98 (d, J = 8.8 Hz, 1H, Ar–H), 7.93 (dd, J = 8.8 Hz, 1H, Ar–H), 8.01 (s, 1H, H-vinyl), 8.54 (d, J = 8.4 Hz, 1H, Ar–H), 11.04 (s, 1H, OH); 13C NMR (75 MHz, DMSO-d6) δ 35.7 (CH2Cl), 56.1 (OCH3), 110.4, 116.4, 117.2, 125.3, 132.8, 148.1, 153.4, 155.2, 162.3 (C=N), 169.2 (C=O).
3-(Chloromethyl)-4-(4-(dimethylamino)benzylidene)isoxazol-5(4H)-one (4o) 1H NMR (400 MHz, CDCl3) δ 3.22 (s, 6H, NMe2), 4.56 (s, 2H, CH2Cl), 6.78 (d, J = 8.6 Hz, 2H, Ar–H), 7.55 (s, 1H, H-vinyl), 8.47 (d, J = 7.2 Hz, 2H, Ar–H); 13C NMR (100 MHz, CDCl3) δ 35.6 (NMe2), 40.2 (CH2Cl), 107.3, 111.7, 121.7, 125.4, 138.3, 150.4, 154.8, 160.5 (C=N), 169.7 (C=O).
3-(Chloromethyl)-4-(3-hydroxybenzylidene)isoxazol-5(4H)-one (4p) 1H NMR (300 MHz, DMSO-d6) δ 4.82 (s, 2H, CH2Cl), 7.04 (d, J = 7.6 Hz, 1H, Ar–H), 7.44 (t, J = 8.0 Hz, 1H, Ar–H), 7.82 (d, J = 7.6 Hz, 1H, Ar–H), 7.86 (s, 1H, H-vinyl), 8.02 (s, 1H, Ar–H), 9.95 (s, 1H, OH); 13C NMR (75 MHz, DMSO-d6) δ 37.3 (CH2Cl), 112.3, 115.2, 119.1, 125.4, 130.8, 136.6, 152.5, 156.5, 164.7 (C=N), 169.6 (C=O).
4-Benzylidene-3-(chloromethyl)isoxazol-5(4H)-one (4q) 1H NMR (400 MHz, DMSO-d6) δ 4.93 (s, 2H CH2Cl), 7.72–7.58 (m, 3H, Ar–H), 8.18 (s, 1H, H-vinyl), 8.41 (d, J = 7.2 Hz, 2H, Ar–H); 13C NMR (100 MHz, DMSO-d6) δ 34.9, 115.6, 129.1, 132.2, 133.8, 134.5, 152.9, 161.6 (C=N), 167.3 (C=O).
4-(4-Hydroxybenzylidene)-3-phenylisoxazol-5(4H)-one (4r) 1H NMR (300 MHz, DMSO-d6) δ 6.96 (d, J = 9.0 Hz, 2H, Ar–H), 7.62–7.70 (m, 6H, Ar–H, H-vinyl), 8.45 (d, J = 9.0 Hz, 2H, Ar–H), 11.2 (s, 1H, OH); 13C NMR (75 MHz, DMSO-d6) δ 113.2, 116.6, 124.9, 127.9, 129.3, 129.7, 131.3, 133.2, 138.5, 153.7, 164.8 (C=N), 169.4 (C=O).
4-(4-Methylbenzylidene)-3-phenylisoxazol-5(4H)-one (4s) 1H NMR (400 MHz, CDCl3) δ 2.45 (s, 3H, CH3), 7.38 (d, J = 8.8 Hz, 2H, Ar–H), 7.59 (s, 1H, H-vinyl), 7.63–7.75 (m, 5H, Ar–H), 8.35 (d, J = 8.8 Hz, 2H, Ar–H).
4-(4-Methoxybenzylidene)-3-phenylisoxazol-5(4H)-one (4t) 1H NMR (300 MHz, DMSO-d6) δ 3.91 (s, 3H, OCH3), 7.14 (d, J = 9.0 Hz, 2H, Ar–H), 7.61–7.72 (m, 5H, Ar–H), 7.74 (s, 1H, H-vinyl), 8.52 (d, J = 8.7 Hz, 2H, Ar–H); 13C NMR (75 MHz, DMSO-d6) δ 56.5 (OCH3), 114.7, 115.1, 126.1, 127.8, 129.3, 129.7, 131.4, 137.8, 153.5, 164.7, 165.1 (C=N), 169.2 (C=O).
4-(4-Hydroxy-3-methoxybenzylidene)-3-phenylisoxazol-5(4H)-one (4u) 1H NMR (300 MHz, DMSO-d6) δ 3.91 (s, 3H, OCH3), 7.08 (d, J = 3.9, 8.7 Hz, 1H, Ar–H), 7.61–7.69 (m, 6H, Ar–H, H-vinyl), 8.21 (dd, J = 1.5, 8.4 Hz, 1H, Ar–H), 8.23 (s, 1H, Ar–H), 9.65 (s, 1H, OH); 13C NMR (75 MHz, DMSO-d6) δ 56.5 (OCH3), 112.2, 114.2, 120.3, 126.5, 127.8, 129.2, 129.8, 130.7, 131.2, 146.7, 153.8, 154.5, 164.6 (C=N), 169.2 (C=O).
4-(4-(Dimethylamino)benzylidene)-3-phenylisoxazol-5(4H)-one (4v) 1H NMR (300 MHz, DMSO-d6) δ 2.24 (s, 3H, CH3), 3.17 (s, 6H, NMe2), 6.65 (s, 1H, H-vinyl), 6.87 (d, J = 9.3 Hz, 2H, ArH), 8.47 (d, J = 9.0 Hz, 2H, ArH); 13C NMR (75 MHz, DMSO-d6) δ 40.3 (NMe2), 107.7, 112.3, 121.4, 128.6, 129.3, 129.5, 130.8, 138.7, 152.2, 155.3, 164.7 (C=N), 170.4 (C=O).
4-Benzylidene-3-phenylisoxazol-5(4H)-one (4w) 1H NMR (300 MHz, DMSO-d6) δ 7.60–7.80 (m, 8H, Ar–H), 7.91 (s, 1H, H-vinyl), 8.42 (dd, J = 1.2, 7.2 Hz, 2H, Ar–H); 13C NMR (75 MHz, DMSO-d6) δ 118.5, 127.5, 129.2, 129.3, 129.8, 131.6, 132.7, 134.2, 134.5, 154.0, 164.4 (C=N), 168.3 (C=O).
Results and discussion
ZnO NPs were synthesized with minor modifications according to the documented procedure [95, 96] and characterized using scanning electron microscopy (SEM) and X-ray diffraction techniques (supporting information). The nanostructure and crystallite size of the particles was confirmed by XRD, SEM, transmission electron microscope (TEM Philips EM 208S), and comparison with the literature [97, 98]. The X-ray diffraction pattern of ZnO NPs (Fig. 3) shows definite line broadening of the X-ray diffraction peaks, showing that the prepared particles were in the nanoscale range. The diffraction peaks located at 31.9, 34.5, 36.2, 47.6, 56.7, 63.1, and 67.8° have been indexed as the spherical to the hexagonal phase of ZnO with high crystallinity [82, 98]. Based on these characteristic peaks in XRD, there is no specific impurity in the synthesized particles.
SEM images of ZnO NPs (see supporting) show that the particles are semirod and semispherical and have accumulated in a compact form. The particles are almost homogeneous and this homogeneity has caused ZnO NPs to play important roles in different activities. The morphology of synthesized ZnO NPs can also be determined based on TEM images (Fig. 4). Accordingly, the particle size is less than 25 nm.
After this, the model reaction (a mixture of 1 equiv. of ethyl 3-oxobutanoate 1a, 4-hydroxybebzaldehyde 2a, and NH2OH·HCl 3) under water solvent at room temperature deprived of using any catalyst was conducted and after 100 min., 30% of product 4a was isolated (Table 1, entry 1). Then, the same three-component reaction was accomplished using nano-ZnO as the catalyst at RT under water solvent. It was decided to use 1 mol% of the catalyst in the reaction. As a result of this reaction, the corresponding product (4a) was obtained with an isolated reaction yield of 40% afterward 80 min (Table 1, entry 2). This was a good result and encouraged us to check other amounts of catalyst. When 2 mol% of nano-ZnO catalyst was utilized, the desired heterocyclic product 4a was attained with 55% isolated reaction yield after 50 min. (Table 1, entry 3). Using 2.5 mol% of the catalyst, the model reaction proceeded to afford the isoxazolone product 4a in 66% isolated reaction yield (Table 1, entry 4). Due to the gradual increase of the catalyst along with the increase in the yield of the reaction and decrease in reaction time, the amount of catalyst was increased to 5 mol%. In this case, the reaction gave the best-isolated reaction yield (98%) after 3 min (Table 1, entry 5). Adding higher amounts of the catalyst did not lead to an improvement in the yield of reaction or reaction time (Table 1, entries 6 and 7). Therefore, the optimal amount of the catalyst was selected as 5 mol%. Besides water solvent, other solvents including ethanol, acetonitrile, dichloromethane, chloroform, and ethyl acetate were also tested using the optimum catalyst loading. None of these solvents had the same positive effect as water on the reaction (Table 1, entries 8–12). When a 1:1 volumetric mixture of water and ethanol was utilized, the corresponding isoxazole-5-one (4a) was achieved in 98% isolated yield and 1.5 min (Table 1, entry 13). This effect was better than a water solvent in terms of reaction time, but since water is cheaper, greener, and safer, it was chosen as the best solvent for other reactions. After all the above-mentioned investigations, we found that the best solvent for the reaction is water, and the best temperature condition is room temperature. Apart from checking different amounts of catalyst loading and solvents, the temperature parameter was also checked in the water solvent. Performing the reaction at temperatures of 40, 50, and 60 °C did not significantly change the time and yield (Table 1, entries 14–16). Considering this result, we avoided checking the reaction at other temperatures. Finally, the model reaction was evaluated under solvent-free conditions, and the corresponding isoxazolone product 4a was separated in 86% yield after 40 min (Table 1, entry 17). Thus, the optimized conditions were determined as nano-ZnO catalyst (5 mol%), water solvent, and RT (Table 1, entry 5).
After determining the optimized conditions of the reaction, the generality of this reaction was evaluated using other substituted aldehyde precursors. The aryl aldehydes containing electron-donating groups underwent hetero-cyclization in 1.5–5.5 min to obtain the corresponding heterocyclic compounds (4b-f) in 85–97% isolated yields (Table 2, entries 2–6). Also, the sterically hindered 2-hydroxybenzaldehyde (2g) successfully participated in the three-component reaction and obtained a 92% isolated yield of compound 4g in 3 min (Table 2, entry 7). When cinnamaldehyde (2i) was employed as an α,β-unsaturated aldehyde precursor, the nano-ZnO catalyzed hetero-cyclization process has occurred and the isoxazolone product 4i obtained with an excellent yield (94%) after 3 min (Table 2, entry 9). When substituted benzaldehydes containing electron-accepting functional groups were used in the reaction, the desired heterocyclic products were not obtained, which is probably due to the destabilizing effect of these substituents. Surprisingly, when a substituted benzaldehyde containing both electron-releasing and electron-accepting groups (2h) was treated with NH2OH·HCl (3) and β-keto ester 1a, the desired heterocyclic 4-(4-hydroxy-3-nitrobenzylidene)-3-methylisoxazole-5(4H)-one (4h) was achieved with 92% isolated reaction yield in 3 min (Table 2, entry 8). To further investigate the generality of the precursor, two other β-keto-esters 1b and 1c were tested in this catalytic hetero-cyclization. When ethyl acetoacetate (1a) was changed to ethyl 4-chloroacetoacetate (1b) or ethyl benzoylacetate (1c), the three-component reactions could furnish the arylideneisoxazol-5(4H)-ones (4k–w) in good to excellent yields (Table 2, entries 11–23).
Recyclability and the ability to reuse the catalyst are important from the point of view of green chemistry [99]. For this reason, the cyclability and reuse of the catalyst were investigated. After the reaction is complete, the crude product along with the catalyst was collected after simple filtration from the reaction mixture and washed with a minimum amount of EtOH. To separate of catalyst from the products, the solid mixture remaining on the filter paper was liquified in hot EtOH and quickly filtered off (The isoxazolone products were easily liquified in hot EtOH and the catalyst remained insoluble.). The remaining ZnO NPs catalyst on the filter paper was washed with EtOH and dried. The recovered nano-catalyst was used in the model reaction under optimized reaction conditions. The experimental results revealed that the catalyst can be recycled up to four times. In four runs, the product was obtained with yields of 90, 85, 76, and 70% after 5, 9, 15, and 20 min, respectively.
According to the literature [65, 104], the possible reaction mechanism is drawn in Scheme 2. Accordingly, the condensation between hydroxylamine and β-ketoester leads to the formation of oxime intermediate. The reaction between the activated aldehyde and the oxime intermediate leads to Knoevenagel condensation and the corresponding intermediate is formed. Intramolecular cyclization and the subsequent emersion of ethanol lead to the formation of the final arylideneisoxazol-5(4H)-one products (Scheme 2).
A comparative study of this work with previously reported works was carried out for the synthesis of 4a (Table 3). The results of these investigations indicate that this work has advantages over some previous catalysts. Among these advantages can be a lower amount of catalyst (compared to entries 1–5, 7–13, 16, 18–23, and 25 in Table 3), shorter reaction times (compared to entries 1–25 in Table 3), relatively higher yields (compared to entries 1–6, 10–15, 17–20, and 22–25 in Table 3), performing the reaction in an aqueous medium (in comparison with entries 20 and 25), avoiding heating or irradiation (in comparison with entries 1, 2, 5, 6, 9, 11, 14, 15, 20, 22, 24, and 25), and one-step preparation of the catalyst instead of several steps (in comparison with entries 9 and 15).
Conclusions
In summary, a multicomponent reaction (MCR) for the synthesis of fully substituted arylideneisoxazol-5(4H)-ones using ZnO NPs has been developed. The MCR proceeded smoothly through the Knoevenagel condensation followed by hetero-cyclization to afford a series of desired heterocyclic compounds with acceptable reaction yields. The screening of solvents by means of a nano-ZnO as the catalyst revealed that water was the best choice solvent. During the synthesis of arylideneisoxazol-5(4H)-ones, using aryl aldehydes with various functional groups, in the case of substituted aldehydes comprising electron-releasing functional groups, excellent results were obtained, while the use of substituted aldehydes containing electron-withdrawing functional groups did not result in the construction of arylideneisoxazol-5(4H)-ones. The nano-ZnO catalyst was recycled and reused for four consecutive runs. This method was environment-friendliness and simple, as well as the used nano-catalyst was nontoxic and stable at room temperature. Short reaction times, aqueous conditions, and recyclability of catalyst all of which were beneficial to the economy and environment.
References
S. Madhavan, S.K. Keshri, M. Kapur, Asian J. Org. Chem. 10, 3127 (2021)
S. Das, K. Chanda, Rsc Adv. 11, 32680 (2021)
P. Pattanayak, T. Chatterjee, J. Org. Chem. 88, 5240 (2023)
M. Fawzi, A. Oubella, A. Bimoussa, F.Z. Bamou, Z.A. Khdar, A. Auhmani, A. Riahi, A. Robert, H. Morjani, M.Y.A. Itto, Comput. Biol. Chem. 98, 107666 (2022)
K. Manvinder, Y. Mohamad, M.S. Dharambeer, S.S. Harvinder, Curr. Org. Synth. 17, 671 (2020)
A. Al-Azmi, M.A. Shalaby, Curr. Org. Chem. 25, 849 (2021)
L. Alonso, K.E. Pianoski, A. Alonso, F.A. Rosa, J. Mol. Struct. 1249, 131604 (2022)
M. Antony, G.L. Balaji, P. Iniyavan, H. Ila, J. Org. Chem. 85, 15422 (2020)
A. Thakur, M. Verma, R. Bharti, R. Sharma, Tetrahedron 119, 132813 (2022)
G.J. Yu, S. Iwamoto, L.I. Robins, J.C. Fettinger, T.C. Sparks, B.A. Lorsbach, M.J. Kurth, J. Agric. Food Chem. 57, 7422 (2009)
M. Gao, J. Zhang, X. Zhang, D. Xu, Z. Hu, J. Yao, Y. Wu, Cryst. Growth Des. 21, 3153 (2021)
Y. Yang, J. Zhao, X. Zhang, Z. Hu, Y. Wu, CrystEngComm 25, 1313 (2023)
D.E. Nánási, A. Kunfi, Á. Ábrahám, P.J. Mayer, J. Mihály, G.F. Samu, É. Kiss, M. Mohai, G. London, Langmuir 37, 3057 (2021)
A. Macchia, A. Eitzinger, J.F. Brière, M. Waser, A. Massa, Synthesis 53, 107 (2021)
I.F. Eckhard, K. Lehtonen, T. Staub, L.A. Summers, Aus. J. Chem. 26, 2705 (1973)
S.S. Wazalwar, A.R. Banpurkar, F. Perdih, J. Mol. Struct. 1150, 258 (2017)
S.J. Kim, J. Yang, S. Lee, C. Park, D. Kang, J. Akter, S. Ullah, Y.J. Kim, P. Chun, H.R. Moon, Bioorg. Med. Chem. 26, 3882 (2018)
S.H. Sukanya, T. Venkatesh, R. Kumar, Y.D. Bodke, Chem. Data Collect. 33, 100713 (2021)
K.B. Badiger, S.Y. Khatavi, K. Kamanna, R.S.C. Med, Chem. 13, 1367 (2022)
A. Saleem, U. Farooq, S.M. Bukhari, S. Khan, A. Zaidi, T.A. Wani, A.J. Shaikh, R. Sarwar, S. Mahmud, M. Israr, F.A. Khan, S.A. Shahzad, ACS Omega 7, 30359 (2022)
M. Ali, U. Saleem, F. Anwar, M. Imran, H. Nadeem, B. Ahmad, T. Ali, T. Ismail, Neurochem. Res. 46, 905 (2021)
T.D. Bhatt, D.G. Gojiya, P.L. Kalavadiya, H.S. Joshi, ChemistrySelect 4, 11125 (2019)
T. Anwar, H. Nadeem, S. Sarwar, H. Naureen, S. Ahmed, A. Khan, M. Arif, Drug Dev. Res. 81, 893 (2020)
A.P. Chavan, R.R. Deshpande, N.A. Borade, A. Shinde, P.C. Mhaske, D. Sarkar, V.D. Bobade, Med. Chem. Res. 28, 1873 (2019)
E.E. Galenko, M.A. Kryukova, M.S. Novikov, A.F. Khlebnikov, J. Org. Chem. 86, 6888 (2021)
C. Loro, L. Molteni, M. Papis, L.L. Presti, F. Foschi, E.M. Beccalli, G. Broggini, Org. Lett. 24, 3092 (2022)
N.M.R. Capreti, I.D. Jurberg, Org. Lett. 17, 2490 (2015)
Y. Wang, D.M. Du, J. Org. Chem. 85, 15325 (2020)
X. Tian, Y. Zhang, H. Dong, W. Ren, Y. Wang, J. Org. Chem. 87, 9593 (2022)
E.E. Galenko, S.A. Linnik, O.V. Khoroshilova, M.S. Novikov, A.F. Khlebnikov, J. Org. Chem. 84, 11275 (2019)
A. Macchia, F.F. Summa, G. Monaco, A. Eitzinger, A.R. Ofial, A. Di Mola, A. Massa, ACS Omega 7, 8808 (2022)
S.H. Wan, X.A. Li, Y.H. Liu, S.T. Liu, Org. Biomol. Chem. 18, 9516 (2020)
Y. Zheng, S. Qian, P. Xu, T. Ma, S. Huang, Adv. Synth. Catal. 364, 3800 (2022)
P. Martinez-Pardo, A. Lavios, A. Sanz-Marco, C. Vila, J.R. Pedro, G. Blay, Adv. Synth. Catal. 362, 3564 (2020)
B.D. Cui, S.W. Li, J. Zuo, Z.J. Wu, X.M. Zhang, W.C. Yuan, Tetrahedron 70, 1895 (2014)
Y. Wang, C. Niu, D.-H. Xie, D.-M. Du, Org. Biomol. Chem. 19, 8572 (2021)
A.F. da Silva, I.A. Leonarczyk, M.A.B. Ferreira, I.D. Jurberg, Org. Chem. Front. 7, 3599 (2020)
W. Xiao, Q.Q. Yang, Z. Chen, Q. Ouyang, W. Du, Y.C. Chen, Org. Lett. 20, 236 (2018)
G. McArthur, S. Abel, G. Volpin, D.M. Barber, Eur. J. Org. Chem. 2022, e202200947 (2022)
E.S. Uvarova, A.V. Kutasevich, E.S. Lipatov, V.S. Mityanov, Org. Biomol. Chem. 21, 651 (2023)
Y.E. Ryzhkova, V.M. Kalashnikova, F.V. Ryzhkov, M.N. Elinson, Molbank 2022, M1317 (2022)
R. Torán, C. Vila, A. Sanz-Marco, M.C. Muñoz, J.R. Pedro, G. Blay, Eur. J. Org. Chem. 2020, 627 (2020)
A. Thakur, M. Verma, P. Setia, R. Bharti, R. Sharma, A. Sharma, N.P. Negi, V. Anand, R. Bansal, Res. Chem. Intermed. 49, 859 (2023)
D.R. Mishra, B.S. Panda, S. Nayak, N.K. Rauta, S. Mohapatra, C.R. Sahoo, R.N. Padhy, Tetrahedron 124, 133015 (2022)
D. Zhang, C. Liu, L. Ren, W. Li, B. Luan, Y. Zhang, ChemistrySelect 8, e202204658 (2023)
L.S.K. Achary, R. Parida, A. Kumar, S. Giri, P. Dash, Mater. Chem. Phys. 285, 126096 (2022)
G. Aleaba, S.K. Asadi, N. Daneshvar, F. Shirini, Polycycl. Aromat. Compd. 42, 7569 (2022)
S.S. Kapale, Y.U. Gadkari, H.K. Chaudhari, Polycycl. Aromat. Compd. (2002). https://doi.org/10.1080/10406638.2022.2096649
K. Hiba, S. Prathapan, K. Sreekumar, Catal. Lett. 152, 2457 (2022)
Z. Ghasemi, A.H. Amale, S. Azizi, S. Valizadeh, J. Soleymani, Rsc Adv. 11, 36958 (2021)
A.B. Barkule, Y.U. Gadkari, V.N. Telvekar, Polycycl. Aromat. Compd. 42, 5870 (2022)
N.T. Hatvate, S.M. Ghodse, Synth. Commun. 50, 3676 (2020)
S. Maddila, L. Devi, P. Muralidhar, K. Nagaraju, S.B. Jonnalagadda, Inorg. Chem. Commun. 143, 109741 (2022)
G.D. Shirole, A.S. Tambe, S.N. Shelke, Indian J. Chem. 59B, 459 (2020)
Z. Daroughezadeh, H. Kiyani, Heterocycles 104, 1625 (2022)
Z. Faramarzi, H. Kiyani, Heterocycles 102, 1779 (2021)
F. Ghorbani, H. Kiyani, S.A. Pourmousavi, Res. Chem. Intermed. 46, 943 (2020)
Z. Faramarzi, H. Kiyani, Polycycl. Aromat. Compd. 43, 3099 (2023)
N. Reihani, H. Kiyani, Curr. Org. Chem. 25, 950 (2021)
B.M. Patil, S.K. Shinde, A.A. Jagdale, S.D. Jadhav, S.S. Patil, Res. Chem. Intermed. 47, 4369 (2021)
S. Gulati, R. Singh, S. Sangwan, Sci. Rep. 11, 23563 (2021)
H.K. Kadam, K. Salkar, A.P. Naik, M.M. Naik, L.N. Salgaonkar, L. Charya, K.C. Pinto, V.K. Mandrekar, T. Vaz, ChemistrySelect 6, 11718 (2021)
P. Kulkarni, J. Indian Chem. Soc. 98, 100013 (2021)
M. Kalhor, S. Samiei, S.A. Mirshokraie, SILICON 13, 201 (2021)
E. Madandar, F.K. Behbahani, Russ. J. Org. Chem. 58, 830 (2022)
S.M.A. Ali, A. Chrouda, M.B. Elamin, I.S. Yahia, H.Y. Zahran, S.H. Zyoud, Appl. Phys. A 128, 897 (2022)
M. Tajbakhsh, M.R. Naimi-Jamal, S. Balalaie, M. Rezaeian, Sci. Rep. 12, 19106 (2022)
R. Nongrum, R. Nongkhlaw, S.P. Majaw, J. Kumari, D. Sriram, R. Nongkhlaw, Sustain. Chem. Pharm. 32, 100967 (2023)
F. Haydari, H. Kiyani, Res. Chem. Intermed. 49, 837 (2023)
S.Z. Tahmasabi, H. Kiyani, H.A. Samimi, Lett. Org. Chem. 20, 167 (2023)
H. Ostadzadeh, H. Kiyani, Org. Prep. Proced. Int. (2023). https://doi.org/10.1080/00304948.2023.2192601
S. Gharehassanlou, H. Kiyani, Indian J. Chem. 61, 515 (2022)
A. Moradi Delfani, H. Kiyani, M. Zamani, Curr. Org. Chem. 26, 1575 (2022)
G.H.C. Oliveira, L.M. Ramos, R.K.C. de Paiva, S.T.A. Passos, M.M. Simões, F. Machado, J.R. Correa, B.A.D. Neto, Org. Biomol. Chem. 19, 1514 (2021)
S. Valizadeh, Z. Ghasemi, A. Shahrisa, M. Pirouzmand, R. Kabiri, React. Func. Polym. 171, 105172 (2022)
H. Atharifar, A. Keivanloo, B. Maleki, Org. Prep. Proced. Int. 52, 517 (2020)
F.K. Damghani, H. Kiyani, S.A. Pourmousavi, Curr. Green Chem. 7, 217 (2020)
J. Jiang, J. Pi, J. Cai, Bioinorg. Chem. Appl. (2018) Article ID 1062562, 18 pages
M. Zare, K. Namratha, S. Ilyas, A. Sultana, A. Hezam, M.A. Surmeneva, R.A. Surmenev, M.B. Nayan, S. Ramakrishna, S. Mathur, K. Byrappa, Acs Food Sci. Technol. 2, 763 (2022)
H. Moradpoor, M. Safaei, H.R. Mozaffari, R. Sharifi, M.M. Imani, A. Golshahe, N. Bashardoust, Rsc Adv. 11, 21189 (2021)
J. Dhatwalia, A. Kumari, A. Chauhan, K. Mansi, S. Thakur, R.V. Saini, I. Guleria, S. Lal, A. Kumar, K.M. Batoo, Materials 15, 3470 (2022)
S. Faisal, H. Jan, S.A. Shah, S. Shah, A. Khan, M.T. Akbar, M. Rizwan, F. Jan, N. Akhtar, A. Khattak, S. Syed, ACS Omega 6, 9709 (2021)
L.E. Shi, Z.H. Li, W. Zheng, Y.F. Zhao, Y.F. Jin, Z.X. Tang, Food Addit. Contam. Part A 31, 173 (2014)
S. Raha, Md. Ahmaruzzaman, Nanoscale Adv. 4, 1868 (2022)
S.A. Al-Ghamdi, T.A. Alkathiri, A.E. Alfarraj, O.M. Alatawi, A.S. Alkathiri, C. Panneerselvam, S. Vanaraj, A.A.A. Darwish, T.A. Hamdalla, A. Pasha, S. Khasim, Res. Chem. Intermed. 48, 4769 (2022)
Y.Y. Chan, Y.L. Pang, S. Lim, W.C. Chong, J. Environ. Chem. Engin. 9, 105417 (2021)
Y. Madan, R. Gupta, J. Heterocycl. Chem. 55, 402 (2018)
H. Narimani, J. Synth. Chem. 1, 62 (2022)
M. Saha, A.R. Das, Curr. Green Chem. 7, 53 (2020)
N.F.H. Mahmoud, A.M. El-Saghier, J. Heterocycl. Chem. 56, 1820 (2019)
K.D. Dhawale, A.P. Ingale, S.V. Shinde, N.M. Thorat, L.R. Patil, Synth. Commun. 51, 1588 (2021)
B.J. Banerjee, Nanostruct. Chem. 7, 389 (2017)
V. Kumar, D. Singh, A.K. Paul, R. Shrivastava, V. Singh, New J. Chem. 43, 18304 (2019)
S.R. Attar, B. Shinde, S.B. Kamble, Res. Chem. Intermed. 46, 4723 (2020)
J.N. Hasnidawani, H.N. Azlina, H. Norita, N.N. Bonnia, S. Ratim, E.S. Ali, Procedia Chem. 19, 211 (2016)
S.S. Kumar, P. Venkateswarlu, V.R. Rao, G.N. Rao, Int. Nano Lett. 3, 30 (2013)
H. Sachdeva, R. Saroj, Sci. World J. 2013, 680671 (2013)
N.M. Shamhari, B.S. Wee, S.F. Chin, K.Y. Kok, Acta Chim. Slov. 65, 578 (2018)
A. Kumar, D. Saxena, M.K. Gupta, Green Chem. 15, 2699 (2013)
H. Kiyani, F. Ghorbani, Res. Chem. Intermed. 41, 2653 (2015)
S.R. Deshmukh, A.S. Nalkar, S.R. Thopate, J. Chem. Sci. 134, 15 (2022)
M. Shanshak, S. Budagumpi, J.G. Małecki, R.S. Keri, Appl. Organomet. Chem. 34, e5544 (2020)
Z. Daroughezadeh, H. Kiyani, Heterocycles 106, 1187 (2023)
H. Kiyani, F. Ghorbani, Res. Chem. Intermed. 42, 6831 (2016)
H. Kiyani, H.A. Samimi, Chiang Mai J. Sci. 44, 1011 (2017)
H. Kiyani, A. Mosallanezhad, Curr. Org. Synth. 15, 715 (2018)
F. Saikh, J. Das, S. Ghosh, Tetrahedron Lett. 54, 4679 (2013)
H. Kiyani, F. Ghorbani, Res. Chem. Intermed. 41, 7847 (2015)
Q.F. Cheng, X.Y. Liu, Q.F. Wang, L.S. Liu, W.J. Liu, Q. Lin, X.J. Yang, Chin. J. Org. Chem. 29, 1267 (2009)
H. Kiyani, H. Darbandi, A. Mosallanezhad, F. Ghorbani, Res. Chem. Intermed. 41, 7561 (2015)
S.B. Kasara, S.R. Thopate, Curr. Organocatal. 6, 231 (2019)
A.B.S. Sampaio, M.S.S. Mori, L.C. Albernaz, L.S. Espindola, C.E.M. Salvador, C.K.Z. Andrade, Catalysts 13, 518 (2023)
Acknowledgements
The authors are thankful to Damghan University Research Council. The corresponding author would also like to thank Shahrekord University.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The first draft revision of the manuscript was written by Dr. HK Synthesis of compounds and conducting the experiments in the laboratory were performed by M.Sc. SA.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
We give our consent for publication.
Ethical approval
The authors declare that the research process did not involve any human or animal experiments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aslanpour, S., Kiyani, H. Rapid synthesis of fully substituted arylideneisoxazol-5(4H)-one using zinc oxide nanoparticles. Res Chem Intermed 49, 4603–4619 (2023). https://doi.org/10.1007/s11164-023-05059-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-05059-7