In this study, pure spinel nanoparticles of copper chromite (CuCr2O4) were synthesized by coprecipitation method. These nanoparticles were characterized by X-ray diffraction, scanning electron microscopy, and FTIR. CuCr2O4 nanoparticles were found to be an efficient catalyst for the synthesis of 4,4'-(arylmethanediyl)bis(3-methyl-1H-pyrazol-5-ols) via pseudo-five-component reaction of aldehydes, hydrazine hydrate, and ethyl acetoacetate. The present methodology offers several advantages such as excellent yields, simple procedure, and mild conditions. The catalyst can be reused at least five times without any obvious change in its catalytic activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pyrazoles are an important class of heterocyclic compounds with prominent properties. Among this class of compounds, pyrazolone derivatives have a broad spectrum of approved biological activity such as anti-inflammatory, antipyretic, antibacterial, gastric secretion stimulatory, antidepressant, and antifilarial activities.1 – 3 Additionally, they are also important intermediates in organic synthesis.4 The conventional chemical approach to 4,4'-(arylmethanediyl)bis-(3-methyl-1H-pyrazol-5-ols) involves the successive Knoevenagel synthesis of the corresponding arylidenepyrazolones and its base-promoted Michael reaction, and also one-pot tandem Knoevenagel–Michael reaction of arylaldehydes with 2 equiv of 3-methyl-1H-pyrazol-5-ol carried out under a variety of reaction conditions.5 – 7 Recently, synthesis of 4,4'-(arylmethanediyl)bis(3-methyl-1H-pyrazol-5-ol) derivatives have been reported using pyridine trifluoroacetate or acetic acid,8 ceric ammonium nitrate,9 phosphomolybdic acid,10 sulfuric acid [3-(3-silicapropyl)sulfanyl]propyl ester,11 silica-bonded N-propyltriethylenetetramine,12 sodium dodecyl sulfate,13 1,3-disulfo-1H-imidazolium tetrachloroaluminate,14 and 1,3,5-tris(hydrogensulfato)benzene.15
Heterogeneous catalytic materials play a very important role in organic synthesis in ways that are economically advantageous and environmentally friendly.16 , 17 They can be recovered easily from the reaction mixture by simple filtration and reused several times, making the process more economically and environmentally viable. From a practical viewpoint, the development of simple, inexpensive, widely applicable, and environmentally benign catalysts and procedures is still an active area of research.
Among various inorganic solids, spinel type mixed oxides (AB2O4) are well known for their rich catalytic action. These oxides are non-toxic, inexpensive, very stable materials with strong resistance to acids and alkalis, they have high melting points, and their nanoparticles possess relatively high surface area. These properties make them suitable for the use as solid heterogeneous catalysts for organic transformations.18 – 20 One of them, copper chromite (CuCr2O4) has been used extensively as a heterogeneous catalyst in many reactions. Among the applications of CuCr2O4 as a catalyst of chemical reactions are oxidation of aniline to azoxybenzene,21 hydrogenation of 2-furfuraldehyde,22 synthesis of pyrazine,23 and the thermal decomposition of ammonium perchlorate.24 These catalytic applications prompted us to test this spinel type mixed oxide, and especially its nanoparticles, as a heterogeneous catalyst.
As a part of our continuing efforts on the development of nanoparticles of various inorganic compounds as catalysts for the synthesis of biologically active heterocyclic compounds,25 – 28 we describe in the present study CuCr2O4 nanoparticles (NP) as a highly efficient catalyst for a facile synthesis of 4,4'-(arylmethanediyl)bis(3-methyl-1H-pyrazol-5-ol) derivatives 1a–i starting directly from aldehydes, hydrazine hydrate, and ethyl acetoacetate in aqueous medium (Scheme 1). Our method showed good to high overall yields, short overall reaction times, and convenient procedure as described below.

Scheme 1
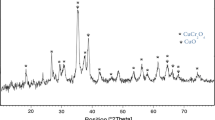
In the preliminary experiments nanocrystalline CuCr2O4 29 was characterized by IR, scanning electron microscopy (SEM), and X-ray diffraction (XRD) analysis. Phase investigation of the crystallized product was performed by XRD, and the powder diffraction pattern of CuCr2O4 nanoparticles is presented in Figure 1. All of the observed diffraction peaks are indexed by the spinel phase structure of CuCr2O4 (JCPDS No. 88-0110) revealing a high phase purity of the product. The average crystallite size was estimated by applying
Debye–Scherrer equation (D = Kλ/βcosθ), where β, the full-width at half-maximum or half-width, is in radians and θ is the position of the maximum of the diffraction peak, K is the so-called shape factor, which usually takes a value of about 0.9, and λ is the X-ray wavelength (1.5406 Å for CuKα). An average size of around 20–30 nm was obtained for these nanoparticles.
The SEM micrograph provides more accurate information on the particle size and morphology of the CuCr2O4 nanoparticles (Fig. 2). The SEM image shows that the nanoparticles have a uniform size and spherical shape. In the FTIR spectra (Fig. 3), the absorption bands at 615, 514 cm–1 were assigned to Cr2O4 2– group. In turn, the bands at 911 and 999 cm–1 refer to the Cr–O bond of the chromate group.30
In the following, we investigated the activity of CuCr2O4 NP as a heterogeneous catalyst in one-pot synthesis of 4,4'-(arylmethanediyl)bis(3-methyl-1H-pyrazol-5-ols) 1a–i in a pseudo-five-component reaction of arylaldehydes, 2 equiv of hydrazine hydrate, and 2 equiv of ethyl acetoacetate. The results are summarized in Table 1 (entries 8–11), together with literature data added for comparison (entries 1 and 3). For this purpose, a simple model reaction using benzaldehyde was carried out in order to establish the feasibility of the synthetic strategy and optimize the reaction conditions. The best product yield with respect to catalyst load was obtained using 6 mol % of CuCr2O4 NP in water (Table 1, entry 10). When the reaction was carried out in the absence of the catalyst, the product yields were good, however a longer reaction time prompted to use a catalyst in this reaction.
Also, it is worth noting that good results were obtained in EtOH (Table 1, entry 2), but to comply with the principles of green chemistry water was chosen as the optimal solvent. Notably, conventional and commercial catalysts (Table 1, entries 4−6) also showed poor activity in this reaction thus demonstrating the advantages of the spinel nanoparticle catalyst. The higher activity of the spinel NP can be attributed to its better accessibility for the substrate molecules owing to their greater surface/volume ratio compared to that of the commercial catalyst which consists of particles with irregular shapes and larger sizes. Furthermore, to elucidate the role of the NP size effect, the CuCr2O4 spinel nanoparticle catalyst was replaced by a CuCr2O4 catalyst with larger particle size at the optimum reaction conditions (Table 1, entry 7). In this experiment, the yield of the product was very modest even with a prolonged reaction time, indicating that the particle size has a considerable influence on the catalyst reactivity.
Having established optimal reaction conditions for the catalytic synthesis on the example of compound 1a, the generality and scope of this protocol was further investigated with a variety of arylaldehydes under the same conditions, and the results are displayed in Table 2. The results obtained using variously substituted aldehydes indicated that a variety of aromatic aldehydes both with electron-donating and electron-withdrawing substituents reacted efficiently to afford the products 1a–i in good to excellent yields. Moreover, a pseudo-nine-component condensation of terephthalaldehyde, hydrazine hydrate, and ethyl acetoacetate in the presence of CuCr2O4 NP under the same conditions afforded 4,4',4'',4'''-[benzene-1,4-diylbis(methanetriyl)]tetrakis-(3-methyl-1H-pyrazol-5-ol) (2) (Scheme 2).

Scheme 2
The structures of the products 1a–i, 2 were fully established on the basis of their 1H, 13C NMR, and FTIR spectra. It is worth mentioning that the 1H NMR spectra showed a signal at 11.20–11.58 ppm assigned to NH group and a signal at 3.40–3.70 ppm assigned to OH group. The latter showed exchange with the signal of water contained in DMSO-d6. This observation differs from that by Soleimani and co-workers [8] where a signal at 3.50–5.50 ppm, assigned to OH and NH groups, was reported.
Based on the above results, a plausible mechanism for the formation of products 1 is proposed in Scheme 3. In the first step, condensation of hydrazine with ethyl acetoacetate gives pyrazolone A in the presence of a catalytic amount of CuCr2O4 NP. In the second step, aldehyde is condensed with pyrazolone A to give arylidene intermediate B. Under the described reaction conditions, intermediate B reacts with another molecule of pyrazolone A to afford the final product 1.

Scheme 3
The recyclability of CuCr2O4 NP was further investigated because the possibility of a repeated use of a heterogeneous catalyst is one of the most important issues for practical applications. After the separation from reaction mixture by filtration, the catalyst had been reused in model reaction for five reaction cycles. It was found that the product yields decreased to a small extent on each reuse (89, 89, 88, 87, and 87% for runs 1–5, respectively).
In summary, we have proposed a facile approach to the synthesis of 4,4'-(arylmethanediyl)bis(3-methyl-1H-pyrazol-5-ol) derivatives in the presence of CuCr2O4 nanoparticles which exhibited excellent catalytic efficiency in aqueous medium. The catalytic activity of the catalyst remains unaltered after five consecutive cycles. Advantages of the presented methodology are efficiency, broad scope, operational simplicity, high yields, and mild reaction conditions.
Experimental
IR spectra were recorded on a FT-IR Magna 550 apparatus in KBr pellets. 1H, 13C NMR spectra were recorded on Bruker Avance spectrometers (400 and 100 MHz, respectively) in DMSO-d6 with TMS as internal standard. Electronionization mass spectra were recorded on a JEOL D-30 instrument at an ionization potential of 70 eV. Elemental analyses were performed on a Carlo ERBA Model EA1108 analyzer. Melting points were determined on an Electrothermal IA9200 apparatus and were not corrected. All the chemicals reagents used in our experiments were of analytical grade and were used without further purification. Powder X-ray diffraction experiment was carried out on a Philips X'pert diffractometer with monochromatized CuKα radiation (λ 1.5406 Å). Microscopic morphology of products was visualized by MIRA 3 TESCAN scanning electron microscope.
CuCr 2 O 4 nanoparticles were prepared according to the procedure reported by Edrissi et al. 29 CuCr2O4 has been produced by coprecipitation method. Cu(NO3)2·6H2O (1.4 g, 0.005 mol,) and Cr(NO3)3·9H2O (4.0 g 0.010 mol) were dissolved in distilled water (50 ml). The mixed solution was subsequently added to 100 ml of distilled water, containing 5% glycerol as capping agent, under stirring. 1.5 M aqueous solution of precipitating agent (NaOAc) was added dropwise until the pH value of solution was adjusted to 10. During the mixing procedure, the temperature of solution was maintained about 60°C. Then the temperature was further increased to 80°C at which the precipitation occurred. The fine precipitates were centrifuged and subsequently washed with distilled water and ethanol several times and then dried in an oven at 60°C for 2 h. Finally, after calcination at 600°C for 5 h CuCr2O4, nanoparticles were obtained. Yield 1.0 g (86%).
Synthesis of 4,4'-(arylmethanediyl)bis(3-methyl-1 H pyrazol-5-ol) derivatives 1a–i (General Method). A solution of hydrazine hydrate (100%, 0.10 ml, 2 mmol), ethyl acetoacetate (0.26 ml, 2 mmol), and CuCr2O4 (0.01 g, 6 mol %) in water (10 ml) was stirred at room temperature for 15 min. Then the appropriate aldehyde (1 mmol) was added and the mixture was stirred at 50°C for the time indicated in Table 1. After completion of the reaction, as indicated by TLC on silica gel, eluent hexane–AcOEt, 7:3, the reaction mixture was allowed to cool to room temperature. The precipitated solid was isolated by filtration. The product was dissolved in hot MeOH, and the catalyst was filtered off. After cooling of the filtrate, the product precipitated. The precipitate was washed with H2O–EtOH, 1:1, to give the product with sufficient purity.
4,4'-[(2-Chlorophenyl)methanediyl]bis(3-methyl-1 H pyrazol-5-ol) (1g). Pale-orange powder. IR spectrum, ν, cm–1: 3409, 2926, 1605, 1534, 1466, 753. 1H NMR spectrum, δ, ppm: 1.92 (6H, s, 2CH3); 3.34 (2H, br. s, 2OH); 5.06 (1H, s, CH); 7.19–7.52 (4H, m, H Ar); 11.23 (2H, br. s, 2NH). 13C NMR spectrum, δ, ppm: 161.1; 141.1; 139.3; 132.7; 131.0; 129.5; 127.9; 126.8; 102.8; 31.7 (CH); 10.9 (2CH3). Mass spectrum, m/z (I rel, %): 320 [M(37Cl)]+ (32), 318 [M(35Cl)]+ (100), 224 (15), 196 (40), 180 (18), 126 (12). Found, %: C 56.59; H 4.70; N 17.62. C15H15ClN4O2. Calculated, %: C 56.52; H 4.74; N 17.58.
4,4'-[(3-Methylphenyl)methanediyl]bis(3-methyl-1 H pyrazol-5-ol) (1h). Pale-orange powder. IR spectrum, ν, cm–1: 3350, 2922, 1602, 1455. 1H NMR spectrum, δ, ppm: 2.05 (6H, s, 2CH3); 2.18 (3H, s, CH3); 2.75 (2H, br. s, 2OH); 4.75 (1H, s, CH); 6.71–7.06 (4H, m, H Ar); 11.58 (2H, br. s, 2NH). 13C NMR spectrum, δ, ppm: 161.5; 143.6; 140.4; 137.0; 128.4; 128.1; 126.6; 125.0; 104.8; 33.0 (CH); 21.6 (CH3); 10.8 (2CH3). Mass spectrum, m/z (I rel, %): 299 [M+1]+ (10), 241 (7), 203 (10), 150 (100), 115 (20). Found: C 64.52; H 6.10; N 18.73. C16H18N4O2 Calculated, %: C 64.41; H 6.08; N 18.78.
4,4'-[(2-Methylphenyl)methanediyl]bis(3-methyl-1 H pyrazol-5-ol) (1i). Pale-orange powder. IR spectrum, ν, cm–1: 2923, 1602, 1529, 1484, 749. 1H NMR spectrum, δ, ppm: 1.79 (6H, s, 2CH3); 2.11 (3H, s, CH3); 3.35 (2H, br. s, 2OH); 4.93 (1H, s, CH); 7.04–7.21 (4H, m, H Ar); 10.73 (2H, br. s, 2NH). 13C NMR spectrum, δ, ppm: 160.8; 141.8; 138.3; 135.8; 130.3; 128.7; 126.0; 125.5; 103.1; 31.4 (CH); 19.6 (CH3), 10.8 (2CH3). Mass spectrum, m/z (I rel, %): 299 [M+1]+ (6), 241 (3), 205 (9), 150 (100), 115 (25). Found: C 64.50; H 6.11; N 18.75. C16H18N4O2 Calculated, %: C 64.41; H 6.08; N 18.78.
4,4',4'',4'''-[Benzene-1,4-diylbis(methanetriyl)]tetrakis-(3-methyl-1 H -pyrazol-5-ol) (2) was synthesized as described above, except that the molar ratio of hydrazine hydrate, ethyl acetoacetate, and terephthalaldehyde was 4:4:1, respectively. Orange powder. IR spectrum, ν, cm–1: 3351, 3112, 1600, 1471, 833. 1H NMR spectrum, δ, ppm: 2.04 (12H, s, 4CH3); 3.94 (4H, br. s, 4OH); 4.70 (2H, s, 2CH); 6.93 (4H, s, H Ar); 11.61 (4H, br. s, 4NH). 13C NMR spectrum, δ, ppm: 161.5; 140.9; 140.4; 127.3; 104.8; 32.8 (2CH); 10.8 (4CH3). Mass spectrum, m/z (I rel, %): 490 [M]+ (2), 481 (2), 467 (6), 455 (58), 438 (26), 425 (26), 411 (11), 395 (27), 382 (100), 356 (82), 340 (96). Found: C 58.72; H 5.36; N 22.80. C24H26N8O4 Calculated, %: C 58.77; H 5.34; N 22.84.
References
Brogden, R. N. Drugs 1986, 32, 60.
Sugiura, S.; Ohno, S.; Ohtani, O.; Izumi, K.; Kitamikado, T.; Asai, H.; Kato, K.; Hori, M.; Fujimura, H. J. Med. Chem. 1977, 20, 80.
Bailey, D. M.; Hansen, P. E.; Hlavac, A. G.; Baizman, E. R.; Pearl, J.; Defelicem, A. F.; Feigenson, M. E. J. Med. Chem. 1985, 28, 256.
Hamama, W. S. Synth. Commun. 2001, 31, 1335.
Sivaprasad, G.; Perumal, P. T.; Prabavathy, V. R.; Mathivanan, N. Bioorg. Med. Chem. Lett. 2006, 16, 6302.
Elinson, M. N.; Dorofeev, A. S.; Nasybullin, R. F.; Nikishin, G. I. Synthesis 2008, 1933.
Niknam, K.; Saberi, D.; Sadegheyan, M.; Deris, A. Tetrahedron Lett. 2010, 51, 692.
Soleimani, E.; Ghorbani, S.; Taran, M.; Sarvary, A. C. R. Chim. 2012, 15, 955.
Sujatha, K.; Shanthi, G.; Selvam, N. P.; Manoharan, S.; Perumal, P. T.; Rajendran, M. Bioorg. Med. Chem. Lett. 2009, 19, 4501.
Phatangare, K. R.; Padalkar, V. S.; Gupta, V. D.; Patil, V. S.; Umape, P. G.; Sekar, N. Synth. Commun. 2012, 42, 1349.
Tayebi, S.; Baghernejad, M.; Saberi, D.; Niknam, K. Chin. J. Catal. 2011, 32, 1477.
Niknam, K.; Sadeghi Habibabad, M.; Deris, A.; Aeinjamshid, N. Monatsh. Chem. 2013, 144, 987.
Wang, W.; Wang, S. X.; Qin, X. Y.; Li, J. T. Synth. Commun. 2005, 35, 1263.
Khazaei, A.; Zolfigol, M. A.; Moosavi-Zare, A. R.; Asgari, Z.; Shekouhy, M.; Zare, A.; Hasaninejad, A. RSC Adv. 2012, 2, 8010.
Karimi-Jaberi, Z.; Pooladian, B.; Moradi, M.; Ghasemi, E. Chin. J. Catal. 2012, 33, 1945.
Sartori, G.; Ballini, R.; Bigi, F.; Bosica, G.; Maggi, R.; Righi, P. Chem. Rev. 2004, 104, 199.
Sakakura, A.; Kawajiri, K.; Ohkubo, T.; Kosugi, Y.; Ishihara, K. J. Am. Chem. Soc. 2007, 129, 14775.
Adkins, H.; Connor, R. J. Am. Chem. Soc. 1931, 53, 1091.
Kawamoto, A. M.; Pardini, L. C.; Rezende, L. C. Aerosp. Sci. Technol. 2004, 8, 591.
Sathiskumar, P. S.; Thomas, C. R.; Madras, G. Ind. Eng. Chem. Res. 2012, 51, 10108.
Acharyya, S. S.; Ghosh, S.; Bal, R. ACS Sustainable Chem. Eng. 2014, 2, 584.
Liu, D.; Zemlyanov, D.; Wu, T.; Lobo-Lapidus, R. J.; Dumesic, J. A.; Miller, J. T.; Marshall, C. L. J. Catal. 2013, 299, 336.
Madhavi Latha, B.; Sadasivam, V.; Sivasankar, B. Catal. Commun. 2007, 8, 1070.
Ping, L.; Zhen, Z.; Hongbin, X.; Zhang, Y. Thermochim. Acta 2012, 544, 71.
Safaei-Ghomi, J.; Zahedi, S. Monatsh. Chem. 2013, 144, 687.
Safaei-Ghomi, J.; Ghasemzadeh, M. A.; Zahedi, S. J. Serb. Chem. Soc. 2013, 78, 769.
Safaei-Ghomi, J.; Ghasemzadeh, M. A.; Zahedi, S. J. Mex. Chem. Soc. 2013, 57, 1.
Safaei-Ghomi, J.; Zahedi, S.; Ghasemzadeh, M. A. Monatsh. Chem. 2014, 145, 1191.
Jia, X.-D.; Duan, H.-F.; Lin, Y.-J.; Cao, J.-G.; Liang, D.-P.; Wu, M.-C. Chem. Res. Chin. Univ. 2012, 28, 999.
Edrissi, M.; Hosseini, S. A.; Soleymani, M. Micro Nano Lett. 2011, 6, 836.
The authors are grateful to University of Kashan for supporting this work by Grant No. 159196/XXI.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(1), 34–38
Rights and permissions
About this article
Cite this article
Safaei-Ghomi, J., Khojastehbakht-Koopaei, B. & Zahedi, S. Copper chromite nanoparticles as an efficient and recyclable catalyst for facile synthesis of 4,4'-(arylmethanediyl)bis(3-methyl-1H-pyrazol-5-ol) derivatives. Chem Heterocycl Comp 51, 34–38 (2015). https://doi.org/10.1007/s10593-015-1656-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1656-y