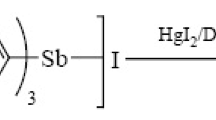Abstract
In the present work, an efficient and convenient one-pot multi-component reaction is reported for the synthesis of highly diversified piperidines by tandem condensation reaction of aromatic aldehydes, anilines, and β-keto esters in the presence of a catalytic amount (15 mol%) of SbI3 in EtOH at 50 °C. The advantages of this protocol are that it is easy to work up and inexpensive, the catalyst and solvent are readily available, and it results in a favorable yield.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The synthesis and chemistry of heterocyclic compounds are appealing areas of research in organic chemistry, in particular because a significant number of natural compounds contain a heterocyclic portion. The application of heterocycles in biology, pharmacology, material sciences, and medicine, as well as agriculture is very well-known [1]. Additionally, the majority of antibiotics and vitamins include heterocyclic rings in their structure [2].
In recent years multi-component reactions (MCRs), defined as reactions in which more than two reactants are combined to give a product that comprises a majority of the involved atoms, have played an outstanding role in synthetic organic chemistry. These reactions are powerful tools for the construction of novel and complex molecular structures with a minimum number of synthetic steps. Distinguishing features of MCRs include high efficiency, simplicity, time-saving processes, and atom economy [3–6].
Piperidines and their analogues are an essential building block for numerous natural products, and for pharmaceutically relevant molecules and organic fine chemicals [7–14]. They also behave as pharmaceutical factors and exhibit diverse biological features [15] such as anti-hypertensive [16], anti-bacterial [17], anti-HIV [18], anti-malarial [19], anti-inflammatory [20] and diagnostic properties [19]. Additionally, piperidines and their derivatives are used as therapeutic agents in the treatment of some diseases such as cancer metastasis [21, 22], diabetes [23, 24], and influenza [25–27]. The applicability of piperidine as a biologically active moiety is represented in Fig. 1.
Due to excessive use of piperidines in various areas over the years, diverse protocols have been reported for the synthesis of substituted piperidines in the presence of a plethora of catalysts such as l-proline [19], InCl3 [14–28], bromodimetheyl sulfonium bromide (BDMSB) [29], ZrOCl2·8H2O [30], VCl3 [31], Cerium Ammonium Nitrate (CAN) [32], BF3. SiO2 [33], Mg(HSO4)2 [34], Zn(HSO4)2 [35], PEG-embedded KBr3 [36], NiCl2.6H2O [37], Bi(NO3)3.5H2O [38], and tetrabutylammonium tribromide (TBATB) [39]. However, some of these procedures suffer from some drawbacks such as unsatisfactory yields, long reaction times, expensive reagents or catalysts, and harsh reaction conditions. Therefore, there is a need to provide a new, effective, and convenient approach in order to eliminate weaknesses and disadvantages of the previous works.
Antimony triiodide is a ruby-red solid compound. Despite the numerous applications of metal halides as catalyst in organic transformations [40, 41], there has been no report on the use of SbI3 as a catalyst for organic reactions. Following our previous works on the synthesis of piperidines [42–48], herein we report for the first time a SbI3-catalyzed synthesis of highly functionalized piperidines via an easy and simple five-component procedure resulting in corresponding products with favorable yields.
Experimental
General
All reagents were purchased from Merck (Darmstadt, Germany) and Acros (Geel, Belgium), and were used as received without further purification. Melting points were measured on an electrothermal 9100 apparatus. The 1H NMR spectra were recorded on a Bruker DRX-300 Avance instrument with CDCl3 as solvent. The mass spectra (MS) were recorded on a shimadzu GCeMS-QP5050 mass spectrometer, operating at an ionization potential of 70 eV.
General procedure for synthesis of highly functionalized piperidine 4a–4o
To a magnetically stirred solution of aromatic amine (2 mmol) in ethanol (5 mL), β-ketoester (1 mmol) and SbI3 (0.015 mmol) were added and allowed to react for 20 min at ambient temperature. Next, the aromatic aldehyde (2 mmol) was added and the temperature was raised to 50 °C. After completion of the reaction, determined through thin-layer chromatography (TLC) monitoring, the reaction mixture was cooled to room temperature. Then the precipitate was filtered off and recrystallized with hot ethanol to give the pure product. Physical and spectral data for selected products are represented as follows.
Ethyl 1,2,6-triphenyl-4-(phenylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate (4a)
White solid; m.p. = 167–172; 1H NMR (300 MHz, CDCl3): \(\delta\) = 1.36 (3H, t, J = 7.2 Hz), 2.68 (1H, dd, J = 20.7, 2.1 Hz), 2.80 (1H, dd, J = 15.2, 5.7 Hz), 4.16–4.27 (1H, m), 4.30–4.38 (1H, m), 5.05 (1H, d, J = 3.3 Hz), 6.17 (2H, t, J = 5.1), 6.34 (1H, s), 6.40 (2H, d, J = 8.4 Hz), 6.47 (1H, t, J = 7.5 Hz), 6.91–7.02 (5H, m), 7.04–7.29 (10H, m), 10.17 (1H, D2O-exchanged). MS (EI, 70 eV): m/z (%) M + 474(45 %), 395(100 %), 289(62), 179(42), 103(38), 76(91), 28(91).
Methyl 1,2,6-triphenyl-4-(phenylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate (4b)
White solid; m.p. = 155–163; 1H NMR (400 MHz, CDCl3): \(\delta\) = 3.86 (3H, s), 5.06 (1H, d, J = 3.9 Hz), 6.20 (2H, dd, J = 9, 1.5 Hz), 6.38 (1H, s), 6.45 (2H, d, J = 8.4 Hz), 6.53 (1H, t, J = 7.2 Hz), 6.96–7.26 (17H, m), 10.18 (1H, D2O-exchanged) ppm. MS (EI, 70 eV): m/z (%) M+ 460 (87 %), 380(100), 178(77), 103(59). 76(89), 43(47), 28(88).
Ethyl 1-(4-bromophenyl)-4-((4-bromophenyl)amino)-2,6-diphenyl-1,2,5,6-tetrahydropyridine-3-carboxylate (4c)
White solid; m.p. = 198–202 °C; 1H MNR (300 MHz, CDCl3): \(\delta\) = 1.50 (3H, t, J = 6.8 Hz), 2.74 (1H, d, J = 15.2 Hz), 2.89 (1H, dd, J = 15.2, 5.6 Hz), 4.35–4.40 (1H, m), 4.46–4.52 (1H, m), 5.13 (1H, d, J = 4 Hz), 6.14 (2H, d, J = 8 Hz), 6.41 (1H, s), 6.42 (2H, d, J = 8 Hz), 7.14–7.34 (14H, m), 10.26 (1H, D2O-exchanged) ppm. MS (EI, 70 eV): m/z (%) M+ 631(89 %), 555(100), 295(75), 216(65), 154(64), 128(45), 77(48), 29(96).
Methyl 2,6-bis(4-bromophenyl)-1-(4-chlorophenyl)-4-((4-chlorophenyl)amino)-1,2,5,6-tetra hydropyridine-3-carboxylate (4 h)
White solid; m.p. = 155–157 °C; 1H MNR (300 MHz, CDCl3): \(\delta\) = 2.63 (1H, dd, J = 15.3, 5.7 Hz), 2.75 (1H, dd, J = 15.3, 5.7 Hz), 3.90 (3H, s), 5.01 (1H, d, J = 3.3 Hz), 6.21 (2H, d, J = 8.7 Hz), 6.30 (2H, d, J = 9.0 Hz), 6.90–7.20 (12H, m), 10.12 (1H, D2O-exchanged). MS (EI, 70 eV): m/z (%) M+ 684(37 %), 531(100), 291(89), 214(20), 110(88), 74(42), 28(99).
Methyl 2,6-bis(4-chlorophenyl)-1-phenyl-4-(phenylamino)-1,2,5,6-tetrahydropyridinr-3-carboxyate (4 k)
White solid; m.p. = 183–187 °C; 1H NMR (300 MHz, CDCl3): \(\delta\) = 2.66 (1H, dd, J = 15.3, 2.7 Hz), 2.75 (1H, dd, J = 15.0, 5.4 Hz), 3.85 (3H, s), 5.02 (1H, d, J = 2.4 Hz), 6.28 (1H, s), 6.32 (2H, dd, J = 7.2, 1.8 Hz), 6.38 (2H, d, J = 8.1 Hz), 6.57 (2H, t, J = 7.2 Hz), 6.98–7.12 (6H, m), 7.15–718 (7H, m), 10.18 (1H, D2O-exchanged). MS (EI, 70 eV): m/z (%) M+ 528(80 %), 416(100), 276(49), 251(89), 103(48), 76(93), 28(90).
Methyl 1-(4-bromophenyl)-4-((4-bromophenyl)amino)-2,6-bis(4-methoxyphenyl)-1,2,5,6-tetrahydropyridine-3-carboxylate (4 l)
White solid; m.p. = 170–174 °C; 1H NMR (300 MHz, CDCl3): \(\delta =\) 2.72 (1H, dd, J = 16.2, 2.1 Hz), 2.86 (1H, dd, J = 20.4, 5.4 Hz), 3.81 (3H, s), 3.83 (3H, s), 3.96 (3H, s), 5.07 (1H, d, J = 3.3 Hz), 6.22 (2H, d, J = 8.4 Hz), 6.31 (1H, s), 6.41 (2H, d, J = 9.0 Hz), 6.85 (4H, dd, 8.7, 3.6 Hz), 7.07 (2H, d, J = 8.4 Hz), 7.14–7.20 (4H, m), 7.24–7.29 (2H, d, J = 8.7 Hz), 10.24 (1H, D2O-exchanged) ppm. MS (EI, 70 eV): m/z (%) M+ 676(7 %), 525(20), 384(93), 285(100), 216(20), 133(21), 75(34).
Results and discussion
In the initial experiment, the reaction of benzaldehyde (2 mmol), aniline (2 mmol), and ethyl acetoacetate for the synthesis of corresponding piperidine 4a was performed in the presence of various Brönsted or Lewis acid as catalysts in EtOH at room temperature (Scheme 1) and results are listed in Table 1.
Obviously from Table 1, among the numerous Lewis/Brönsted acids tried in this reaction, only SbI3 afforded a product with a satisfactory yield. With SbI3 as a known suitable catalyst, the model reaction was then conducted to optimize other conditions, and the obtained results are summarized in Table 2.
Optimization for the amount of catalyst was carried out and showed that increasing the SbI3 loading up to 15 mol% significantly enhanced the reaction yield (Table 2, Entries 1–4). Further increase in the amount of catalyst did not exhibit any effect on efficiency (Table 2, Entry 5). In the next step, reaction temperature changes were fulfilled in order to find the best conditions, and our resulting observations identified 50 °C as the preferred temperature for the subsequent reactions. An increase in the temperature to 60 °C did not demonstrate a remarkable improvement in the yield, and once the reaction reached 70 °C, it actually resulted in a loss of efficiency (Table 2, Entries 6–9). On the other hand, replacement of ethanol with other solvents such as methanol, EtOAc, CH3CN, and DMF decreased the yield. Utilization of H2O or a 1:1 mixture of EtOH/H2O also lowered the efficiency of the model reaction.
With the completion of the optimization process, in order to confirm the generality and versatility of our protocol, several derivatives of highly-substituted piperidine were successfully prepared from the reaction of substituted benzaldehydes, substituted anilines, and ethyl/methyl acetoacetate catalyzed by SbI3 , the results of which are presented in Table 3.
Evidently, numerous aromatic aldehyde and amine derivatives containing electron-releasing and electron-withdrawing groups pushed the reactions forward to afford corresponding products with satisfactory yields. Additionally, both methyl and ethyl acetoacetate were found to afford piperidine derivatives successfully. Further, the consistency of the observed melting point with literature reports affirmed our synthesis of piperidine derivatives. Based on the provided data, the efficiency of our presented protocol and specifically SbI3 was such that all derivatives of piperidines were obtained with high yield without any dependency on reactants (Scheme 2).
An acceptable mechanism for the formation of piperidine is depicted in Scheme 3.
Based on a mechanism that has previously been presented in the literature [50], as a Lewis acid SbI3 first accelerates the formation of enaminone 6 and imine 7 intermediates via coordination with carbonyl groups. Then, Knoevenagel condensation of enaminone 5 with aldehyde leads to obtaining intermediate 6a, which is in equilibrium with reactive form 6b. Eventually, an aza-Diels–Alder reaction of 6b with the prepared imine in step 1 gives the intended piperidine.
Conclusion
In brief, a one-pot five-component reaction has been developed for the synthesis of highly substituted piperidines via a reaction of aromatic aldehydes, aromatic amines, and β-keto esters in EtOH at 50 °C. In this approach, for the first time, SbI3 was exhibited as a beneficial catalyst and facilitated the formation of substituted piperidines. The above protocol also offered several advantages such as mild reaction conditions, simple purification steps, an inexpensive catalyst, and the absence of toxic solvents, as well as its generality for various aromatic aldehydes, amines, and β-keto esters.
References
R. Dua, S. Shrivastava, S.K. Sonwane, S.K. Srivastava, Adv. Biol. Res. 5, 120 (2011)
A. Rahmati, N. Pashmforosh, J. Iranian Chem. Soc. 12, 993 (2014)
A. Domling, ACS Chem. Rev. 106, 17 (2006)
F. Lieby-Muller, C. Simon, T. Constantieux, J. Rodriguez, QSAR Comb. Sci. 25, 432 (2006)
G.V.M. Sharma, K.L. Reddy, P.S. Lakshmi, P.R. Krishn, Synthesis 1, 55 (2006)
L.M. Wang, J. Sheng, L. Zhang, J.W. Han, Z.Y. Fan, H. Tian, C.T. Qian, Tetrahedron 61, 1539 (2005)
J.W. Daly, T.F. Spande, H.M. Garraffo, J. Nat. Prod. 68, 1556 (2005)
P.S. Watson, B. Jiang, B. Scott, Org. Lett. 2, 3679 (2000)
P.M. Dewick, Medicinal natural products a biosynthetic approach, 2nd edn. (Wiley, New York City, 2002), p. 307
C. Viegas, J.V.C. Bolzani, M. Furlan, E.J. Barreiro, M.C.M. Young, D. Tomazela, M.N. Eberlin, J. Nat. Prod. 67, 908 (2004)
S. Kobayashi, M. Ueno, R. Suzuki, H. Ishitani, H.S. Kim, Y. Wataya, J. Org. Chem. 64, 6833 (1999)
M.C. Desai, S.L. Lefkowitz, P.F. Thadeio, K.P. Longo, R.M. Srider, J. Med. Chem. 35, 4911 (1992)
J. Breman, A. Egan, G. Keusch, Am. J. Trop. Med. Hyg. 64, iv (2001)
P.A. Clark, A.V. Zaytzev, A.C. Whitwood, Tetrahedron Lett. 48, 5209 (2007)
S. Petit, J.P. Nallet, M. Guillard, J. Dreux, R. Chermat, M. Poncelet, C. Bulach, P. Simon, C. Fontaine, M. Barthelmebs, J.L. Imbs. Eur. J. Med. Chem. 26, 19 (1991)
H. Bin, A.M. Crider, J.P. Stables, Eur. J. Med. Chem. 36, 265 (2001)
Y. Zhou, V.E. Gregor, B.K. Ayida, G.C. Winters, Z. Sun, D. Murphy, G. Haley, D. Bailey, J.M. Froelich, S. Fish, S.E. Webber, T. Hermann, D. Wall, Bioorg. Med. Chem. Lett. 17, 625 (2009)
S. Imamura, Y. Nishikawa, T. Ichikawa, T. Hattori, Y. Matsushita, S. Hashiguchi, N. Kanzaki, Y. Iizawa, M. Baba, Y. Sugihara, Bioorg. Med. Chem. 13, 397 (2009)
M. Misra, S.K. Pandey, V.P. Pandey, J. Pandey, R. Tripathi, R.P. Tripathi, Bioorg. Med. Chem. 17, 625 (2009)
R. Gitto, L. De Luca, S. Ferro, F. Occhiuto, S. Samperi, G. De Sarro, E. Russo, L. Ciranna, L. Costa, A. Chimirri, Chem. Med. Chem. 3, 1539 (2008)
Y. Nishimura, T. Satoh, H. Adachi, S. Kondo, T. Takeuchi, M. Azetaka, H. Fukuyasu, Y. Iizuka, J. Med. Chem. 40, 2626 (1997)
N. Zitzmann, A.S. Mehta, S. Carrouee, T.D. Butters, F.M. Platt, J. Mc Cauley, B.S. Blumberg, R.A. Dwek, T.M. Block, Proc. Nat. Acad. Sci. (PNAS) USA 96, 11878 (1999)
G.S. Jacob, Curr. Opin. Struct. Biol. 5, 605 (1995)
J.L. Treadway, P. Mendys, D.J. Hoover, Opin. Invest. Drugs 10, 439 (2001)
C.U. Kim, W. Lew, M.A. Williams, H. Liu, L. Zhang, S. Swaminathan, N. Bischofberger, M.S. Chen, D.B. Mendel, C.Y. Tai, W.G. Laver, R.C. Stevens, J. Am. Chem. Soc. 119, 681 (1997)
M. Von Itzstein, W.Y. Wu, G.B. Kok, M.S. Pegg, J.C. Dyason, B. Jin, T.V. Phan, M.L. Smythe, H.F. White, S.W. Oliver, P.M. Colman, J.N. Varghese, D.M. Ryan, J.M. Woods, R.C. Bethell, V.J. Hothman, J.M. Camreon, C.R. Penn, Nature 369, 418 (1993)
P. Chand, P.L. Kotian, A. Dehghani, Y. El-Kattan, T.H. Lin, T.L. Hutchison, Y. Sudhakar, Babu, S. Bantia, A.J. Elliott. J. Montgomery J. Med. Chem. 44, 4379 (2009)
P.A. Clark, A.V. Zaytzev, A.C. Whitwood, Synthesis 21, 3530 (2008)
A.T. Khan, T. Parvin, L.H. Choudhury, J. Org. Chem. 73, 8398 (2008)
S. Mishra, G. Rina, Tetrahedron Lett. 52, 2857 (2011)
S. Pal, L.H. Choudhury, T. Parvin, Mol. Divers. 16, 129 (2012)
H.J. Wang, L.P. Mo, Z.H. Zhang, ASC Comb. Sci. 13, 181 (2011)
R. Ramachandran, S. Jayanthi, Y.T. Jeong, Tetrahedron 68, 363 (2012)
M.R. Mohammad Shafiee, B.H. Najafabadi, M. Ghashang, Res. Chem. Intermed. 39, 3753 (2013)
M. Ghashang, Lett. Org. Chem. 9, 497 (2012)
S. Verma, S.L. Jain, B. Sain, Beilstein J. Org. Chem. 7, 1334 (2011)
M.R. Mohammad Shafiee, B.H. Najafabadi, M. Ghashang, J. Chem. Res. 36, 336 (2012)
G. Brahmachari, S. Das, Tetrahedron Lett. 53, 1479 (2012)
A.T. Khan, M. Lal, M.M. Khan, Tetrahedron Lett. 51, 4419 (2010)
C.S. Schwalm, M.A. Ceschi, D. Russowsky, J. Braz. Chem. Soc. 22, 623 (2011)
K. Fagnon, M. Lautens, Angew. Chem. Int. Ed. 41, 26 (2002)
S.S. Sajadikhah, N. Hazeri, M.T. Maghsoollou, S.M. Habibi-Khorassani, A.C. Willis, Res. Chem. Intermed. 40, 723 (2014)
J. Aboonajmi, M.R. Mousavi, M.T. Maghsoodlou, N. Hazeri, A. Masoumnia, Res. Chem. Intermed. 41, 1925 (2015)
S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, S.J. Shams-Najafi, Monatsh. Chem. 143, 939 (2012)
S.S. Sajadikhah, N. Hazeri, M.T. Maghsoodlou, S.M. Habibi-khorassani, A. Beigbabaei, A.C. Willis, J. Iranian Chem. Soc. 10, 863 (2013)
J. Aboonajmi, M.T. Maghsoodlou, N. Hazeri, M. Lashkari, M. Kangani, Res. Chem. Intermed. 41, 8057 (2015)
Z. Madanifar, M.T. Maghsoodlou, M. Kangani, N. Hazeri, Res. Chem. Intermed. 41, 9863 (2015)
N. Hazeri, J. Aboonajmi, A. Masoumnia, M. Safarzaei, Iranian J. Org. Chem. 4, 917 (2012)
G. Brahmachari, C.Y. Choo, P. Ambure, K. Roy, Bio. Med. Chem. 23, 4567 (2015)
B. Umamahesh, V. Sathesh, G. Ramachandran, M. Sathishkumar, K. Sathiyanarayanan, Catal. Lett. 142, 895 (2012)
Acknowledgments
The authors thank the University of Sistan and Baluchestan for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chahkamali, F.O., Faghihi, M.R. & Maghsoodlou, M.T. Introduction of antimony triiodide (SbI3) as a new and efficient catalyst for synthesis of polyfunctionalized piperidines. Res Chem Intermed 42, 8109–8117 (2016). https://doi.org/10.1007/s11164-016-2582-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2582-z