Abstract
A transition metal/ligand/additive/promoter-free synthesis of 3-methyl-4-arylmethylene-isoxazol-5(4H)-ones and the Biginelli-like synthesis is carried out in a natural acidic medium of Averrhoa bilimbi extract (ABE) with cleaner and facile approach smentioned here. The isoxazol-5(4H)-ones and 11-acetyl-2-methyl-5,6-dihydro-2H-2,6-methanobenzo[g][1,3,5]-oxadiaazocin-4(3H)-ones are synthesized, respectively, under aerobic conditions at room temperature and at reflux temperature of ethanol. This eco-friendly and economically cheap, non-toxic acidic catalytic media is obtained from the renewable resource, and its dynamic phase is confirmed by the optical microscopy, DLS technique, and with critical micelle concentration (c.m.c.) measurements. The notable advantages are excellent yields of the obtained products, versatility in handling substrates, reuse of the catalyst, use of no hazardous organic solvents, and minimization of waste or side products. So, the reported procedure is simple, evergreen, and a sound alternative to the existing protocols for isoxazol-5(4H)-one synthesis and for Biginelli-like synthesis as well.
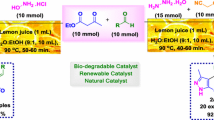
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The selection of an appropriate reaction medium implementing solvent or solvent mixture is of paramount importance besides task-specific reaction catalysis and energy requirements, in the synthetic research field. One important aspect of green chemistry is the selection of solvents with minimum toxicity, pollution, or energy demand, practically which is achieved by the replacement of hazardous substrates in chemical processes with relatively ecologically benign ones. From this perspective, water is the most studied solvent from a green chemistry standpoint, and it is the solvent selected by nature do carry out the biological processes. Reactions in the aqueous media are generally environmentally safe, simple to handle, comparatively cheaper to operate, and especially important in the industry [1, 2]. As an effect, using water as a reaction medium is worthwhile to be explored and is still an active field of research opening a pace to new reactivity and devoted to accomplishing greener chemical processes [3, 4].
However, in most of the catalytic processes, the volatile organic solvents employed as reaction media often creating a great deal of safety and health, environmental issues due to their flammable and toxic nature [5, 6]. The use of eco-compatible reaction medium which drives the transformation towards the target product side with higher yield with lesser energy consumption is sustainable and is welcomed [7]. In organic synthesis, the use of eccentric organic solvents has its rewards like synthetic competence by stabilizing the catalyst, curating the mode of product formation, facilitating reaction workup, efficacy in reaction environment, etc., rather than just confining them towards the green chemistry and sustainability facet [8]. By this synthetic approach, nowadays, there is the use of neoteric media like biomass-derived solvents, ionic liquids, deep eutectic solvents, and supercritical carbon dioxide, etc. [9]. In parallel to this, the use of wastewater, water extracts of fruits and vegetable juices, table salt, bile salts, etc., are acting as bio-catalysts and as solvents also proved to be rate enhancing in the organic synthetic conversions [10,11,12,13].
Nowadays, using surfactants in the organic synthetic protocol has become one of the most exciting research endeavours in facilitating the aqueous mediated reaction and also in compatibility with various catalysts [14]. The surfactant-aided catalysis has several advantages along with solubilizing the organic components from the reaction, e.g. product isolation, catalyst recycling, found to increase the rate of reaction in most of the cases. Furthermore, it can tolerate the drastic reaction conditions like high temperature/pressure, too [15]. The efficacy of aqueous-mediated reaction is enhanced by the well-known type of acid catalysis which is the amalgamation of an acid catalysts with the designed surfactants, Lewis Acid-Surfactant-Combined (LASC) and Brǿnsted Acid-Surfactant Combined (BASCs) class of catalysts [16,17,18,19]. Also, the applicability of the designed surfactants to the various acid/base/ionic liquid catalysed organic transformations in different reaction media is the eminent one [20,21,22,23,24]. Hitherto, micellar catalysis is a well-known alternative approach to traditional synthetic methodologies, and the engraining of micellar media has been studied in detail for many syntheses with various reaction conditions [25,26,27,28,29,30,31]. Here, the mentioned catalytic utilization of the fruit extract viz., extract of fruits of Averrhoa bilimbi (ABE), is distinguished as a micellar bio-catalytic acidic medium [32] providing the effective, efficient and non-toxic renewable reaction medium which converts the reacting substrates to products with clean and simple approach.
Functionalized isoxazoles are important heterocyclic scaffolds that have long been used as ‘building blocks’ in synthetic organic chemistry and used as a framework in bioorganic research [33,34,35,36,37,38,39]. The various isoxazol-5(4H)-ones found to have applications in the field of chemotherapy and agrochemicals (Fig. 1) due to their properties, such as antibacterial [40,41,42], antifungal [43, 44], antitumor [45], anti-HIV [46], anti-obesity [47], CDP-ME kinase inhibitor [48], fungicidal [49], insecticidal [50, 51], antiviral [52], and antiandrogens [53, 54]. Some isoxazol-5(4H)-ones have been studied as new conjugated donor–acceptor molecules as well as for applications in optical field (e.g. planar waveguide amplifiers, plastic lasers, light-emitting diodes and luminescent probes) [55].
Biginelli reaction is one of the most widely studied reactions in synthetic organic chemistry [56,57,58,59,60,61,62]. This reaction used to synthesize the tetrahydropyrimidines. Under the Biginelli reaction conditions, any aliphatic or aromatic aldehyde when condensed with an acetoacetic ester and urea or thiourea reported to give conventional 1,2,3,4-tetrahydropyrimidine (DHPM). But with the use of salicylaldehyde, the product obtained is not the conventional pyrimidine but oxygen bridged pyrimidine [62,63,64,65]. With the use of acetylacetone (or ethyl acetoacetate) as active methylene species, reaction termed as “Biginelli-type reaction” was used to synthesize the oxygen bridged pyrimidines which are synthetic intermediates in organic synthesis [66,67,68,69].
The previous reports in the literature for the synthesis of 3-methyl-4-arylmethylene-isoxazol-5(4H)-one use the variety of catalysts like inorganic salts viz., sodium benzoate [70], sodium silicate [71], potassium iodide [72], sodium hypophosphite (SHP) [73], etc., different acid catalysts like boric acid [74], DL-tartaric acid [75], 2-hydroxy-5-sulphobenzoic acid (HSBA) [76], salicylic acid [77], lemon juice [78], bases like pyridine [79], nano-sized MgO [80], ionic liquids [81, 82], clays [83], microwave [84] and ultrasound [85, 86]-assisted synthesis. Despite the numerous reports for synthesis of isoxazol-5(4H)-one, these methods have affected with serious limitations like harsh reaction conditions, longer reaction time, toxic chemical catalysts and media and tedious workup procedures. Similarly, the Biginelli-like reaction has several synthetic reports with different used catalysts and reaction conditions viz., acetic acid [67], NaHSO4 under solvent-free conditions [63] and under microwave conditions [64], Baker’s yeast [62], MgBr2 [65], benzyl triethylammonium chloride [68], etc. These synthetic procedures have suffered from drawbacks of limited substrate scope, drastic reaction conditions, low product yield, etc.
In the context of development of different synthetic sustainable methodologies, the search for more ecologically benign forms of catalysis is in central attention and one of the foremost contenders for environmentally suitable options is the class of biodegradable and renewable materials [87,88,89]. The prominent examples of these bio-catalysed reactions are using papaya bark ash [90], animal bone [91], bael fruit ash (BFA) [92], eggshell [87], fruit juices like coconut juice [93], Tamarindus indica fruit juice [94], pineapple juice [95], lemon juice [78, 96], etc., and also various water extracts like of Acacia concinna pods [97], Water extract of onion peel (WEOP) [98], water extract of banana (WEB) [99], etc., are well recognized. The increased use of these naturally sourced catalysts, nowadays, serving as better alternatives to the chemically generated catalysts in chemical processes.
For this report, we have mindset to explore the synthetic applications of naturally sourced catalysts in organic reactions. A tree, Averrhoa bilimbi Linn., belongs to the family oxalidaceae and it is native of India [100]. The fruits of plants known as bilimbi possess the medicinal properties against scurvy, diarrhoea, hepatitis and inflammatory conditions [101], and also, the syrup made from these fruits is used in treatment of febrile excitement, haemorrhages and internal haemorrhoids and in bilious colic and hepatitis [102, 103], hyperlipidaemia [104]. The physico-chemical studies revealed that fruit contains the carbohydrates, proteins, amino acids, tannins, hydrolysable tannins, bitter principles, essential oils, valepotriates, coumarins, flavonoids and terpenes, as the principle constituents [105].
With this perspective in mind and continuing our insight of employing of various natural catalysts in organic synthesis [25, 96, 106, 107], we here incorporated the use of an eco-friendly and highly economical, non-hazardous Averrhoa bilimbi Extract (ABE) as a catalytic micellar medium in which has strong acidic pH 1.38, and it is found to be an efficient, simple and clean as compared to commercial chemical catalysts with an exemplar canopy from the BASC class. With best of our knowledge, there is no report for the synthesis of 4-(4-arylbenzylidene)-3-methylisoxazol-5(4H)-ones and 11-acetyl-2-methyl-5,6-dihydro-2H-2,6-methanobenzo[g][1,3,5]-oxadiaazocin-4(3H)-ones (Scheme 1) in the micellar medium in the literature.
Results and discussion
To evaluate the effect of ABE-catalyst on multi-component condensation reaction of aldehyde, hydroxylamine hydrochloride and ethyl acetoacetate, we investigated a model reaction of equimolar quantities of 4-hydroxybenzaldehyde, 1b (1.0 mmol), ethyl acetoacetate, 2 (1.0 mmol), hydroxylamine hydrochloride, 3 (1.0 mmol) loaded into a 25-mL round-bottom flask, and the results are incorporated in Table 1.
To optimize the reaction conditions, as an initial task, the reaction was conducted under solvent and catalyst-free conditions at room temperature, but it showed no formation of target product, confirmed with TLC after 7 h (Table 1, entry 1). Without addition of catalyst, using water as a solvent, there was formation of a product in a trace amount detected on TLC (Table 1, entry 2) which indicated the need of a catalyst for propagating the reaction. So, we added ABE to the reaction mixture in an increasing amount from 0.5 to 5.0 mL at room temperature which had shown significant increase in the product yield without addition of any solvent (Table 1, entry 3–8). We observed that addition of only 0.5 mL of the ABE catalyst to the reaction furnished the 53% of the product after 3.0 h (Table 1, entry 3). While on adding the 1.0 mL ABE to the reaction mixture, it gave the product with a higher yield than with 0.5 mL ABE-catalyst after 2.0 h (Table 1, entry 4). So, we continued the addition of ABE in an increasing amount to the reaction mixture, to improve the product yield. Further, with 2.0 mL of ABE-catalyst to the reaction furnished the 92% of the product yield after 2.0 h (Table 1, entry 5), showed that reaction worked well in the ABE-catalytic medium. Further with increase in the amount of ABE-catalyst to the reaction mixture with the addition of 3.0 mL of ABE-catalyst reaction furnished the 97% of the product yield after 2.0 h (Table 1, entry 6), traced the efficient conversion of reactants to the target product. But, still further more addition of catalyst was not actually helpful in increasing the product yield (Table 1, entry 7–8). Thus, 3.0 mL of ABE is the optimum amount of bio-catalytic medium which pushes the reaction forward to give 3-methyl-4-arylmethylene-isoxazol-5(4H)-one for this one-pot three-component protocol. Here, we also screened the different solvents for this synthetic reaction which on comparison emphasized that the protic solvents are more efficient in delivering the product rather than the aprotic ones (Table 1, entry 9–14) and also, no solvent is better in delivering the product as like using ABE-itself as a reaction medium.
The obtained product after completion of the reaction was washed with the cold distilled water and then separated by filtration. For most of the products, reaction mixture after completion showed a single spot on the TLC plate for a target product only, indicated pure product formation. These isolated pure products were further analysed and confirmed by their physical constants, and 1H-NMR, 13C-NMR, and MS (ESI) spectroscopy techniques with structural evidences.
Here, it is pertinent to mention that the reaction mixture was found to be a colloidal solution or having a turbid emulsion like nature. On studying this reaction mixture by Photon correlation spectroscopy (PCS), commonly known as dynamic light scattering (DLS) technique, it was observed that there was the presence of colloidal aggregates or micelles in solution which have nearly spherical shape (Fig. 2a) and has average particle size 64 nm (Fig. 2b) turning the reaction mixture turbid. These spherical micellar aggregates in the reaction medium were leading to cause effective collisions among the reacting substrates to form the target product by subsequent steps within short reaction time and hence found to enhance the rate of reaction. This rate enhancing effect in ABE is actually in harmonious to the acidic nature of it pushing the reaction in a forward direction.
The high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (HPLC–Q-TOF–MS/MS) technique is a sensitive, high-speed and established technique with shorter analysis time and greater accuracy of the m/z value [108, 109]. Thus, the bilimbi fruit extract, ABE, is assayed with the help of the HPLC–Q-TOF–MS/MS. Literature says that bilimbi fruit contains the high levels of oxalic acid, 8.57–10.32 mg/g [110] along with a very large proportion of aliphatic acids (47.8%), among which the hexadecanoic acid (20.4%) and (Z)-9-octadecenoic acid (10.2%) predominated [111]. The obtained analytical results support this and also confirmed that ABE contains different volatile organic compounds viz., aliphatic acids (which are numerically more) and linear alcohols (Fig. 3), lipids, aldehydes, amides, phosphonates, and ammonium salts, etc. These compounds were identified or tentatively characterized based on their MS/MS spectra. The surface activity of ABE may be found due to the cumulative effect of such long chain amphiphilic molecules of aliphatic fatty acids, alcohols, ammonium salts, phosphonates, lipids, etc. contains in it. Due to effect of this, in ABE micellar media reaction proceeds effectively within short time to form desired product offering a synergistic effect to the acidic nature of ABE. The use of bio-based substances/materials as a catalyst is widespread and has been investigated in detail for various reactions in aqueous media [11, 89, 112, 113]. The analytical details of HPLC–Q-TOF–MS/MS analysis of catalyst ABE have been provided in supplementary information.
In order to verify the specific effect of the acid from ABE on this synthetic reaction, we employed the commercial analytical grade chemicals like oxalic acid, vitamin C, hexadecanoic acid, tridecanoic acid, dodecanoic acid, stearic acid, etc. which are present in ABE (as per LC–MS analysis), treated as a catalytic promoter in the model reaction under the optimized reaction conditions for the synthesis of 4b. In the said experiment, the model reaction was firstly tested with oxalic acid as a catalyst, a major proportionate acid from ABE, the reaction yielded the 78% of product after 4.0 h (Table 2, entry 1). Next, by using ascorbic acid (vitamin C) as a catalyst, 74% of product is obtained after the time of 3.5 h (Table 2, entry 2). The catalytic micellar medium of the dodecanoic acid and the tridecanoic acid found to give 72 and 73% yield, respectively (Table 2, entry 3 and 4). Subsequently, the hexadecanoic acid and the stearic acid delivered the target product in corresponding 73 and 75% of product yield after 4.0 h (Table 2, entry 5 and 6). But these volatile aliphatic fatty acids affected the nature of the product which needs purification with re-recrystallization. Furthermore, to know whether these aliphatic acids have any synergistic effects in micellar reaction medium, these acids were collectively added to the reaction mixture of test reaction as a catalyst (imitating the ABE catalytic medium), and the obtained results was compared with the ABE as a catalyst (Table 1, entry 6). Here, the results predicted the micellar environment created by natural, non-toxic ABE bio-surfactant sounds better effective than the artificial mixture of chemical compounds which weighs more to the importance of ABE as a medium.
The exact mechanism in transforming the reacting substrates to the target product is not studied, but according to the results obtained, we proposed here a plausible reaction mechanism (Fig. 4) in the micellar acidic medium of ABE. Briefly, a Brǿnsted acidic ABE medium activates aromatic aldehyde, 1, ethyl acetoacetate, 2 and hydroxylamine hydrochloride, 3. The amphiphilic molecules from ABE arranged themselves to form the colloidal spherical aggregates with lipophilic centre and hydrophilic environment, where, firstly, from the reaction mixture, due to their high affinity to form a stable product, ethyl acetoacetate, 2 and hydroxylamine hydrochloride, 3 collides to form their cyclized adduct A by the dehydrative cyclization. Further the aromatic aldehyde, 1, and the enol form of the cyclized adduct, A’ as under acidic environment undergoes the Knoevenagel condensation reaction to form the intermediate, B which after dehydration gives the 4-(4-arylbenzylidene)-3-methylisoxazol-5(4H)-one, 4, a desired product.
After optimizing the reaction conditions, we moved towards the elaboration of substrate scope for the protocol, where different aromatic aldehydes were treated with ethyl acetoacetate and hydroxylamine hydrochloride in the ABE micellar media for the appropriate time and the results are shown in Table 3. The reaction holds good for a wide variety of different substituents on aromatic aldehyde to deliver the target product as 3-methylisoxazol-5(4H)-ones with higher yield. The noteworthy observation is that the yields varied because of the difference in the substitution group on aromatic aldehydes. The aromatic aldehydes with an electron-donating groups afforded the target products with high yields and purity (Table 3, entries 2–7) as compared to the electron-withdrawing substituents. Also, heterocyclic aldehydes viz., thiophene-2-carboxaldehyde, endured smoothly with ethyl acetoacetate and hydroxylamine hydrochloride giving high yield (Table 3, entry 18) but pyridine-3-carboxaldehyde just reacted to form the condensed product 3-methyl-4-(pyridine-3-ylmethylene)isoxazol-5(4H)-one (Table 3, entry 19) in trace amount, showed on TLC.
We also studied the recyclability of this ABE bio-surfactant media for the target reaction in the 3-methyl-4-arylmethylene-isoxazol-5(4H)-one synthesis to scale up the protocol for practical and industrial applications. To get the parameter of recyclability, after completion of the model reaction judged by the TLC, the product was extracted with the addition of the ethyl acetate so as to separate it from reaction mixture. Then, aqueous phase of ABE-catalytic media was reused for another run in the next reaction mixture to form the product. The ABE extract is recycled up to four runs effectively with negligible loss in the target product, 4b, yield as shown in Fig. 5. This recycled ABE emulsion reaction media was tested with pH meter each time, so as to check the stability and it was found to be in the range of 1.38 to 1.84. There is no significant loss in the catalytic activity of the media in the first four cycles but further, the marginal loss in catalytic activity was observed after the fourth run may be due to the trivial alterations and loss in some ABE-media in the tested conditions also due to the organic contaminations, too.
This successful accomplishment of 3-methyl-4-arylmethylene-isoxazol-5(4H)-ones in ABE micellar medium encourages us to use this catalytic medium in the synthesis of 11-acetyl-2-methyl-5,6-dihydro-2H-2,6-methanobenzo[g][1,3,5]-oxadiazocin-4(3H)-ones (8a-m) (Scheme 1) which have few reports for their synthetic procedure. Initially, we chose salicylaldehyde, acetylacetone, and urea as model reactants in equimolar amount so as to check the optimum temperature, effect of catalyst, catalytic amount and solvent effect in the progress of the reaction. The results are recorded in Table 4.
To inspect these reaction parameters for the formation of a diazabicyclo-heterocyclic compound, the reactants were taken in a 25-mL round-bottom flask and stirred at room temperature without the addition of any solvent, initially, resulted in no product formation on TLC after 6.0 h (Table 4, entries 1 to 3). So, we tried the use of ethanol as a non-toxic solvent to reaction mixture with 1.0 mL of ABE catalyst showed that there was a trace product formation at room temperature turning the reaction medium colloidal in nature (Table 4, entry 4). By this effect, further addition of 2.0 and 5.0 mL of ABE catalyst in ethanolic medium, there was product with low yield of 32 and 44% consecutively (Table 4, entry 5 and 6). So, to improve the yield of the product changing the temperature condition of the reaction with ethanol as a solvent under reflux condition, the reaction forwarded with a low increase in the amount of catalyst as 0.5, 1.0, and 2.0 mL (Table 4, entry 7–9), which showed the result for increase in the product yield. Here, for 2.0 mL of ABE catalyst, reaction showed formation of 95% the product after 2.5 h (Table 4, entry 9), But still further addition of ABE-catalyst was not much effective (Table 4, entry 10–12). Thus, the 2.0 mL of ABE is sufficient to enhance the reactivity of reactants in ethanolic medium to deliver the desired product. To test the progress of the reaction without catalyst under reflux condition of ethanol, we conducted the model reaction under given reaction conditions but it failed to give the target product after 7.0 h (Table 4, entry 13). Further, tested with the polar protic solvents like lower alcohols viz., methanol, iso-propanol and t-butanol as co-solvents in the reaction medium and using 1.0 mL of ABE as a catalyst furnishes the 92, 90 and 90% of the yield of the product respectively at reflux temperature of cosolvents (Table 4, entry 14–16). Using water as a solvent at the reflux of temperature in a reaction was accomplishing the product yield of 54% after 5 h (Table 4, entry 17). The polar aprotic solvents viz., dimethyl sulphoxide (DMSO), dichloromethane (DCM) and acetonitrile (CH3CN) furnished the product yield as 36, 34, and 40% successively after 4.0 h under the refluxing temperature condition (Table 4, entry 18–20). For non-polar solvent like toluene the reaction got completed, shown on TLC, by accomplishing the target product with 32% yield after 5.0 h (Table 4, entry 21). These results emphasized that the polar protic solvents are effectively proceeded to the diazabicyclo heterocycle synthesis than the aprotic and the non-polar ones.
Thus, at the reflux temperature of ethanol as a solvent with 2.0 mL of ABE catalyst, the test reaction giving us the 95% of the product yield, so with these chosen optimized reaction conditions the product was obtained where the completion of the reaction judged by the TLC with the n-hexane: ethyl acetate (7:3) solvent system. The obtained product was separated from the reaction mixture as a solid by adding 10 mL of cold distilled water to it. This crude product was thoroughly washed with distilled water and purified by recrystallization from 96% ethanol and distilled water. The obtained pure product was confirmed by its physical constant, and spectral analyses like FT-IR, 1H-NMR, 13C-NMR, and EI-MS.
With these standard conditions in hand, some other aldehydes including electron-withdrawing, electron-donating, and neutral groups on the salicylaldehyde were used to expand the utility of ABE as a catalyst and to test the generality and scope of the protocol. As shown in Table 5, the yields are exceptionally good for diazacyclo derivatives (8a-m) irrespective of the structural variations in aldehydes, handling both urea as well as thiourea effectively.
The addition of ABE to the reaction medium made it turbid emphasized that there was the formation of colloidal particles in the solution after the addition of ethanol, too. The 2.0 mL of ABE in 1.0 mL of ethanol furnishes the product effectively suggesting that it is the concentration of ABE in ethanolic medium to push the reaction forward successfully (Table 4, entry 10). This effect of colloidal reaction mixture with ABE catalyst in alcoholic medium is explained with the critical micelle concentration (c.m.c.) of ethanolic ABE solution which is found to be 55%, measured with the specific conductivity parameter, indicated in Fig. 6. Further, increase in the concentration of ABE in a reaction was not helpful to enhance the efficiency of reaction much (Table 4, entry 11 and 12), justifying that the concentration above c.m.c. delivering the desired product, effectually. Here, the formation of micelles is providing a synergistic effect to the acidic activity of the catalyst ABE, by acting as a reaction centre for the reacting molecules. Hence, ABE catalysis in an ethanolic medium is considered to be an example of the micellar catalysis.
On comparing on the account of the efficiency of our method for the synthesis of 3,4-disubstituted-isoxazole-5(4H)-ones and for the synthesis of 11-acetyl-2-methyl-5,6-dihydro-2H-2,6-methanobenzo[g][1,3,5]-oxadiaazocin-4(3H)-ones with other reported methods, the results are portrayed in Table 6. Each of these methods has its own advantages, but some of them suffer from disadvantages such as very poor yield, long reaction time, use of organic solvents and employment of expensive catalysts from which not anyone is natural sourced. So, the present method furnishes a naturally sourced catalyst, green reaction medium, utilises shorter reaction time and a small quantity of this inexpensive, readily available, non-toxic catalyst is sufficient to get a good yield of the expected product with the clean procedure.
Experimental section
General remarks
All chemicals were of reagent grade and procured from Sigma-Aldrich Pvt. Ltd., and were used without any further purification. Analytical thin-layer chromatography (TLC) was carried out on precoated Merck silicagel 60 F254 aluminium plates. Melting points were recorded on DBK programmable melting point apparatus and are uncorrected. The HPLC-Q-TOF–MS/MS was carried out with the use of an Agilent 1290 LC system coupled to column Q-TOF–MS with dual ESI source. FT-IR spectra were obtained using potassium bromide pellets on Bruker ALPHA FT-IR spectrometer. The 1H-NMR and 13C-NMR spectra of synthesized compounds were recorded on a Bruker AC (300 and 400 MHz for 1H-NMR and 13C-NMR) spectrometer using CDCl3 and DMSO as a solvent. Chemical shifts (δ) are expressed in parts per million (ppm) values with tetramethylsilane (TMS) as the internal reference. DLS measurements were performed on the Malvern Zetasizer Nano ZS90 instrument. The specific conductivity is measured on the EQUIP-TRONICS conductivity meter model NO EQ-660A. Measurement of optical micrograph is on an ordinary compound microscope by taking a turbid reaction mixture tested under 100 × magnification.
Preparation of Averrhoa bilimbi Extract (ABE)
Averrhoa bilimbi fruits known as bilimbi which are fresh and mature were collected from the botanical garden, Department of Botany, Shivaji University, Kolhapur. These fruits (Fig. 7a) were thoroughly washed with water, deseeded and then cut into small pieces with knife (Fig. 7b). These pieces were crushed with the help of mortar and pestle to get the turbid juice which is filtered through the muslin cloth to get fibre-free extract (Fig. 7c). This obtained extract was stored at the temperature of 0–5 °C, and it is found to be stable for several days.
Synthesis of 4-(4-arylbenzylidene)-3-methylisoxazol-5(4H)-ones (4a-t):
A mixture of aryl aldehyde (1a–t), (1.0 mmol), hydroxylamine hydrochloride 2 (0.07 g, 1.0 mmol) and ethyl acetoacetate 3 (0.130 g, 1.0 mmol) was stirred in 3.0 mL of ABE at room temperature for the indicated time shown in Table 3. After completion of the reaction which is monitored by TLC, showed single spot for the product. The solid pure product was isolated by simple filtration and washed with water (5 mL) to remove the catalyst. If on TLC indicated product is not pure, then further purification was performed by recrystallization from 96% EtOH and distilled water mixture. The structural analyses of the obtained pure products and hence confirmation of the synthesized derivatives was with FT-IR, 1H-NMR, 13C-NMR and MS (ESI) spectral techniques which has been given in the supplementary information.
The analytical and spectral data of the unreported compounds is given as follows,
4-((4-hydroxynaphthalen-1-yl) methylene)-3-methylisoxazol-5(4H)-one (4 g):

Yellow solid. IR(KBr) ῡmax (Fig. 22): 2999, 2948, 2841, 1729, 1594, 1492, 1377, 1128, 1029, 990, 881 cm–1; 1H-NMR (CDCl3, 300 MHz) (Fig. 23): δ 1.38(t, 3H, -CH3), 2.18(s, 3H, -CH3), 4.05(q, 2H, -OCH2-), 6.86(d, 1H, Ar–H), 7.37(s, 1H, = CH), 7.52(d, 1H, Ar–H), 7.68(s, 1H, Ar–H), 8.54(s, 1H, Ar–H), 10.12(bs, 1H, -OH); 13C-NMR (CDCl3 + DMSO, 300 MHz) (Fig. 24): δ 11.08(C-1), 14.19(C-2), 63.77(C-3), 113.64(C-4), 115.33(C-5), 116.53(C-6), 124.77(C-7), 131.43(C-8), 146.44(C-9), 150.60(C-10), 153.69(C-11), 161.21(C-12), 168.24(C-13); MS (EI) m/z (Fig. 25): 247.28 [M+].
4-(4-isopropylbenzylidene)-3-methylisoxazol-5(4H)-one (4i):

Yellow solid. (KBr) ῡmax (Fig. 26): 2982, 1713, 1573, 1478, 1364, 1253, 1190, 1068, 989, 756 cm–1; 1H-NMR (CDCl3, 400 MHz) (Fig. 27): δ 1.29 (d, 6H, 2 × -CH3), 2.29 (s, 3H, -CH3), 2.99 (sep, 1H, -CH), 7.37 (d, 1H, Ar–H), 7.41 (s, 1H, = CH), 8.31 (d, 2H, Ar–H); 13C-NMR (CDCl3, 400 MHz): δ 11.07(C-1), 23.31(C-2), 33.26(C-3), 111.35(C-4), 116.37(C-5), 118.36(C-6), 126.84(C-7), 137.49(C-8), 154.17(C-9), 161.55(C-10), 168.74(C-11); MS (EI) m/z: 230.14 [M + 1] + .
4-(2,4-dichlrobenzylidene)-3-methylisoxazol-5(4H)-one (4j):

White solid. IR(KBr) ῡmax: 2957, 1721, 1652, 1617, 1518, 1453, 784 cm–1; 1H-NMR (CDCl3 + DMSO, 300 MHz) (Fig. 28): δ 2.29 (s, 3H, -CH3), 3.88 (s, 6H, -OCH3), 6.89 (d, 1H, Ar–H), 7.13 (dd, 1H, Ar–H), 8.03 (s, 1H, = CH), 8.73 (d, 1H, Ar–H); 13C-NMR (CDCl3 + DMSO, 300 MHz) (Fig. 29): δ 10.34(C-1), 122.53(C-2), 125.85(C-3), 126.44(C-4), 126.81(C-5), 127.96(C-6), 128.45(C-7), 130.16(C-8), 143.58(C-9), 160.30(C-10), 171.11(C-11); MS (EI) m/z (Fig. 30): 256.64 [M +] + .
4-(2-nitrobenzylidene)-3-methylisoxazol-5(4H)-one (4 l):

Orange solid. IR(KBr) ῡmax: 2957, 1721, 1652, 1617, 1518, 1453, 784 cm–1; 1H-NMR (CDCl3, 300 MHz) (Fig. 31): δ 2.29 (s, 3H, -CH3), 3.88 (s, 6H, -OCH3), 6.89 (d, 1H, Ar–H), 7.13 (dd, 1H, Ar–H), 8.03 (s, 1H, = CH), 8.73 (d, 1H, Ar–H); 13C-NMR (CDCl3, 300 MHz) (Fig. 32): δ 11.63(C-1), 56.09(C-2, C-3), 111.91(C-4), 115.52(C-5), 117.99(C-6), 121.50(C-7), 124.63(C-8), 143.71(C-9), 153.15(C-10), 154.86(C-11); MS (EI) m/z (Fig. 33): 232.38 [M+].
4-(4-hydroxy-3-ethoxybenzylidene)-3-methylisoxazol-5(4H)-one (4q):

Orange yellow solid. IR(KBr) ῡmax (Fig. 36): 3212, 2940, 1731, 1619, 1563, 1509, 1601, 1313, 1282, 1158, 1035, 935 cm–1; 1H-NMR (CDCl3, 300 MHz) (Fig. 37): δ 2.34 (s, 3H, -CH3), 7.56 (s, 1H, = CH), 7.59 (d, 1H, Ar–H), 7.64 (d, 1H, Ar–H), 7.67 (d, 1H, Ar–H), 7.86 (s, 1H, Ar–H), 7.86 (s, 1H, Ar–H), 7,89(d, 1H, Ar–H), 7.93(s, 1H, Ar–H), 8.47(dd, 1H, Ar–H), 8.86(s, 1H, -OH); 13C-NMR (CDCl3 + DMSO, 300 MHz) (Fig. 38): δ 11.68(C-1), 119.44(C-2), 127.07(C-3), 127.78(C-4), 127.95(C-5), 128.44(C-6), 128.73(C-7), 129.55(C-8), 129.87(C-9), 130.05(C-10), 132.74(C-11), 136.67(C-12), 149.77(C-13), 161.14(C-14), 168.05(C-15); MS (EI) m/z: 254.39 [M + 1] + .
4-(2-chlorobenzylidene)-3-methylisoxazol-5(4H)-one (4n):

White solid. IR(KBr) ῡmax: 2957, 1721, 1652, 1617, 1518, 1453, 784 cm–1; 1H-NMR (CDCl3, 300 MHz): δ 2.29 (s, 3H, -CH3), 3.88 (s, 6H, -OCH3), 6.89 (d, 1H, Ar–H), 7.13 (dd, 1H, Ar–H), 8.03 (s, 1H, = CH), 8.73 (d, 1H, Ar–H); 13C-NMR (CDCl3, 300 MHz): δ 11.44(C-1), 125.09(C-2), 126.83(C-3), 127.76(C-4), 129.11(C-5), 129.62(C-6), 133.19(C-7), 136.50(C-8), 124.63(C-9), 143.71(C-10), 163.12(C-10), 174.81(C-11); MS (EI) m/z: 221.46 [M +].
Synthesis of 11-acetyl-2-methyl-5,6-dihydo-2H-2,6-methanobenzo[g][1,3,5]-oxadiazocin-4(3H)-ones (8a-8 m):
The components of reaction viz., substituted salicylaldehyde, 5(a-m), (1.0 mmol), urea/thiourea, 6 (1.0 mmol), acetylacetone, 7 (1.0 mmol) and ABE catalyst (2.0 mL) in ethanol (1.0 mL) was taken in a 25 mL of a round bottom flask and was heated at the reflux temperature of ethanol. The progress of the reaction was monitored by TLC with n-hexane: ethyl acetate (7:3) solvent system. After completion of the reaction, ice-cold distilled water is added to the reaction mixture to get a solid product. Then, the product was washed with distilled water, filtered and recrystallized by ethanol to get the pure product. The pure products are analysed with spectroscopic techniques viz., FT-IR, 1H-NMR, 13C-NMR and EI-MS to interpret and confirm their structures.
11‐acetyl‐7‐chloro-2‐methyl‐5,6‐dihydro‐2H‐2,6-methanobenzo-[g][1,3,5]-oxadiazocin-4(3H)-one (8e):

Brown powder, IR(KBr) ῡmax: 3227, 1738, 1535, 1492, 1320, 1182, 1084, 917, 840 cm–1; 1H-NMR (DMSO, 300 MHz) (Fig. 50): δ 2.06(s, 3H, CH3), 2.27(s, 3H, CH3), 3.37(m, 1H, -CH), 5.49(d, 1H, -CH), 6.82(d, 1H, Ar–H), 6.90(d, 1H, Ar–H), 7.10 (dd, 1H, Ar–H), 9.19 (s, 1H, NH), 10.10 (s, 1H, NH); 13C-NMR (DMSO, 300 MHz) (Fig. 51): δ 18.75(C-1), 29.80(C-2), 48.74(C-3), 107.88(C-4), 117.31(C-5), 122.53(C-6), 126.58(C-7), 128.28(C-8), 131.81(C-9), 148.42(C-10), 152.11(C-11), 153.27(C-12), 194.61(C-13); MS (EI) m/z (Fig. 52): 281.93[M + 1]; Anal. Calcd. For C13H13N2O3Cl: C, 55.62; H, 4.67; N, 9.98. Found: C, 54.85; H, 3.62; N, 10.03.
1-(7-chloro‐2‐methyl‐4‐thioxo‐3,4,5,6‐tetrahydro-2H-2,6-methanobenzo[g][1,3,5]-oxadiazocin-11-yl)-ethanone (8f).

Yellow powder. IR(KBr) ῡmax (Fig. 53): 3246, 1712, 1538, 1482, 1317, 1170, 1088, 910, 862, 824, 675 cm–1; 1H-NMR (DMSO, 300 MHz) (Fig. 54): δ 1.67 (s, 3H, CH3), 2.18 (s, 3H, CH3), 3.39 (d, 1H, -CH), 4.80 (s, 1H, -CH), 6.86 (d, 2H, Ar–H), 7.23 (t, 1H, Ar–H), 8.98 (s, 1H, -NH), 9.15(s, 1H, NH); 13C-NMR (DMSO, 300 MHz) (Fig. 55): δ 22.63(C-1), 29.11(C-2), 46.98(C-3), 47.57(C-4), 81.97(C-5), 118.37(C-6), 124.00(C-7), 126.08(C-8), 128.34(C-9), 129.31(C-10), 149.55(C-11), 176.77(C-12), 203.11(C-13); MS (EI) m/z (Fig. 56): 296.77[M +]; Anal. Calcd. For C13H13N2O2ClS: C, 52.61; H, 4.42; N, 9.44. Found: C, 50.54; H, 3.50; N, 7.36.
11-acetyl-10-bromo-8-chloro-2-methyl-5,6-dihydro-2H-[2,6]methanobenzo[g][1,3,5]oxadiazocin-4(3H)-one (8 k).

Yellow powder. IR(KBr) ῡmax: 3246, 1757, 1538, 1462, 1357, 1194, 1088, 935, 818, 785 cm–1; 1H-NMR (DMSO, 300 MHz): δ 1.54 (s, 3H, CH3), 2.09 (s, 3H, CH3), 3.67 (d, 1H, -CH), 4.82 (s, 1H, -CH), 6.76 (d, 1H, Ar–H), 7.13 (d, 1H, Ar–H), 8.95 (s, 1H, -NH), 9.17(s, 1H, NH); 13C-NMR (DMSO, 300 MHz): δ 21.33(C-1), 31.98(C-2), 47.57(C-3), 78.97(C-4), 83.37(C-5), 117.50(C-6), 123.08( C-7), 123.34(C-8), 127.11(C-9), 129.86 (C-10), 147.38(C-11), 156.23(C-12), 198.76(C-13); MS (EI) m/z: 358.77[M + 1]; Anal. Calcd. For C13H12N2O3BrCl: C, 43.42; H, 3.36, N, 7.79; Br, 22.22, Cl, 9.86; Found: C, 41.61; H, 2.32, N, 7.79; Br, 20.05, Cl, 8.63.
1-(10-bromo‐8-chloro-2‐methyl‐4‐thioxo‐3,4,5,6‐tetrahydro-2H-2,6-methanobenzo[g][1,3,5]-oxadiazocin-11-yl)-ethanone (8 l).

Yellow powder. IR(KBr) ῡmax: 3250, 1723, 1554, 1461, 1392, 1130, 1017, 947, 819, 672 cm–1; 1H-NMR (DMSO, 300 MHz): δ 1.51 (s, 3H, CH3), 2.23 (s, 3H, CH3), 3.31 (d, 1H, -CH), 4.78 (s, 1H, -CH), 6.74 (d, 1H, Ar–H), 7.03 (d, 1H, Ar–H), 8.97 (s, 1H, -NH), 9.19(s, 1H, NH); 13C-NMR (DMSO, 300 MHz): δ 21.47(C-1), 32.11(C-2), 48.54(C-3), 79.57(C-4), 83.58(C-5), 118.65(C-6), 124.08(C-7), 124.38(C-8), 127.84(C-9), 129.72(C-10), 148.13(C-11), 174.54(C-12), 202.11(C-13); MS (EI) m/z: 373.77[M +]; Anal. Calcd. For C13H12N2O2BrClS: C, 41.56; H, 3.22; N, 9.44; Br, 21.27; Cl, 9.44; S, 8.54; Found: C, 39.56; H, 2.21; N, 8.29; Br, 20.08; Cl, 8.37; S, 7.23.
11‐acetyl‐(9-diethylamino)-2‐methyl‐5,6-dihydro-2H-2,6-methanobenzo-[g][1,3,5]-oxadiazocin-4(3H)-one (8 m).

Brown black solid. IR(KBr) ῡmax: 2999, 2948, 2841, 1729, 1594, 1492, 1377, 1128, 1029, 990, 881 cm–1; 1H-NMR (DMSO, 300 MHz) (Fig. 57): δ 1.09(t, 9H, 2 × -CH3 and -CH3), 2.38(s, 3H, -CH3), 3.37 (q, 5H, 2 × -N-CH2 and-CH), 4.73 (d, 1H, -CH), 6.04 (s, 1H, Ar–H), 6.33 (dd, 1H, Ar–H), 7.41 (d, 1H, Ar–H), 9.59 (s, 1H, -NH), 11.25(s, 1H, -NH); 13C-NMR (DMSO, 300 MHz) (Fig. 58): δ 12.38(C-1), 12.39(C-1), 20.82(C-2), 29.13(C-4), 44.08(C-5), 44.09(C-5), 76.58(C-6), 81.83(C-7), 95.88(C-8), 104.43(C-9), 111.18(C-10, C-11), 133.95(C-12), 153.76(C-13), 163.38(C-14), 190.61(C-15); MS (EI) m/z: 319.39 [M + 1] + ; Anal. Calcd. For C17H23N3O2S: C, 64.33; H, 7.30, N, 13.24; Found: C, 61.44; H, 6.59; N, 12.08.
Conclusion
We have described an efficient and facile protocol for the synthesis of 3-methyl-4-arylmethylene-isoxazol-5(4H)-ones and of 11-acetyl-2-methyl-5,6-dihydro-2H-2,6-methanobenzo[g][1,3,5]-oxadiaazocin-4(3H)-ones in an ethanolic media with cheaper, natural acidic Averrhoa bilimbi extract (ABE) environment. This resourceful, high yield and simple work-up procedure ascertains the ABE catalytic medium as beneficial over synthetic chemical catalysts. The use of non-toxic, neoteric, mild catalytic ABE micellar medium obtained from a renewable resource leads to the resolution to the issues like use of undesirable solvents, decreasing our environmental footprints, involving simpler processes while providing advantageous economical benefits. All of these characteristics of this protocol working with ABE bio-surfactant makes it much more competitive than previously reported ones.
References
A. Meijer, S. Otto, J.B.F.N. Engberts, J. Org. Chem. 63, 8989 (1993)
C. Li, J. Chem. Rev. 93, 2023 (1993)
C.J. Li, L. Chen, Chem. Soc. Rev. 35, 68 (2006)
T. Kitanosono, K. Mosuda, P. Xu, S. Kobayashi, Chem. Rev. 118, 679 (2018)
C. Jimenez-Gonzales, A.D. Curzons, D.J.C. Constable, V.L. Cunningham, Int. J. Life Cycle Assess. 9, 114 (2004)
R.A. Sheldon, Green Chem. 9, 1273 (2007)
G. Yanlong, Green Chem. 14, 2091 (2012)
C.K.Z. Andrade, L.M. Alves, Curr. Org. Chem. 9, 195 (2005)
S.E. Hooshmand, S.E. Heidari, R. Sedghi, R.S. Verma, 21, 381 (2019)
S. Fiorito, V.A. Taddeo, S. Genovese, F. Epifano, Tetrahedron Lett. 57, 4795 (2016)
R. Pal, Open J. Org. Chem. 1(4), 47 (2013)
A. Garg, A. Ali, K. Damarla, A. Kumar, D. Sarma, Tetrahedron lett. 59, 4031 (2018)
S. Hazra, A.K. Kushawaha, D. Yadav, P. Dolui, M. Deb, A.J. Elias, Green Chem. 21, 1929 (2019)
M. Shiri, M.A. Zolfigol, Tetrahedron 65, 587 (2009)
S. Shirakawa, S. Kobayashi, Org. Lett. 9, 311 (2007)
K. Manabe, S. Iimura, X.-M. Sun, S. Kobayashi, J. Am. Chem. Soc. 124, 11971 (2002)
K. Manabe, Y. Mori, T. Wakabayashi, S. Nagayama, S. Kobayashi, J. Am. Chem. Soc. 122, 7202 (2000)
K. Manabe, Y. Mori, S. Kobayashi, Tetrahedron 57, 2537 (2001)
H. Miura, S. Kameyama, D. Komori, T. Shishido, J. Am. Chem. Soc. 141, 1636 (2019)
S. Handa, D.J. Lippincott, D.H. Aue, B.H. Lipshutz, Angew. Chem. Int. Ed. 53, 10658 (2014)
C. Duplais, A. Krasovskiy, B.H. Lipshutz, Organometallics 30, 6090 (2011)
T. Nishikata, A.R. Abela, B.H. Lipshutz, Angew. Chem. Int. Ed. 49, 781 (2010)
J. Kraïem, T. Ollevier, Green Chem. 19, 1263 (2017)
X. Shang, S. Zhao, W. Chen, C. Chen, H. Qiu, Chem. - Eur. J. 20, 1825 (2014)
S.T. Morbale, S.D. Jadhav, M.B. Deshmukh, S.S. Patil, RSC Adv. 5, 84610 (2015)
R. Selke, J. Holz, A. Riepe, A. Borner, Chem. Eur. J. 4, 769 (1998)
I.B. Blagoeva, M.M. Toteva, N. Ouarti, M.F. Ruasse, J. Org. Chem. 66, 2123 (2001)
H. Firouzabadi, N. Iranpoor, A. Garzan, Adv. Synth. Catal. 347, 1925 (2005)
C. Larpent, E. Bernard, F.B. Menn, H. Patin, J. Mol. Catal. A: Chem. 116, 227 (1997)
R. Selke, J. Holz, A. Riepe, A. Borner, Chem. d Eur. J. 4, 769 (1998)
L.J.P. van den Broeke, V.G. de Bruijn, J.H.M. Heijnen, J.T.F. Keurentjes, Ind. Eng. Chem. Res. 40, 5240 (2001)
B.M. Patil, S.R. Mali, B.M. Patil, S.S. Patil, Current Sci. 118(6), 931 (2020)
T.M.V.D. Pinho e Melo, Curr. Org. Chem. 9, 925 (2005)
L. Carlsen, D. Dopp, H. Dopp, F. Duus, H. Hartmann, S. Lang-Fugmann, B. Schulze, R.K. Smalley, B.J. Wakefield, Houben-Weyl, Methods in Organic Chemistry; ed. by E Schaumann, (Georg Thieme Verlag: Stuttgart, Germany, 1992)
B. Frolund, A.T. Jorgensen, L. Tagmose, T.B. Stensbol, H.T. Vestergaad, C. Engblom, U. Kristiansen, C. Sanchez, P. Krogsgaard-Larsen, T. Liljefors, J. Med. Chem. 45, 2454 (2002)
C. Chen, X. Zhu, Y. Wu, H. Sun, G. Zhang, W. Zhang, Z. Gao, J. Mol. Catal. A: Chem. 395, 124 (2014)
K. Tanabe, W.F. Hölderich, Appl. Catal. A 181(2), 399 (1999)
A. Corma, H. Garcia, Chem. Rev. 103, 4307 (2003)
M.R. Leach, Lewis Acid/Base Reaction Chemistry (Meta-Synthesis, Brighton, 1999)
S.S. Wazalwar, A.R. Banpurkar, F. Perdih, J. Mol. Struct. 1150, 258 (2017)
A.K. Oraby, K.R.A. Abdellatif, M.A. Abdelgawad, K.M. Attia, L.N. Dawe, P.E. Georghiou, Chem. Select 3, 3295 (2018)
Y.K. Kang, K.J. Shin, K.H. Yoo, Bioorg. Med. Chem. Lett. 10, 95 (2000)
A.R. Banpurkar, S.S. Wazalwar, F. Perdih, Bull. Chem. Soc. Ethiop. 32, 249 (2018)
V.S. Konkala, P.K. Dubey, J. Heterocycl. Chem. 54, 2483 (2017)
K.R. Reddy, P.S. Rao, G.J. Dev, Y. Poornachandra, C.G. Kumar, P.S. Rao, B. Narsaiah, Bioorg. Med. Chem. Lett. 24, 1661 (2014)
S. Breuer, M.W. Chang, J. Yuan, B.E. Torbett, J. Med. Chem. 55, 4968 (2012)
B. Kafle, N.G. Aher, D. Khadka, H. Park, H. Cho, Chem. Asian J. 6, 2073 (2011)
M. Tang, S.I. Odejinmi, Y.M. Allette, H. Vankayalapati, K. Lai, Bioorg. Med. Chem. 19, 5886 (2011)
ŞG. Kömürcü, S. Rollas, N. Yilmaz, A. Çevikbaş, Drug Metabol. Drug Interact. 12, 161 (1995)
W. Hallenbach, O. Guth, T. Seitz, H.J. Wrolowsky, P. Desbordes, U. Wachendorff-Neumann, P. Dahmen, E. Voerste, P. Lösel, O. Malssm, R. Rama, H. Hadano, US Patent, Pub. No.: US 2012/0065063A1 (2012)
W. Hallenbach, O. Guth, T. Seitz, H.J. Wrolowsky, P. Desbordes, U. Wachendorff-Neumann, P. Dahmen, E. Voerste, P. Lösel, O. Malssm, R. Rama, H. Hadano, WIPO Patent Application WO/2011/161035A1 (2011)
Y.S. Lee, S.M. Park, B.H. Kim, Bioorg. Med. Chem. Lett. 19(4), 1126 (2009)
T. Ishioka, A. Kubo, Y. Koiso, K. Nagasawa, A. Itai, Y. Hashimoto, Bioorg. Med. Chem. 10, 1555 (2002)
T. Ishioka, A. Tanatani, K. Nagasawa, Y. Hashimoto, Bioorg. Med. Chem. Lett. 13, 2655 (2003)
A.F. da Silva, A.A.G. Fernandes, S. Thurow, M.L. Stivanin, I.D. Jurberg, Synthesis 50, 2473 (2018)
W. Su, J. Li, Z. Zheng, Y. Shen, Tetrahedron Lett. 46, 6037 (2005)
J. Azizian, A.A. Mohammadi, M. Kohshari, A.R. Karimi, M.R. Mohammadizadeh, J. Heterocycl. Chem. 44, 455 (2007)
E. Rafiee, H. Jafari, Bioorg. Med. Chem. Lett. 16, 2463 (2006)
J. Lu, Y. Bai, Synthesis 4, 466 (2002)
J. Lu, Y. Bai, Z. Wang, B. Yang, H. Ma, Tetrahedron Lett. 41, 9075 (2000)
N.Y. Fu, Y.F. Yuan, Z. Cao, S.W. Wang, J.T. Wang, C. Peppe, Tetrahedron 58, 4801 (2002)
A. Kumar, R.A. Maurya, Tetrahedron Lett. 48, 4569 (2007)
Q. Cheng, Q. Wang, X. Xu, M. Ruan, H. Yao, X. Yang, J. Heterocycl. Chem. 47, 624 (2010)
Q. Cheng, Q. Wang, T. Tan, N. Chen, M. Shuaib, J. Heterocycl. Chem. 49, 1352 (2012)
H. Salehib, Q.R. Li, Q.X. Guo, Chin. J. Chem. Physics 19(1), 84 (2006)
W.S. El-Hamouly, H.A. Tawfik, E.M.H. Abbas, Green Chem. Lett. Rev. 2(4), 213 (2009)
W.S. El-Hamouly, A.M.A. El-Khamry, E.M.H. Abbas, Indian J Chem. 45B, 2091 (2006)
D.S. Bose, M. Sudharshan, S.W. Chavhan, ARKIVOC iii, 228 (2005)
J. Svetlík, L. Veizerová, V. Kettmann, Tetrahedron Lett. 49, 3520 (2008)
Q. Liu, Y.N. Zhang, Bull. Korean Chem Soc. 32(10), 3559 (2011)
Q. Liu, R. Wu, J. Chem. Res. 35, 598 (2011)
M. Asiyeh, H. Kiyani, Orbital: Electron J. Chem. 10(2), 133 (2018)
A.P. Tayde, R.P. Pawar, R.V. Khobare, C.B. Mane, N.P. Tayde, Int. J. Innov. Sci. Res. Tech. 4(11), 358 (2019)
H. Kiyani, F. Ghorbani, Res. Chem Intermed. 41, 2653 (2015)
A.U. Khandebharad, S.R. Sarda, C.H. Gill, B.R. Agrawal, Res. J. Chem. Sci. 5(5), 27 (2015)
H. Kiyani, H. Darbandi, A. Mosallanezhad, F. Ghorbani, Res. Chem. Intermed. 41, 7561 (2013)
A. Mosallanezhad, H. Kiyani, Curr. Organocatal. 6, 28 (2019)
R.H. Vekariya, K.D. Patel, H.D. Patel, Res. Chem. Intermed. 42, 7559 (2016)
Q. Cheng, X. Xu, Q. Wang, L. Liu, W. Liu, Q. Lin, X. Yang, Chin. J. Org. Chem. 29(8), 1267 (2009)
H. Kiyani, F. Ghorbani, Res. Chem. Intermed. 42, 6831 (2016)
T. Lohar, A. Kumbhar, M. Barge, R. Salunkhe, J. Mol. Liq. A 224, 1102 (2016)
S. Farahi, N. Nowrouzi, M. Irajzadeh, Iran J. Sci. Technol. Trans. Sci. 42, 1881 (2018)
J. Safari, M. Ahmadzadeh, Z. Zarnegar, Catal Commun. 86, 91 (2016)
S. Ningaiah, B. Vrushabendra, Chandra, C. Javarashetty, D. Shridevi, J. Applicable Chem. 7(6), 1582 (2018)
Q. Cheng, X. Xu, Q. Wang, L. Liu, W. Liu, Q. Lin, X. Yang, Chin. J. Org. Chem. 29, 1267 (2009)
M. Ahamadzadeh, Z. Zarnegar, J. Safari, Green Chem. Lett. Rev. 11(2), 78 (2018)
E. Mosaddegh, A. Hassankhani, Catal. Commun. 33, 70 (2013)
M.G. Dekamin, M. Azimoshan, L. Ramezani, Green Chem. 15, 811 (2013)
B.H. Zhou, J. Yang, M.H. Li, Y.L. Gu, Green Chem. 13, 2204 (2011)
M. Sarmah, A. Dewan, M. Mondal, A.J. Thakur, U. Bora, RSC Adv. 6, 28981 (2016)
Y. Riadi, R. Mamouni, R. Azzalou, R. Boulahjar, Y. Abrouki, M. El Haddad, S. Routier, G. Guillaumet, S. Lazar, Tetrahedron Lett. 51, 6715 (2010)
S.K. Shinde, M.U. Patil, S.A. Damate, S.S. Patil, Res. Chem. Intermed. 44(3), 1775 (2018)
A.M. Fonseca, F.J. Monte, M.C.F. Oliveira, M.C.M. Mattos, G.A. Cordell, R. Braz-Filho, T.L.G. Lemos, J. Mol. Catal. B: Enzyme 57, 78 (2009)
R. Pal, Int. J. Chemtech. Appl. 2, 26 (2013)
S.S. Patil, S.D. Jadhav, M.B. Deshmukh, Indian J. Chem. 52B, 1172 (2013)
M.B. Deshmukh, S.S. Patil, S.D. Jadhav, P.B. Pawar, Synth. Commun. 42, 1177 (2012)
S. Pore, G. Rashinkar, K. Mote, R. Salunkhe, Chem. Biodiver. 7, 1796 (2010)
P.W. Chia, B.S. Lim, K. Chen Tan, F.S.J. Yong, S.-Y. Kan, J. King Saud. Uni.-Sci. 31(4), 642 (2018)
B. Saikia, P. Borah, N.C. Barua, Green Chem. 17, 4533 (2015)
M.P. Corrêa, Diccionario Das Plantas Úteis Do Brasil e Das Exoticas Cultivadas (Imprensa Nacional, Rio de Janeiro, 1926), p. 307
S.H. Goh, C.H. Chuah, J.S.L. Mok, E. Soepadmo, Malaysian Medicinal Plants for the Treatment of Cardiovascular Diseases (Pelanduk Publication, Malaysia, 1995)
C.P. Khare, Indian Medicinal Plants e an Illustrated Dictionary (Verlag Berlin/Heidelberg, Springer, 2007)
K.R. Kirtikar, B.D. Basu, Indian Medicinal Plants, 2nd edn. (Allahabad, India, Lalit Mohan Basu, 1984)
S. Ambili, A. Subramonian, N.S. Nagaranjan, Planta. Med. 75, 55 (2009)
A.G. Patil, S.P. Koli, D.A. Patil, J. Pharm. Res. 6, 145 (2013)
S. Mali, S. Shinde, S. Damate, S. Patil, R. Soc, Open Sci. 5, 170333 (2018)
S.T. Morbale, S.K. Shinde, S.A. Damate, M.B. Deshmukh, S.S. Patil, Lett. Org. Chem. 15, 57 (2018)
E. Sieniawska, T. Baj, R. Sawicki, A. Wanat, K.K. Wojtanowski, G. Ginalska, G. Zgorka, J. Szymanska, Oxid. Med. Cell. Longevity, 1, 11 (2015)
F. Bunghez, M.A. Rotar, R.M. Pop, F. Romanciuc, F. Csernatoni, F. Fetea, Z. Diaconeasa, C. Socaciu, Bull. UASVM Food Sci. Tech. 72(1), 36 (2015)
V.L.A.G. De Lima, E.D.A. Mélo, L.D.S. Lima, Rev. Bras. Frutic. Jaboticabal-SP 23(2), 421 (2001)
K.C. Wong, S.N. Wong, J. Essen, Oil Res. 7(6), 691 (1995)
P.R. Boruah, A.A. Ali, M. Chetia, B. Saikia, D. Sarma, Chem. Commun. 51, 11489 (2015)
P.R. Boruah, A.A. Ali, B. Saikia, D. Sarma, Green Chem. 17, 1442 (2015)
F. Saikh, J. Das, S. Ghosh, Tetrahedron Lett. 54, 4679 (2013)
R.H. Vekariya, H.D. Patel, Indian J. Chem. 56B, 890 (2017)
D. Setamdideh, J. Mex. Chem. Soc. 59(3), 191 (2015)
A.P. Chavan, A.B. Pinjari, P.C. Mhaske, J. Heterocycl. Chem. 00, 00 (2014)
A. Ahad, M. Farooqui, Int. J. Chem. Tech. Res. 10(6), 269 (2017)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, B.M., Shinde, S.K., Jagdale, A.A. et al. Fruit Extract of Averrhoa bilimbi: A Green Neoteric Micellar Medium for Isoxazole and Biginelli-Like Synthesis. Res Chem Intermed 47, 4369–4398 (2021). https://doi.org/10.1007/s11164-021-04539-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04539-y