Abstract
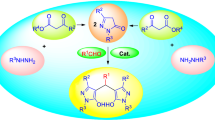
4-Sulfophthalic acid (4-H3SPA) solution 50 wt% in H2O has been effectively catalyzed the synthesis of a series of biologically relevant bis(indolyl)methanes by the electrophilic substitution of indole derivatives on aldehyde compounds and 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s by condensing 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one with various aldehydes under aqua conditions at room temperature. 3,3′-(Arylmethylene)-bis-4-hydroxycoumarins have also been synthesized in the presence of 0.1 mL (0.262 mmol) of 4-H3SPA solution 50 wt% in H2O at 80 °C. The procedure is simple and the expected bis-heterocyclic compounds were isolated in good to excellent yields. The present protocol provides the benefits of convenience, mild reaction conditions, eco-friendliness, and no use of hazardous organic solvents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bis(indolyl)methanes (BIMs) are an important class of bis-heterocyclic compounds which have been studied owing to their applications in industry and pharmaceutics. They are present in numerous biologically important natural products and synthetic compounds [1, 2]. As previously reported, some of BIMs exhibited biological activities, including antibacterial [3], antifungal [4], anti-inflammatory [5], DNA damaging [6], antihyperlipidemic [7], anticancer [8], topoisomerase IIa inhibitory [9], tranquilizing [10], antileishmanial [11], and antibiotics [12]. Moreover, heterocycles having bis(indolyl)methane units act as dietary supplements [13], colorimetric chemosensors [14–17] and pH indicators [18].
Generally, BIMs were prepared using the three-component reaction (3CR) of indole derivatives and numerous aldehydes or ketones. So far, due to a variety of applications of BIMs, several synthetic methods have been developed for their construction. Resins [19], acidic organocatalysts [20–24], biocatalysts [25, 26], solid acid catalysts [27–34], Lewis acids [35–40], protic acids [41], ionic liquids [42, 43], supported Bronsted acids [8, 44], deep eutectic solvents [45], fruit juice natural catalysts [46, 47], and nanomaterials [48–56] have been utilized for the synthesis of these nitrogen-containing heterocyclic compounds. Furthermore, microwave heating [57–60] and ultrasonic waves [61, 62] are valuable tools to the synthesis of these bisheterocycles. BIMs have also been prepared in the presence of sulfonic acid-containing compounds [63–76], catalyst- and/or solvent-free [77–81] and aqua-mediated conditions [82–84]. Therefore, the synthesis of BIMs in an environmentally benign solvent is always attractive.
Besides, 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s as an important class of pyrazolones, have been used as anti-inflammatory [85], antipyretic [86], gastric secretion stimulatory [87], antidepressant [88], antibacterial [89], antifilarial [90], antiviral [91], antioxidant [92], and hypoglycemic [93] agents. These molecules have also been investigated as fungicides [94], pesticides [95], insecticides [96], dyestuffs [97], chelating, as well as extracting reagents for different metal ions [98]. The synthesis of 4,4′-(Arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s have been carried out using several catalysts, including sulfuric acid ([3-(3-silicapropyl)sulfanyl]propyl)ester [99], 1,3,5-tris(hydrogensulfato) benzene (THSB) [100], Ce(SO4)2.4H2O [101], 2-hydroxy ethylammonium propionate (2-HEAP) [102], CuCr2O4 nanoparticles [103], Fe(SO4)2.(NH4)2.6(H2O) [104], CsF [105], [HMIM]HSO4 under ultrasound irradiation [106], silica sulfuric acid (SSA) [107], ZnAl2O4 nanoparticles [108], diammonium hydrogen phosphate [109], 12-tungstophosphoric acid (H3PW12O40) [110], phosphomolybdic acid [111], nano n-propylsulphonated γ-Fe2O3 [112], and N-methylimidazolium perchlorate ([MIm]ClO4) [113]. Catalyst-free in refluxing EtOH [114], catalyst- and solvent-free under heating [115] are the other methods for the preparation of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) derivatives. More recently, a comprehensive survey of the various catalysts, reagents, and conditions/techniques for the synthesis of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s has been reported by Gouda [116].
The 3,3′-(arylmethylene)-bis-(4-hydroxycoumarins), on the other hand, play a prominent role in organic chemistry due to a myriad of biological activities of their derivatives, including antibacterial [117], HIV-1 integrase inhibitory [118], anticoagulant [119], urease inhibitory [120], proliferation inhibition of K-562 [121] and α-glucosidase inhibitory [122]. They are also attractive heterocyclic molecules, which have been used for the development of fluorescent and colorimetric sensors [123], as well as photoluminescence probes [124] in recent years. Moreover, 3,3′-methylenebis-4-hydroxycoumarin (often known as dicoumarol), is a type of biscoumarins occurring naturally in moldy clover [125]. Biscoumarins also show optical properties since they are highly efficient laser dyes [126].
The synthesis of biscoumarins is based on the condensation two equivalents of 4-hydroxycoumarin with various (het)aryl and α,β-unsaturated aldehydes in the presence of a catalyst. So far, many catalysts have been used for the preparation of these compounds, for example triethylammonium bromide (TEAB) [117], sodium dodecyl sulfate (SDS) [127], poly(4-vinylpyridinium) perchlorate [128], Bi(NO3)3·5H2O [129], propane-1,2,3-triyltris(hydrogen sulfate) [130], tris(hydrogensulfato) boron [B(HSO4)3] [131], sulfated titania \(\left( {{{{\text{TiO}}_{2} } \mathord{\left/ {\vphantom {{{\text{TiO}}_{2} } {{\text{SO}}_{4}^{2 - } }}} \right. \kern-0pt} {{\text{SO}}_{4}^{2 - } }}} \right)\) [132], NaHSO4/SiO2/indion 190 resin [133], ionic liquids [134–136], silica sulforic acid nanoparticles [137], choline hydroxide [138], Zn(Proline)2 [139], CuO–CeO2 nanocomposite [140], nano-silica chloride [141], nano-MgO [142], trichloroacetic acid [143], tetrabutylammonium hexatungstate [144], titania sulfonic acid (TiO2–SO3H) [145], as well as LiClO4 [146] have been reported to catalyze the aforementioned condensation reaction. Furthermore, microwave heating [147, 148], sonochemically mediated catalyst-free condensation [149, 150] and organic solid state reactions [151] were also reported for the preparation of biscoumarins.
Because of the role of biological and pharmacological related to the above mentioned bis-heterocyclic compounds, the development of convenient, efficient, inexpensive and eco-friendly new methodologies using readily available reagents to the synthesis of these types of heterocyclic dimers is of interest. Water is common solvent, which have the advantages of being green, clean and readily biodegradable. This solvent has been utilized as an environmentally attractive medium for some chemical transformations [152]. Because of the importance the potential of these drug-like heterocyclic compounds, we report a successful one-vessel reaction for synthesis of bis(indolyl)methanes, bispyrazoles and biscoumarins under eco-friendly reaction conditions (Scheme 1).
Experimental
All chemicals were purchased from Alfa Aesar and Aldrich as well as were used without further purification, with the exception of liquid aldehydes which were distilled before using. All solvents were distilled before using. The products were characterized by comparison of their physical data with those of known samples or by their spectral data. Melting points were measured on a Büchi 510 melting point apparatus and are uncorrected. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded at ambient temperature on a Bruker AVANCE DRX-400 MHz using deuterated chloroform (CDCl3) or dimethyl sulfoxide (DMSO)-d 6 as solvent. Fourier-transform infrared (FT-IR) spectra were recorded on a PerkinElmer RXI spectrometer. Progress of reactions was monitored by thin layer chromatography (TLC) analysis on Merck pre-coated silica gel 60 F254 aluminum sheets, visualized by ultraviolet (UV) light.
General procedure for the synthesis of bis(indolyl)methanes (4a–r)
Aldehyde 1 (1 mmol), indole derivative 2 (2 mmol), and 4-H3SPA solution 50 wt% in H2O (0.3 mL, 0.787 mmol) and water (5 mL) was stirred at room temperature. After completion of the reaction, as confirmed by TLC analysis, the resulting precipitated product was filtered off, washed with distilled water (2 × 5 mL), and dried to afford the corresponding products in high purity. The filtrate containing the catalyst was used as such for exploring the reusability of the catalyst. The catalyst is soluble in water while the products are insoluble in water. Spectral data for 3a and 3k were as follows:
3,3′-Benzylidenebis(2-methyl-1H-indole) ( 3a ) IR (KBr, cm−1): 3398, 3051, 2921, 2860, 1615, 1461, 1305, 1218, 1016, 746, 594; 1H NMR (400 MHz, CDCl3): δ = 7.68 (s, 2H), 7.22-7.11 (m, 7H), 6.94 (t, J = 6.8 Hz, 2H), 6.93 (d, J = 8.4 Hz, 2H), 6.75 (t, J = 7.6 Hz, 2H), 5.91 (s, 1H), 1.98 (s, 6H); 13C NMR (100 MHz, CDCl3): δ = 143.5, 134.9, 131.6, 128.9, 128.7, 128.1, 126.0, 120.4, 119.2, 119.1, 113.2, 110.0, 39.6, 12.5.
3,3′-(Phenylmethylene)bis(1H-indole) ( 3 k ) IR (KBr, cm−1): 3392, 3056, 2922, 2347, 1603, 1454, 1328, 1218, 1093, 1014, 748, 702, 595; 1H NMR (400 MHz, CDCl3): δ = 7.88 (s, 2H), 7.37 (d, J = 8.0 Hz, 2H), 7.34-7.13 (m, 9H), 6.99 (t, J = 7.6 Hz, 2H), 6.63 (d, J = 1.6 Hz, 2H), 5.86 (s, 1H); 13C NMR (100 MHz, CDCl3): δ = 144.1, 136.1, 128.7, 128.5, 127.2, 126.2, 124.1, 121.9, 119.8, 119.2, 118.9, 111.1, 40.2.
General procedure for the synthesis of 4,4′-(arylmethylene)-bis-(3-methyl-1-phenyl-1H-pyrazol-5-ol) derivatives (5a–q)
Aldehyde 1 (1 mmol), 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 4 (2 mmol), 4-H3SPA solution 50 wt% in H2O (0.3 mL, 0.787 mmol) and water (5 mL) was stirred at room temperature. After completion of the reaction, the resulting precipitated product was filtered off, washed with distilled water (2 × 5 mL), and dried to afford the corresponding products in high purity. The filtrate containing catalyst was used as such for exploring the reusability of the catalyst. Spectral data for 5e and 5 h were as follows:
4,4′-[(4-Methylphenyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) ( 5e ) IR (KBr, cm−1): 3432, 2921, 1600, 1501, 1408, 1294, 1026; 1H NMR (400 MHz, DMSO-d 6): δ = 13.38 (br, 2H), 7.69 (d, J = 7.7 Hz, 4H), 7.42 (t, J = 7.7 Hz, 4H), 7.20 (t, J = 7.7 Hz, 2H), 7.12 (d, J = 7.6 Hz, 2H), 7.07 (d, J = 8.3 Hz, 2H), 4.88 (s, 1H), 2.30 (s, 6H), 2.23 (s, 3H); 13C NMR (DMSO-d 6, 100 MHz): δ = 154.5, 146.8, 139.7, 135.5, 129.5, 129.2, 128.0, 125.4, 124.7, 122.8, 120.5, 33.3, 21.1, 13.2.
4,4′-[(4-Nitrophenyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) ( 5 h ) IR (KBr, cm−1): 3422, 3056, 2972, 2920, 1604, 1580, 1504, 1348, 1108; 1H NMR (400 MHz, CDCl3): δ = 13.52 (br, 2H), 8.28 (d, J = 8.5 Hz, 2H), 7.72 (d, J = 7.8, 4H), 7.65 (d, J = 8.3, 2H), 7.38 (d, J = 7.3, 4H), 7.20 (t, J = 7.1 Hz, 2H), 5.46 (s, 1H), 2.29 (s, 6H); 13C NMR (CDCl3, 100 MHz): δ = 151.5, 148.6, 145.4, 145.1, 139.3, 131.1, 129.6, 128.1, 121.9, 121.6, 119.5, 31.9, 14.2.
General procedure for the synthesis of 3,3′-(arylmethylene)-bis-(4-hydroxycoumarins) (7a–q)
A mixture of aldehyde 1 (1 mmol), 4-hydroxycoumarin 6 (2 mmol), 4-H3SPA solution 50 wt% in H2O (0.1 mL, 0.262 mmol) and water (5 mL) in a round-bottomed flask was heated at 80 °C. Upon completion of the reaction, as confirmed by TLC analysis, the mixture was cooled to room temperature. After completion of the reaction, the mixture was cooled to room temperature and water was added. Then, the resulting precipitated product was filtered, washed with water (2 × 5 mL), and dried. The pure products were obtained by recrystallization from aqueous ethanol. The filtrate containing catalyst was used as such for exploring the reusability of the catalyst. Spectral data for 7c and 7f were as follows:
3,3′-((4-Methoxyphenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) ( 7c ) IR (KBr, cm−1): 3440, 3072, 3002, 1668, 1604, 1565, 1510,1454, 1353, 1258, 1180, 1094, 907, 828, 768; 1H NMR (400 MHz, CDCl3): δ = 11.53 (s, 1H), 11.31 (s, 1H), 8.02 (dd, 2H, J = 8.4 Hz), 7.65 (t, 2H, J = 8.2 Hz), 7.31-7.42 (m, 4H), 7.14 (d, 2H, J = 8.7 Hz), 6.89 (d, J = 8.7 Hz, 2H), 6.05 (s, 1H), 3.81 (s, 3H).
3,3′-((4-Chlorophenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) ( 7f ) IR (KBr, cm−1): 3069, 2680, 2610, 1669, 1619, 1600, 1491, 1455, 1353, 1310, 1267, 1185, 1096, 922, 910, 822, 790; 1H NMR (400 MHz, CDCl3): δ = 11.59 (s, 1H), 11.37 (s, 1H), 8.11 (d, 1H, J = 7.74 Hz), 8.03 (d, 1H, J = 7.8 Hz), 7.66-7.69 (m, 2H), 7.41-7.46 (m, 4H), 7.32 (d, 2H, J = 1.9, 8.6 Hz), 7.19 (dd, 2H, J = 0.9, 8.5 Hz), 6.08 (s, 1H); 13C NMR (100 MHz, CDCl3): δ = 169.6, 167.3, 166.4, 165.1, 152.9, 152.7, 134.3, 133.5, 133.1, 129.2, 128.4, 125.4, 124.8, 117.2, 117.1, 117.0, 116.8, 105.7, 104.1, 36.2.
Results and discussion
In order to evaluate the optimization of the reaction conditions, a model reaction was performed using benzaldehyde (1a) and 2-methylindole (2a) at room temperature (r.t.) and the progress of the reaction was monitored by TLC analysis. The results are summarized in Table 1. It was found that when the reaction conducted in water without any catalyst the reaction did not proceed even until 24 h (Table 1, entry 1). Performing the same reaction in the presence of catalytic amounts of 4-sulfophthalic acid (4-H3SPA, 50 wt% in H2O) in water led to a 76 % yield of 3a after 15 min (Table 1, entry 2). After obtaining the corresponding product, the various volumes of 4-H3SPA aimed at the completion of reaction was evaluated. The reaction was performed using 0.2 mL and 0.3 mL of the catalyst (Table 1, entries 3–4). It was observed that 0.3 mL of the 4-H3SPA loading provided maximum yield (89 %) in 15 min. An additional increase of the volume catalyst loading to 0.4 mL did not improve the yield (Table 1, entry 5). A number of other common solvents, viz. ethanol, n-hexane, EtOAc and CH2Cl2 were tested (Table 1, entries 6–9). Solvent optimization studies indicated that water was the best solvent. It was observed that the product 3a was formed in 60 % yield in the solvent-free conditions (Table 1, entry 10). Satisfactory results were not achieved from the reactions at other temperatures. For this reason we have not mentioned in Table 1.

The scope of the reaction was studied for indoles (2a–b) and various aldehydes under the optimal reaction conditions. Representative results are listed in Table 2. Substituted benzaldehydes with both electron-donating substituents (Table 2, entries 2–5 and 11–14) and electron-withdrawing groups (Table 2, entries 6–9, 16, 17 and 19) at the ortho, meta, and para positions on the phenyl ring contributed well in this 3CR toward the synthesis of BIMs. The aryl aldehydes containing electron-withdrawing substituents required longer reaction times compared its electron-donating counterparts. Furan-2-carbaldehyde (1j), as a heterocyclic aldehyde, was also reacted with indoles (2a–b) and the desired product was isolated in good yields (Table 2, entries 10 and 18).
After the successful application of 4-H3SPA catalyst in the synthesis of BIMs, we decided to use it in the condensation of 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (4) with a wide variety of aldehydes (1a–q) leading to 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s (5a–q). To obtain the best reaction conditions, the condensation of benzaldehyde (1a) with 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (4) as the model reaction, was explored using different amounts of 4-H3SPA and various solvents such as water, ethanol, n-hexane, EtOAc and CH2Cl2 as well as solvent-free conditions. The best results were obtained in water using 0.3 mL of 4-H3SPA at r.t. The scope of the catalyst was extended using catalyst with different aromatic aldehydes to prepare a series of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol) derivatives (Table 3). Various aromatic aldehydes containing electron-withdrawing substituents, electron-releasing substituents and halogens on their aromatic rings as well as heteroaromatic aldehydes were utilized successfully in this condensation reaction, and gave the corresponding products (5a–q) in good to high yields and relatively shorter reaction times.
In the next part of this contribution on the application of catalytic activity 4-H3SPA in the synthesis of other bis-heterocyclic compounds, 4-hydroxycoumarin (6) as a suitable precursor was used in the condensation with aldehydes (1a-q). This reaction leads to the synthesis of 3,3′-(arylmethylene)-bis-(4-hydroxycoumarins). To accomplish this goal and to achieve the best reaction conditions, benzaldehyde (1a) was treated with 4-hydroxycoumarin (6), and various conditions including the amounts of catalyst, solvents, and reaction temperatures were investigated. The optimum conditions were obtained using a 0.1 mL catalyst loading, benzaldehyde (1a, 1 mmol), 4-hydroxycoumarin (6, 2 mmol) and at 80 °C under aqueous conditions. After the optimization of the reaction conditions, a variety of substituted benzaldehydes (Table 4, entries 2–10, 12 and 14–16) containing several functional groups and heterocyclic aldehydes (11, 13 and 17) have been successfully utilized and the corresponding 3,3′-(arylmethylene)-bis-(4-hydroxycoumarins) were obtained in good to excellent isolated yields.
The comparison of the catalytic performance of 4-H3SPA with some of the reported catalysts is shown in Table 5. According to the data presented in Table 5, the synthesis of these bis-heterocycles using 4-H3SPA catalyst is comparable in terms of yields and reaction times. It does not require the use of some hazardous organic solvents such as toluene, acetonitrile, and dichloromethane, and also avoids the preparation of the catalyst.
Reusability of the reaction media was conducted using the model reaction under the optimal conditions. After completion of the reaction, the resulting solid product was separated out by simple filtration. To the filtrate that contains the catalyst, benzaldehyde (1a) and 2-methylindole (2a) were added without additional catalyst loading. The reaction mixture was stirred at r.t. for the required times (Table 6). The reaction media was reused as such for the subsequent reactions up to four runs.
Based on the proposed mechanisms in the literature, a plausible reaction mechanism for these reactions is shown in Scheme 2. It can be assumed that the reaction starts with the activation of an oxygen atom of the carbonyl group of the aldehydes, followed by the nucleophilic attack of heterocyclic compounds (A) on activated aldehydes (8) and departure of water which led to the formation of alkene intermediates 11. The condensation of the second molecule of A with the alkene intermediates 11 and then elimination of proton from bis-heterocyclic intermediates 12 leads to the formation of the desired bis-heterocycles (3, 5 and 7).
Conclusions
A series of bis-heterocycles such as bis(indolyl)methanes, 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s, and 3,3′-(arylmethylene)-bis-4-hydroxycoumarins were synthesized using 4-H3SPA as an efficient catalyst. The experimental procedure is simple, convenient, and has the ability to a wide range of substrates, which affords a series of diversity bis-heterocycles. The use of 4-H3SPA in these reactions is included notable features such as clean reaction profiles, minimization of waste, operational simplicity, non-toxicity, shorter reaction times, easy experimental work-up procedure, high yields of the products, and avoids usage of organic solvents. These advantages make the present protocol an interesting alternative to the previously reported methods for the synthesis of BIMs, 3,3′-(arylmethylene)-bis-(4-hydroxycoumarins), as well as 4,4′-(arylmethylene)-bispyrazoles.
References
M. Shiri, M.A. Zolfigol, H.G. Kruger, Z. Tanbakouchian, Chem. Rev. 110, 2250 (2010)
P.P. Kaishap, C. Dohutia, Int. J. Pharm. Sci. Res. 4, 1312 (2013)
S. Sarva, J.S. Harinath, S.P. Sthanikam, S. Ethiraj, M. Vaithiyalingam, S.R. Cirandur, Chin. Chem. Lett. 27, 16 (2016)
G. Sivaprasad, P.T. Perumal, V.R. Prabavathy, N. Mathivanan, Bioorg. Med. Chem. Lett. 16, 6302 (2006)
K. Sujatha, P.T. Perumal, D. Muralidharan, M. Rajendran, Indian J. Chem. 48B, 267 (2009)
T. Osawa, M. Namiki, Tetrahedron Lett. 24, 4719 (1983)
K.V. Sashidhara, A. Kumar, M. Kumar, A. Srivastava, A. Puri, Bioorg. Med. Chem. Lett. 20, 6504 (2010)
M. Mari, A. Tassoni, S. Lucarini, M. Fanelli, G. Piersanti, G. Spadoni, Eur. J. Org. Chem. 2014, 3822 (2014)
Y. Gong, G.L. Firestone, L.F. Bjeldanes, Mol. Pharmacol. 69, 1320 (2006)
J. Povszasz, G.P. Katalin, S. Foleat, B. Malkovics, Acta Phys. Acad. Sci. Hung. 29, 299 (1996)
S.B. Bharate, J.B. Bharate, S.I. Khan, B.L. Tekwani, M.R. Jacob, R. Mudududdla, R.R. Yadav, B. Singh, P.R. Sharma, S. Maity, B. Singh, I.A. Khan, R.A. Vishwakarma, Eur. J. Med. Chem. 63, 435 (2013)
M. Kobayashi, S. Aoki, K. Gato, K. Matsunami, M. Kurosu, I. Kitagawa, Chem. Pharm. Bull. 42, 2449 (1994)
C. Bonnesen, I.M. Eggleston, J.D. Hayes, Cancer Res. 61, 6120 (2001)
R. Martinez, A. Espinosa, A. Tarraga, P. Molina, Tetrahedron 64, 2184 (2008)
R. Pegu, R. Mandal, A.K. Guha, S. Pratihar, New J. Chem. 39, 5984 (2015)
X. He, S. Hu, K. Liu, Y. Guo, J. Xu, S. Shao, Org. Lett. 8, 333 (2006)
D. Sain, C. Kumari, A. Kumar, S. Dey, Supramol. Chem. 28, 239 (2016)
A. Khorshidi, N. Mardazad, Z. Shaabanzadeh, Tetrahedron Lett. 55, 3873 (2014)
R. Surasani, D. Kalita, K.B. Chandrasekhar, Green Chem. Lett. Rev. 6, 113 (2013)
A. Ganesan, J. Kothandapani, J.B. Nanubolu, S.S. Ganesan, RSC Adv. 5, 28597 (2015)
A.K. Mallik, R. Pal, C. Guha, H. Mallik, Green Chem. Lett. Rev. 5, 321 (2012)
M. El-Sayed, K. Mahmoud, A. Hilgeroth, Curr. Chem. Lett. 3, 7 (2014)
H. Veisi, R. Gholbedaghi, J. Malakootikhah, A. Sedrpoushan, B. Maleki, D. Kordestani, J. Heterocycl. Chem. 47, 1398 (2010)
K. Karthikeyan, G. Sivaprasad, Org. Prep. Proced. Int. 47, 449 (2015)
D. Sun, G. Jiang, Z. Xie, Z. Le, Chin. J. Chem. 33, 409 (2015)
Z.B. Xie, D.Z. Sun, G.F. Jiang, Z.G. Le, Molecules 19, 19665 (2014)
K. Ravi, B. Krishnakumar, M. Swaminathan, Res. Chem. Intermed. 41, 5353 (2015)
A.V. Reddy, K. Ravinder, V.L.N. Reddy, T.V. Goud, V. Ravikanth, Y. Venkateswarlu, Synth. Commun. 33, 3687 (2003)
J.S. Yadav, M.K. Gupta, R. Jain, N.N. Yadav, B.V.S. Reddy, Monatsh. Chem. 141, 1001 (2010)
B.Y. Giri, B.L.A.P. Devi, K. Vijayalakshmi, R.B.N. Prasad, N. Lingaiah, P.S.S. Prasad, Indian J. Chem. 51B, 1731 (2012)
M.M. Meshram, N.N. Rao, P.B. Thakur, B.C. Reddy, P. Ramesh, Indian J. Chem. 52B, 814 (2013)
R. Vaid, M. Gupta, O.S. Chambyal, R. Gupta, J. Chem. Sci. 127, 987 (2015)
L.Z. Fekri, M. Nikpassand, M. Kohansal, Rus. J. Gen. Chem. 85, 2861 (2015)
D. Talukdar, A.J. Thakur, Green Chem. Lett. Rev. 6, 55 (2013)
D. Liang, W. Huang, L. Yuan, Y. Ma, J. Ma, D. Ning, Catal. Commun. 55, 11 (2014)
N.C. Ganguly, P. Mondal, S.K. Barik, Green Chem. Lett. Rev. 5, 73 (2012)
S.J. Ji, M.F. Zhou, D.G. Gu, Z.Q. Jiang, T.P. Loh, Eur. J. Org. Chem. 1584, 37 (2004)
C.C. Silveira, S.R. Mendes, M.A. Villetti, D.F. Back, T.S. Kaufman, Green Chem. 14, 2912 (2012)
A.C. Shaikh, C. Chen, J. Chin. Chem. Soc. 58, 899 (2011)
A. Swetha, B.M. Babu, H.M. Meshram, Tetrahedron Lett. 56, 1775 (2015)
M. Auria, Tetrahedron 47, 9225 (1991)
M. Kalantari, Arab. J. Chem. 5, 319 (2012)
S.A.R. Mulla, A. Sudalai, M.Y. Pathan, S.A. Siddique, S.M. Inamdar, S.S. Chavan, R.S. Reddy, RSC Adv. 2, 3525 (2012)
E.L. Armstrong, H.K. Grover, M.A. Kerr, J. Org. Chem. 78, 10534 (2013)
S. Handy, N.M. Westbrook, Tetrahedron Lett. 55, 4969 (2014)
P. Rammohan, Indian J. Chem. 53B, 763 (2014)
P. Rammohan, Int. J. Org. Chem. 3, 136 (2013)
K. Ghodrati, S.H. Hosseini, R. Mosaedi, C. Karami, F. Maleki, A. Farrokhi, Z. Hamidi, Int. Nano Lett. 3, 13 (2013)
R. Pegu, K.J. Majumdar, D.J. Talukdar, S. Pratihar, RSC Adv. 4, 33446 (2014)
A. Shaabani, R. Afshari, S.E. Hooshmand, A.T. Tabatabaei, F. Hajishaabanha, RSC Adv. 6, 18113 (2016)
D.A. Reddy, J. Choi, S. Lee, R. Ma, T.K. Kim, RSC Adv. 5, 67394 (2015)
S. Sobhani, R. Jahanshahi, New J. Chem. 37, 1009 (2013)
H. Mahmoudi, A.A. Jafari, S. Saeedi, H. Firouzabadi, RSC Adv. 5, 3023 (2015)
M. Kour, S. Paul, New J. Chem. 39, 6338 (2015)
P.K. Chhattise, S.S. Arbuj, K.C. Mohite, S.V. Bhavsar, A.S. Horne, K.N. Handore, V.V. Chabukswar, RSC Adv. 4, 28623 (2014)
B. Sadeghi, F. Amiri-Tavasoli, A. Hassanabadi, Synth. React. Inorg. Met.Org. Nano Met. Chem. 45, 1396 (2015)
D.W. Zhang, Y.M. Zhang, Y.L. Zhang, T.Q. Zhao, H.W. Liu, Y.M. Gan, Q. Gu, Chem. Pap. 69, 470 (2015)
M. Zahran, Y. Abdin, H. Salama, Arkivoc xi, 256 (2008)
S.R. Mendes, S. Thurow, F. Penteado, M. da Silva, R.A. Gariani, G. Perinb, E.J. Lenardão, Green Chem. 17, 4334 (2015)
M. Imam Uddin, J.R. Buck, M.L. Schulte, D. Tang, S.A. Saleh, Y.Y. Cheung, J. Harp, H.C. Manning, Tetrahedron Lett. 55, 169 (2014)
S.S. Sonar, S.A. Sadaphal, A.H. Kategaonkar, R.U. Pokalwar, B.B. Shingate, M.S. Shingare, Bull. Korean Chem. Soc. 30, 825 (2009)
A.P.G. Nikalje, S.I. Shaikh, World J. Pharm. Pharmaceut. Sci. 3, 1282 (2014)
J.T. Li, M.X. Sun, G.Y. He, X.Y. Xu, Ultrasound Sonochem. 18, 412 (2011)
F. Shirini, N.G. Khaligh, Chin. J. Catal. 34, 1890 (2013)
K. Niknam, D. Saberi, M. Baghernejad, Phosphor. Sulfur Silicon Relat. Elem. 185, 875 (2010)
S.M. Baghbanian, Y. Babajani, H. Tashakorian, S. Khaksar, M. Farhang, C. R. Chimie 16, 129 (2013)
F. Shirini, N.G. Khaligh, O.G. Jolodar, Dyes Pigments 98, 290 (2013)
H. Alinezhad, A.H. Haghighi, F. Salehian, Chin. Chem. Lett. 21, 183 (2010)
A. Zare, F. Bahrami, M. Merajoddin, M. Bandari, A.R. Moosavi-Zare, M.A. Zolfigol, A. Hasaninejad, M. Shekouhy, M.H. Beyzavi, V. Khakyzadeh, M. Mokhlesi, Z. Asgari, Org. Prep. Proced. Int. 45, 211 (2013)
S.A. Sadaphal, S.S. Sonar, M.N. Ware, M.S. Shingare, Green Chem. Lett. Rev. 1, 191 (2008)
M. Seddighi, F. Shirini, M. Mamaghani, RSC Adv. 3, 24046 (2013)
V.J. Rani, K.V. Vani, C.V. Rao, Synth. Commun. 42, 2048 (2012)
W.J. Li, X.F. Lin, J. Wang, G.L. Li, Y.G. Wang, Synth. Commun. 35, 2765 (2005)
M. Kidwai, R. Chauhan, D. Bhatnagar, Arab. J. Chem. (2014). doi:10.1016/j.arabjc.2014.05.009
S.S. Ekbote, K.M. Deshmukh, Z.S. Qureshi, B.M. Bhanage, Green Chem. Lett. Rev. 4, 177 (2011)
S.R. Sheng, Q.Y. Wang, Y. Ding, X.L. Liu, M.Z. Cai, Catal. Lett. 128, 418 (2009)
K.L. Dhumaskar, S.G. Tilve, Green Chem. Lett. Rev. 5, 353 (2012)
V.D. Patil, G.B. Dere, P.A. Rege, J.J. Patil, Synth. Commun. 41, 736 (2011)
A. Kundu, A. Ganguly, K. Dhara, P. Patra, N. Guchhait, RSC Adv. 5, 53220 (2015)
D.K. Sharma, A. Hussain, M.R. Lambu, S.K. Yousuf, S. Maiety, B. Singh, D. Mukherjee, RSC Adv. 3, 2211 (2013)
N. Baig, G.M. Shelke, A. Kumar, A.K. Sah, Catal. Lett. 146, 333 (2016)
N. Seyedi, M. Kalantari, J. Sci. I. R. Iran 24, 205 (2013)
H. Hikawa, Y. Yokoyama, RSC Adv. 3, 1061 (2013)
N. Gupta, D. Goyal, Chem. Heterocyl. Compd. 51, 4 (2015)
S. Sugiura, S. Ohno, O. Ohtani, K. Izumi, T. Kitamikado, H. Asai, K. Kato, J. Med. Chem. 20, 80 (1977)
L.C. Behr, R. Fusco, C.H. Jarboe, in The Chemistry of Heterocyclic Compounds, Pyrazoles, Pyrazolines, Pyrazolidines, Indazoles and Condensed Rings, ed. by A. Weissberger (Interscience Publishers, New York, 1967)
C.E. Rosiere, M.I. Grossman, Science 113, 651 (1951)
D.M. Bailey, P.E. Hansen, A.G. Hlavac, E.R. Baizman, J. Pearl, A.F. Defelice, M.E. Feigenson, J. Med. Chem. 28, 256 (1985)
R.N. Mahajan, F.H. Havaldar, P.S. Fernandes, J. Indian Chem. Soc. 68, 245 (1991)
P.M.S. Chauhan, S. Singh, R.K. Chatterjee, Indian J. Chem. Sect B: Org. Chem. Incl. Med. Chem. 32, 858 (1993)
K. Sujatha, G. Shanthi, N.P. Selvam, S. Manoharan, P.T. Perumal, M. Rajendran, Bioorg. Med. Chem. Lett. 19, 4501 (2009)
X. Yang, P. Zhang, Y. Zhou, J. Wang, H. Liu, Chin. J. Chem. 30, 670 (2012)
N. Das, A. Verma, P.K. Shrivastava, S.K. Shrivastava, Indian J. Chem. 47B, 1555 (2008)
D. Singh, D. Singh, J. Indian Chem. Soc. 68, 165 (1991)
M. Londershausen, Pestic. Sci. 48, 269 (1996)
H.A. Lubs (ed.), The Chemistry of Synthetic Dyes and Pigments (American Chemical Society, Washington, DC, 1970)
A.D. Garnovskii, A.I. Uraev, V.I. Minkin, Arkivoc iii, 29 (2004)
M. Abbasi-Tarighat, E. Shahbazi, K. Niknam, Food Chem. 138, 991 (2013)
S. Tayebi, M. Baghrnejad, D. Saberi, K. Niknam, Chin. J. Catal. 32, 1477 (2011)
Z. Karimi-Jaberi, B. Pooladian, M. Moradi, E. Ghasemi, Chin. J. Catal. 33, 1945 (2012)
E. Mosaddegh, M.R. Islami, Z. Shojaie, Arab. J. Chem. (2013). doi:10.1016/j.arabjc.2013.02.016
Z. Zhou, Y. Zhang, Green Chem. Lett. Rev. 7, 18 (2014)
J. Safaei-Ghomi, B. Khojastehbakht-Koopaei, S. Zahedi, Chem. Heterocycl. Compd. 51, 34 (2015)
K. Eskandari, B. Karami, S. Khodabakhshi, M. Farahi, Lett. Org. Chem. 12, 38 (2015)
K.M. Khan, M.T. Muhammad, I. Khan, S. Perveen, W. Voelter, Monatsh. Chem. 146, 1587 (2015)
H. Zang, Q. Su, Y. Mo, B. Cheng, Ultrason. Sonochem. 18, 68 (2011)
K. Niknam, S. Mirzaee, Synth. Commun. 41, 2403 (2011)
J. Safaei-Ghomi, B. Khojastehbakht-Koopaei, H. Shahbazi-Alavi, RSC Adv. 4, 46106 (2014)
C. Yang, L. Pang, A. Wang, Asian J. Chem. 23, 749 (2011)
A. Vafaee, A. Davoodnia, M. Pordel, Res. Chem. Intermed. 41, 8242 (2015)
K.R. Phatangare, V.S. Padalkar, V.D. Gupta, V.S. Patil, P.G. Umape, N. Sekar, Synth. Commun. 42, 1349 (2012)
S. Sobhani, Z. Pakdin-Parizi, R. Nasseri, J. Chem. Sci. 125, 975 (2013)
N.G. Khaligh, S.B.A. Hamid, S.J.J. Titinchi, Chin. Chem. Lett. 27, 104 (2016)
M.N. Elinson, O.O. Sokolova, R.F. Nasybullin, Heterocycl. Commun. 21, 97 (2015)
A.D. Gupta, R. Pal, A.K. Mallik, Green Chem. Lett. Rev. 7, 404 (2014)
M.A. Gouda, J. Heterocycl. Chem. (2015). doi:10.1002/jhet.2313
D. Qu, J. Li, X.H. Yang, Z.D. Zhang, X.X. Luo, M.K. Li, X. Li, Molecules 19, 19868 (2014)
H. Zhao, N. Neamati, Y. Pommier, T.R. Burke Jr., Heterocycles 45, 2277 (1997)
I. Manolov, C. Maichle-Moessmer, I. Nicolova, N. Danchev, Arch. Pharm. Chem. Life Sci. 339, 319 (2006)
K.M. Khan, S. Iqbal, M.A. Lodhi, G.M. Maharvi, M.I. Choudhary, Z. Ullah, A.U. Rahman, S. Perveen, Bioorg. Med. Chem. 12, 1963 (2004)
O. Talhi, M. Schnekenburger, J. Panning, D.G.C. Pinto, J.A. Fernandes, F.A. Almeida Paz, C. Jacob, M. Diederich, A.M. Silva, Bioorg. Med. Chem. 22, 3008 (2014)
K.M. Khan, F. Rahim, A. Wadood, N. Kosar, M. Taha, S. Lalani, A. Khan, M.I. Fakhri, M. Junaid, W. Rehman, M. Khan, S. Perveen, M. Sajid, M.I. Choudhary, Eur. J. Med. Chem. 81, 245 (2014)
A.K. Mahapatra, K. Maiti, P. Sahoo, P.K. Nandi, J. Lumin. 143, 349 (2013)
J. Li, Z. Hou, F. Li, Z.D. Zhang, Y. Zhou, X.X. Luo, M.K. Li, J. Mol. Struct. 1075, 509 (2014)
A.D. Gupta, S. Samanta, R. Mondal, A.K. Mallik, Bull. Korean Chem. Soc. 33, 4239 (2012)
H. Ammar, S. Abid, S. Fery-Forgues, Dyes Pigments 78, 1 (2008)
H. Mehrabi, H. Abusaidi, J. Iran. Chem. Soc. 7, 890 (2010)
F. Shirini, S. Esmaeeli-Ranjbar, M. Seddighi, Chin. J. Catal. 35, 1017 (2014)
S. Zahiri, M. Mokhtary, J. Taibah Univ. Sci. 9, 89 (2015)
R. Rezaei, F. Moezzi, M.M. Doroodmand, Chin. Chem. Lett. 25, 183 (2014)
Z. Karimi-Jaberi, M.R. Nazarifar, B. Pooladian, Chin. Chem. Lett. 23, 781 (2012)
B. Karmakar, A. Nayak, J. Banerji, Tetrahedron Lett. 53, 4343 (2012)
V. Padalkar, K. Phatangare, S. Takale, R. Pisal, A. Chaskar, J. Saudi Chem. Soc. 19, 42 (2015)
K. ParvanakBoroujeni, P. Ghasemi, Z. Rafienia, Monatsh. Chem. 145, 1023 (2014)
W. Li, Y. Wang, Z. Wang, L. Dai, Y. Wang, Catal. Lett. 141, 1651 (2011)
A. Tzani, A. Douka, A. Papadopoulos, E.A. Pavlatou, E. Voutsas, A. Detsi, A.C.S. Sustain, Chem. Eng. 1, 1180 (2013)
B. Sadeghi, T. Ziya, J. Chem. 2013, 5, Article ID 179013 (2013). doi:10.1155/2013/179013
A. Zhu, S. Bai, L. Li, M. Wang, J. Wang, Catal. Lett. 145, 1089 (2015)
Z.N. Siddiqui, F. Farooq, Catal. Sci. Technol. 1, 810 (2011)
J. Albadi, A. Mansournezhad, S. Salehnasab, Res. Chem. Intermed. 41, 5713 (2015)
R. Karimian, F. Piri, A. Safari, S.J. Davarpanah, J. Nanostruct. Chem. 3, 52 (2013)
J. Safaei-Ghomi, F. Eshteghal, M.A. Ghasemzadeh, Acta Chim. Slov. 61, 703 (2014)
Z. Karimi-Jaberi, M.R. Nazarifar, Eur. Chem. Bull. 3, 512 (2014)
A. Davoodnia, Bull. Korean Chem. Soc. 32, 4286 (2011)
F. Shirini, M. Abedini, S.A. Kiaroudi, Phosphorus Sulfur Silicon Relat. Elem. 189, 1279 (2014)
E. Sheikhhosseini, Trend. Mod. Chem. 3, 34 (2012)
S. Qadir, A.A. Dar, K.Z. Khan, Synth. Commun. 38, 3490 (2008)
A.D. Gupta, S. Samanta, R. Mondal, A.K. Malik, Chem. Sci. Trans. 2, 524 (2013)
G. Cravotto, G.M. Nano, G. Palmisano, S. Tagliapietra, Synthesis 2003, 1286 (2003)
A. Hasaninejed, M. Rasekhi Kazerooni, A. Zare, ACS Sustain Chem. Eng. 1, 679 (2013)
H. Hagiwara, N. Fujimoto, T. Suzuki, M. Ando, Heterocycles 53, 549 (2000)
W. Zhang, B. Cue (eds.), Green Techniques for Organic Synthesis and Medicinal Chemistry, 1st edn. (Wiley-VCH, New York, 2012)
Acknowledgments
Damghan University is acknowledged for provision of facilities and materials.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banari, H., Kiyani, H. & Pourali, A. Efficient synthesis of bis(indolyl)methanes, bispyrazoles and biscoumarins using 4-sulfophthalic acid. Res Chem Intermed 43, 1635–1649 (2017). https://doi.org/10.1007/s11164-016-2720-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2720-7