Abstract
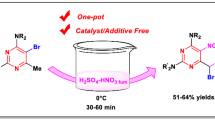
An unexpected and selective synthetic technique was devised for 3-bromo-2-arylimidazo[1,2-a]pyridine and 3-formyl-2-arylimidazo[1,2-a]pyridine derivatives synthesis, utilizing DMSO and copper(II) bromide under time-controlled settings. Initially, the imidazopyridines underwent bromination, followed by the subsequent conversion of the brominated imidazopyridines into formylated imidazopyridines. This innovative technique has various advantages over previous ones, such as sequential synthesis of brominated and formylated imidazo[1,2-a]pyridines and, for the first time, utilizing CuBr2 and DMSO as dual active agents simultaneously. This practical process can moreover produce two widely used products in the medical and pharmaceutical realm which increases its efficiency and adaptability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The heterocyclic compound is significantly important for its use in agrochemistry and modern pharmaceuticals. It is estimated that among small-molecule drugs approved by the FDA, approximately 59% contain a nitrogen heterocycle in their structure [1]. Applications for nitrogen heterocycles can be found in the realm of organic chemistry, material science, optics, and organometallic chemistry [2]. An analysis of sales of the top 25 pharmaceuticals revealed that 12 of these products were classified as small-molecule therapeutics and possessed heterocyclic moieties. These moieties typically consisted of nitrogen atoms and collectively generated an annual revenue over $50 billion USD [3]. In this regard, bicyclic systems containing a fused imidazole ring and a bridgehead nitrogen, like imidazo[1,2-a]pyrazine, imidazo[1,2-a]pyrimidine and imidazo[1,2-a]pyridine, are present in several pharmaceutical compounds, showcasing a diverse range of biological functionalities [4]. In recent years, considerable attention increased in the field of chemistry for imidazo[1,2-a]pyridine synthesis [4]. Within medicinal chemistry, demonstrated interest in the fused bicyclic imidazo[1,2-a]pyridine moiety has led to a wide range of activities including: analgesic, antibacterial, antituberculosis, antifungal, antiulcer, anti-inflammatory, antiprotozoal, antiviral, anticancer, antitumor, anticonvulsant, anthelmintic, antipyretic, and antiepileptic properties [5, 6]. Selected pharmaceutical examples include Olprinone, Zolpidem, Alpidem, and Zolimidine (Fig. 1) [7].
These major classes of heterocyclic compounds are also found in many bioactive molecules as well as natural products [8]. In organic synthesis arena, consideration has been given to conditions-based divergence in organic synthesis as a practical and effective method for discovering novel organic reactions, resulting in the production of interesting compounds from the same starting reactants by merely varying the conditions, for instance, controlling the reaction time [9, 10]. Using specific catalysts and solvents or modern alternative energy inputs, for example, could direct the course of the reaction along distinct pathways, leading to the formation of distinct scaffolds [11]. DMSO could play an imperative fulfillment in conditions-based divergence syntheses [12]. A highly important, high-polarity reaction medium in organic synthetic chemistry is dimethyl sulfoxide. Dimethyl sulfoxide is extensively deployed on account of its low toxicity, thermal stability, low price, and high boiling point [12]. Interestingly, DMSO recently emerged as an atom economical substrate in precious transformations [13]. DMSO can properly play a multiple key role as an organic solvent, an oxidant, –SMe/–CH2SMe source, a Me source, a –CN source, and a ligand [14]. Chemicals commonly applied for usage in preparation of multiple biologically active compounds are formyl group-containing organic compounds. DMSO was recently introduced as an organic synthesis formyl group source [15]. Usage of TMEDA, DMSO, DMF, and anilines as carbon sources developed multiple formylation processes, transforming the formation of carbon–carbon bonds in preparation of formyl heteroarene molecules [16]. The Duff reaction, Reimer–Tiemann reaction, Gattermann–Koch reaction, Vilsmeier–Haack, Rieche, and Friedel–Craft acylation are also powerful and privileged synthetic routes leading to formylated products [16, 17]. In addition, either aryl or alkyl organic halides are notable motifs often found in many biologically active compounds, which serve as versatile building blocks for constructing various complex molecules [18]. Tremendous attention has been given recently to C3-brominated imidazo[1,2-a]pyridines, utilized routinely as critical skeletons or precursors for necopidem, alpidem, and zolpidem [19]. In light of the significances of 3-formyl-imidazo[1,2-a]pyridines along with C3-brominated imidazo[1,2-a]pyridines as an efficient and valuable substrates, there is a tremendous amount of attention to introducing new synthetic methods for their use [20]. In past years, copper(II)acetate-catalyzed, iron(III)-catalyzed, and photocatalyst were used for the formylation of imidazo[1,2-a]pyridines [16, 21, 22] and hydrogen halide, copper-mediated, electrochemical, ultrasound, photocatalytic, transition-metal, oxidants, and various Br sources for the bromination of imidazo[1,2-a]pyridines were used [18, 19, 23,24,25,26,27,28,29,30]. During our unexpected time-controlled synthetic route, 2-arylimidazo[1,2-a]pyridines subsequently brominated and then regenerated formylated 2-arylimidazo[1,2-a]pyridines.
Results and discussion
In continuation of our research interests on heterocyclic compounds [31,32,33,34,35,36,37,38,39,40,41,42,43,44], we devised an unexpected new time-controlled method of C-3 bromination and C-3 formylation of 2-phenylimidazo[1,2-a]pyridines using copper with dual activity as a catalyst and Br source and also DMSO with dual activity as a solvent and as a reagent (Fig. 2).
Investigation commenced with a 2-phenylimidazo[1,2-a]pyridine 1a to find the best conditions for reaction. First, various solvents such as ethyl acetate, chloroform, dioxane, acetonitrile, toluene, DMSO, and DMF were investigated. Among these, DMSO was the best solvent (Table 1, entries 1–9). This reaction was investigated at different temperatures of 80, 100, 120 and 140 °C, with the highest yield found at 120 °C (Table 1, entries 10–11). Different bases such as DABCO, K2CO3, KOtBu, and Na2CO3 were investigated. Among these, the best base was DABCO (Table 1, entries 10–14). Different sources of Br were tested such as CuBr2, CuBr2/KBr, and CuBr; and the best source of Br was CuBr2 (Table 1, entries 11, 14, and 15). We proceeded with the bromination of 2-phenylimidazo[1,2-a]pyridines which were used with CuBr2 as source Br, DABCO as base, and DMSO as solvent at 120 °C for 4 h, which provided 2a with 90% yield (Table 1, entry 14). Notably, less than 2 equiv. of CuBr2 and the 4 h reaction could not be completed.
The scope of bromination of 2-phenylimidazo[1,2-a]pyridine provided with an optimized reaction condition. Brominated products 1a–g containing, donating, and withdrawing electron substituents on both the aryl and the heteroaryl sides generated good to satisfactory yields (68–90%) (Scheme 1). This method worked appropriately yielding 3-bromo-2-phenylimidazo[1,2-a]pyrimidine 1h in 81% (Scheme 1).
Remarkably, a new spot on the thin layer chromatography (TLC) plate was observed when the reaction time was inadvertently extended. Subsequent inquiry revealed that the formyl-containing product was synthesized through a serendipitous time-dependent domino reaction. The preferred mode in proceeding with the formylation of 2-phenylimidazo[1,2-a]pyridines was used with CuBr2 as a catalyst, DABCO as a base, and DMSO as a reagent at 120 °C for 24 h, which provided 3a with 76% yield (Scheme 2).
Various substituted 2-arylimidazo[1,2-a]pyridines 1 were compatible in this formylation reaction and delivered the corresponding products in 57–87% yields (Scheme 2). In general, the 2-phenylimidazo[1,2-a]pyridines with electron-donating (Me and Ph) or electron-withdrawing (Cl and Br) groups in benzene moiety all could partake this transformation to give the corresponding 2-phenylimidazo[1,2-a]pyridine-3-carbaldehydes in good to high yields (3a-e).
It is noteworthy that the steric effect on the reaction was also apparent. Large heteroaryl instead of benzene ring afforded moderate yields in 62 and 64%, respectively, for 3g and 3h. In addition, 2-phenylimidazo[1,2-a]pyrimidine was also tested, providing products 3i in 57% yields.
As to making crystal clear the reaction mechanism, Fig. 3 proposes a plausible mechanism of these bromination and formylation processes. First, starting material 1 through intermediate A with losing HBr generates intermediate B. Then, intermediate B by treatment of extra CuBr2 formed intermediate C and then reductive elimination of intermediate C yields the product 2. Methyl radical which derived from DMSO under Cu(II) and air reacts with radical intermediate D obtained from 2 under Cu(II) and air to form intermediate E. Single electron transfer oxidation of intermediate E generates intermediate radical F followed by coupling with O2, and the elimination of H2O is converted 3 [16, 45]. Notably, this reaction under Ar gas did not work and also using O2 instead of air could not give better yield. To establish a plausible mechanism, prepared 2a was exposed into the optimized reaction condition and worked well to yield 3a in high yield.
Conclusions
The aforementioned processes were performed via C(sp2)-H functionalization procedure by employing simultaneously CuBr2 and DMSO as dual active agents for the first time through a time-controlled selective synthetic route. Initially, the imidazo[1,2-a]pyridines underwent bromination, followed by the subsequent conversion of the brominated imidazo[1,2-a]pyridines into formylated imidazo[1,2-a]pyridines. Notably, bromination and formylation of imidazo[1,2-a]pyridines have been separately reported but there is no report on subsequent conversion of brominated imidazo[1,2-a]pyridines to the formylated ones. The identification of the generated compounds was conducted using analytical techniques including FT-IR, 1H-NMR, 13C-NMR, and HRMS. The present methodology offers a straightforward approach for the formylation and bromination of imidazo[1,2-a]pyridines, causing the result of the formation of two distinct products using a single protocol. It is desirable that this approach be utilized not just in academic institutions, but also in industrial and pharmaceutical facilities.
References
V.M. Muzalevskiy, Z.A. Sizova, A.V. Shastin, V.G. Nenajdenko, Eur. J. Org. Chem. 2019, 4034 (2019)
Z. Tashrifi, M. Mohammadi-Khanaposhtani, B. Larijani, M. Mahdavi, Eur. J. Org. Chem. 2020, 269 (2020)
C. Cabrele, O. Reiser, J. Org. Chem. 81, 10109 (2016)
R. Rawat, S.M. Verma, Synth. Commun. 50, 3507 (2020)
S. Redon, A.R.O. Kosso, J. Broggi, P. Vanelle, Tetrahedron Lett. 58, 2771 (2017)
J. Panda, B.P. Raiguru, M. Mishra, S. Mohapatra, S. Nayak, ChemSelect 7, 202103987 (2022)
Y. Yu, Z. Su, H. Cao, Chem. Rec. 19, 2105 (2019)
D. Ghosh, S. Ghosh, A. Hajra, Adv. Synth. Catal. 363, 5047 (2021)
S.E. Hooshmand, H. Yazdani, C. Hulme, Eur. J. Org. Chem. 2022, 202200569 (2022)
Y.-Z. Ji, J.-Y. Zhang, H.-J. Li, C. Han, Y.-K. Yang, Y.-C. Wu, Org. Biomol. Chem. 17, 4789 (2019)
M. Wang, Q. Zhou, X. Zhang, X. Fan, Adv. Synth. Catal. 365, 1255 (2023)
H. Lu, Z. Tong, L. Peng, Z. Wang, S.-F. Yin, N. Kambe, R. Qiu, Top. Curr. Chem. (Z) 380, 55 (2022)
S. Kalari, A.U. Shinde, H.B. Rode, J. Org. Chem. 86, 17684 (2021)
Z. Zhang, Q. Tian, J. Qian, Q. Liu, T. Liu, L. Shi, G. Zhang, J. Org. Chem. 79, 8182 (2014)
X.-F. Wu, K. Natte, Adv. Synth. Catal. 358, 336 (2016)
H. Cao, S. Lei, N. Li, L. Chen, J. Liu, H. Cai, S. Qiu, Chem. Commun. 51, 1823 (2015)
H. Fei, J. Yu, Y. Jiang, H. Guo, J. Cheng, Org. Biomol. Chem. 11, 7092 (2013)
Z. Zhou, Y. Yuan, Y. Cao, J. Qiao, A. Yao, J. Zhao, W. Zuo, W. Chen, A. Lei, Chin. J. Chem. 37, 611 (2019)
H. Jiang, D. Guo, Y. Zhang, Q.-P. Shen, S. Tang, J. You, Y. Huo, H. Wang, Q.-W. Gui, Synthesis 52, 2713 (2020)
R. Shankar, J.A. Tali, B. Sharma, G. Kumar, Y. Rasool, Y. Sharma, Org. Biomol. Chem. 21, 7267 (2023)
S. Xiang, H. Chen, Q. Liu, Tetrahedron Lett. 57, 3870 (2016)
G. Kibriya, A.K. Bagdi, A. Hajra, Org. Biomol. Chem. 16, 3473 (2018)
S. Song, X. Sun, X. Li, Y. Yuan, N. Jiao, Org. Lett. 17, 2886 (2015)
X. Zhou, H. Yan, C. Ma, Y. He, Y. Li, J. Cao, R. Yan, G. Huang, J. Org. Chem. 81, 25 (2016)
W. Jian, H. Wang, K. Du, W. Zhong, J. Huang, ChemElectroChem 6, 2733 (2019)
J.H. Lee, H.I. Jung, D.Y. Kim, Synth. Commun. 50, 197 (2020)
R.J. Reddy, A. Shankar, A.H. Kumari, Asian J. Org. Chem. 8, 2269 (2019)
P. Katrun, C. Kuhakarn, Tetrahedron Lett. 60, 989 (2019)
D.R. Indukuri, G.R. Potuganti, M. Alla, Synlett 30, 1573 (2019)
S. Zhu, B. Wang, H. Li, W. Xiao, F. Teng, H. Shen, Q. Gui, Z. Li, H. Jiang, Chem. Select 5, 12329 (2020)
M. Tajik, M. Shiri, F.H. Hussain, Y.L. Nosood, B. Baeiszadeh, Z. Amini, R. Bikas, A. Pyra, RSC Adv. 13, 16963 (2023)
S.E. Hooshmand, Z. Amini, M. Shiri, A. Al-Harrasi, J. Fluor. 34, 1131 (2024)
M. Shiri, Z. Gholami-Koupaei, F. Bandehali-Naeini, M.-S. Tonekaboni, S. Soheil-Moghaddam, D. Ebrahimi, S. Karami, B. Notash, Synthesis 52, 3243 (2020)
M. Shiri, M. Fathollahi-Lahroud, Z. Yasaei, Tetrahedron 73, 2501 (2017)
M. Shiri, M. Ranjbar, Z. Yasaei, F. Zamanian, B. Notash, Org. Biomol. Chem. 15, 10073 (2017)
M. Shiri, S.Z. Mirpour-Marzoni, Z. Bozorgpour-Savadjani, B. Soleymanifard, H.G. Kruger, Monatsh. Chem. 145, 1947 (2014)
M. Shiri, R. Pourabed, V. Zadsirjan, E. Sodagar, Tetrahedron Lett. 57, 5435 (2016)
M. Shiri, M. Heydari, V. Zadsirjan, Tetrahedron 73, 2116 (2017)
M. Shiri, Z. Faghihi, H.A. Oskouei, M.M. Heravi, S. Fazelzadeh, B. Notash, RSC Adv. 6, 92235 (2016)
M. Shiri, M.M. Heravi, V. Zadsirjan, M. Ghiasi, S.A. Shintre, N.A. Koorbanally, T. Singh, J. Iran. Chem. Soc. 16, 1517 (2019)
M. Shiri, Z. Bozorgpour-Savadjani, J. Iran. Chem. Soc. 12, 389 (2015)
M. Shiri, B. Farajpour, Z. Bozorgpour-Savadjani, S.A. Shintre, N.A. Koorbanally, H.G. Kruger, B. Notash, Tetrahedron 71, 5531 (2015)
V. Zadsirjan, M. Shiri, M.M. Heravi, T. Hosseinnejad, S.A. Shintre, N.A. Koorbanally, Res. Chem. Intermed. 43, 2119 (2017)
B. Soleymanifard, M.M. Heravi, M. Shiri, M.A. Zolfigol, M. Rafiee, H.G. Kruger, T. Naicker, F. Rasekhmanesh, Tetrahedron Lett. 53, 3546 (2012)
H. Chen, Y. Wang, Q. Liu, Y. Guo, S. Cao, Y. Zhao, Chin. J. Chem. 40, 2313 (2022)
Acknowledgements
We are grateful for financial support provided by Alzahra University and the Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iranfar, S., Shiri, M., Majedi, S. et al. A time-controlled selective bromination and formylation of 2-arylimidazo[1,2-a]pyridines. J IRAN CHEM SOC 21, 2469–2475 (2024). https://doi.org/10.1007/s13738-024-03084-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-024-03084-w