Abstract
The development of alternative, sustainable sources of fuels is necessary for addressing the increasing world’s energy demand and global warming crisis. Butanol, an important and promising biofuel candidate, is generally produced from heterotrophic microorganisms by carbohydrate fermentation. Cyanobacteria can serve as an alternative feedstock for third and fourth-generation biofuel production. Biofuel and biochemical synthesis from CO2 using photosynthetic organisms is an attractive approach. These prokaryotic photosynthetic microorganisms have been used as an energy feedstock and also genetically engineered for direct conversion of CO2 into butanol. This review aims to highlight the crucial aspects of photosynthetic biobutanol synthesis. The review explores the recent advances in biobutanol production from cyanobacteria. It reviews the recent and classical cyanobacterial genetic modification approaches and the advances in synthetic biology toolboxes of cyanobacteria. It discusses both third and fourth-generation butanol synthesis while emphasizes more on genetic modifications used in metabolic engineering and their effects on butanol production. Challenges associated with strain development, cultivation, butanol toxicity, and recovery have also been discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fossil fuels are the major energy sources, but the concerns over CO2 emission and climate change elicited the need for the development of alternatives. Biofuels such as biodiesel, bioethanol, biobutanol, and bioalkanes are promising alternatives to fossil fuels. In the present time, butanol, the next-generation biofuel, has its use as a fuel additive or gasoline substitute due to their similarity in their characteristics (Lee et al. 2008). Apart from that, butanol is an important chemical feedstock used for the synthesis of butyl acrylate and methacrylate esters, butyl glycol ether, butyl acetate, etc., and even as a solvent for the preparation of pharmaceutical products such as antibiotics, vitamins, and hormones (Kirschner 2006; Lee et al. 2008). It is used as an efficient diluent solvent in the oil industry, perfume industry and as an extracting agent (food-grade) in the flavor industry.
Butanol is a better fuel additive than ethanol due to its higher energy content, lower hygroscopicity, and corrosiveness. There are four isomeric structures of butanol depending on the hydroxyl group position: n-butanol (1-butanol), sec-butanol (2-butanol), isobutanol (2-methyl-1-propanol), and tert-butanol (2-methyl-2-propanol). Out of all the isomers, n-butanol and isobutanol are mainly studied as potential biofuel candidates due to their advantageous properties. Butanol can be blended in higher ratios with gasoline due to their similar properties, and due to its high boiling point, the rate of burning is also slower than ethanol (Sarathy et al. 2012; Ndaba et al. 2015). Butanol has 90% of gasoline’s energy density and can be used as a 100% replacement for gasoline (Hönig et al. 2014). It suits the present time infrastructure for storage, transport, used in standard engines without modification, and may be used as jet fuel (Brownstein 2014).
Butanol is naturally produced by microorganisms with acetone and ethanol in a mixture by ABE fermentation. Louis Pasteur, in 1861 first reported butanol synthesis from microbes, and in 1915, the use of Clostridium acetobutylicum for acetone-butanol synthesis was patented, following which industrial production of butanol started in 1916 (Pasteur 1862; Sauer 2016). Clostridium strains are used for the first large-scale butanol production in the UK in 1912 by ABE fermentation, where a mixture of solvents (acetone, butanol, and ethanol), organic acids (acetic acid, lactic acid, and butyric acid), and gases (CO2 and H2) (Mansur et al. 2010) are produced. Commercial Clostridium strain produced 14 to 20 gL−1 butanol in batch fermentation (Green 2011), but the use of anaerobic heterotrophic bacteria for biofuel production is not economical. Therefore, many other non-native microbial strains have been genetically modified to produce butanol (Nawab et al. 2020). The major disadvantage of heterotrophic hosts for biofuel production is the requirement of the constant supply of costly organic carbon sources. Agricultural biomass utilization for biobutanol production also needs rigorous pretreatment and inhibitor removal steps, making the process cumbersome.
Microalgae/cyanobacteria are photosynthetic microorganisms that can serve both as autotrophic non-native host or energy feedstock for butanol production and require no additional carbon and arable land for growth. Advancement in synthetic biology and the availability of modern genetic tools have led to the engineering of cyanobacterial chassis for light-driven biofuel production. Cyanobacterial strain improvement and the development of cultivation systems, and optimization of process parameters are presently required for scaling up butanol production.
Advances in biobutanol production and challenges associated with the utilization of heterotrophic native and non-native butanol producers have been discussed considerably (Zheng et al. 2009; Ou et al. 2015; Moon et al. 2016; Nanda et al. 2017; Kolesinska et al. 2019; Nawab et al. 2020). However, a detailed review of photosynthetic biobutanol production is still not available. In this review, recent advances in cyanobacteria-based butanol production are summarized. This review will discuss genetic modification of cyanobacteria for butanol production as well as challenges associated with large-scale production of butanol via cyanobacteria. It will focus on solutions for improving the process and highlight the possible areas for researchers to emphasize for utilizing the potential of cyanobacteria for industrial-scale biobutanol production.
2 Cyanobacteria: a platform for biobutanol production
2.1 Cyanobacteria
Cyanobacteria are Gram-negative microorganisms, the oldest photosynthetic prokaryote, which originated more than 3 billion years ago (Hedges et al. 2001). Cyanobacteria require CO2, sunlight, and minimal nutrients for growth and convert 3–9% of solar energy into biomass (Lau et al. 2015). Cyanobacteria have been studied for many decades by scientists due to their attractive features. These organisms survived varied and harsh environments from ancient times and were responsible for transforming our atmosphere and still contributing majorly to global carbon fixation (Field et al. 1998). Cyanobacteria do not require fermentable sugars and arable lands for their cultivation as they can fix and utilize dissolved CO2 at elevated concentrations under submerged conditions (Sheehan et al. 1998; Dismukes et al. 2008). On the other hand, the capability of cyanobacteria to grow in diverse locations with minimal nutrients and CO2 provides a scope of mass-cultivation in unproductive lands. Further, atmospheric nitrogen fixation by several cyanobacteria provides an additional advantage of survival and product synthesis even under nitrogen-deficient conditions. The simple cell structure and genetic makeup of this prokaryote make genetic modification comparatively easier than plants (Koksharova and Wolk 2002). Due to all these advantages, much work has been done in the past decade to develop cyanobacteria as a photosynthetic cell factory to synthesize biofuels, bioplastics, and other value-added compounds from CO2, light, and inorganic nutrients (Machado and Atsumi 2012; Lai and Lan 2015; Katayama et al. 2018). Cyanobacteria have also been utilized as single-cell protein or a source of valuable metabolites, including fatty acids, terpenoids, phytohormones, polysaccharides, phycocolloides, phenolic and photoprotective compounds, and cyanotoxins (Raja et al. 2008; Singh et al. 2017). This direct and indirect chemical synthesis ability of these photosynthetic prokaryotes makes them a promising candidate for sustainable industrial biofuel production. Eukaryotic photosynthetic microorganisms although accumulate lipids in reasonable amounts for biofuel production (Pate et al. 2011), but the requirement of intracellular product recovery and lack of synthetic biology tools for strain improvement still makes biofuel production from eukaryotic algae remain unfeasible for industries. Development and improvement of cyanobacterial model strains and modern synthetic biology tools will further provide an opportunity to utilize photosynthetic microorganisms for biofuel production. The high photosynthetic efficiency, fast growth rates, ease of genetic manipulation, low nutrient requirements, and the ability to grow in wastewater make this organism an ideal choice for developing it as an industrial biochemical ‘producer’/ an alternative and eco-friendly platform for biofuel production.
2.2 Alternative carbon and energy source utilization
The oxygenic photosynthetic reaction occurs by absorption of light energy by the cyanobacterial pigments leading to the synthesis of major organic carbon compounds with the evolution of oxygen as a byproduct. The light energy absorbed in the visible range (400–700 nm) is utilized to synthesize organic molecules from inorganic carbon and water. The abundantly available solar energy can therefore be harnessed by the cyanobacterial cells for biomass and product synthesis. Butanol production from heterotrophs requires a continuous supply of organic carbon sources, which are also utilized for biomass generation, thereby increasing the cost of the product.
2.3 Cyanobacterial nutritional requirement
Cyanobacteria utilize radiant energy for biomass generation and valuable chemical production is possible using readily available natural resources like CO2, water, and essential nutrients (N, P, S, K, Fe, etc.). Cultivation of cyanobacteria is done in a variety of culture mediums, including Pringsheim’s medium (Pringsheim 2016), BG11 medium (Rippka et al. 1979), Fogg’s medium (Fogg and Thake 1987), and others, with BG11 and BG110 being the most widely used ones. The most important nutrients for cyanobacterial growth are carbon, nitrogen, and phosphorus. For cyanobacterial cultivation, primarily inorganic nutrients are used, but some organic forms can also be utilized. These photosynthetic microorganisms take up carbon in the inorganic form (CO2). The carbon content in a species may vary depending upon the species type and culture condition from 17.5 to 65% by dry weight but generally contain 50% carbon (Grobbelaar 2004). The CO2 is fixed by the Calvin cycle, where most species possess a C3 pathway while some have C3-C4 intermediate photosynthesis (Roberts et al. 2007; Xu et al. 2012). RuBisCO, the carbon fixing enzyme of the C3 cycle, can use the only CO2 as a substrate for carbon fixation (Price et al. 2008). Although both CO2 and HCO3− are taken up by many cyanobacterial species, some may selectively utilize only CO2 or HCO3− (Camiro-Vargas et al. 2005). CO32− can also be used as a source of inorganic carbon by extremely alkaliphilic cyanobacteria (Mikhodyuk et al. 2008). Some species of cyanobacteria are also able to utilize the organic nutrients heterotrophically or mixotrophically as energy and carbon sources (Chojnacka and Marquez-Rocha 2004; Perez-Garcia et al. 2011). The type of organic nutrient supplementation such as monosaccharides, acetic acid, glycerol, and urea (Chen and Zhang 1997; Hsieh and Wu 2009a, b; Heredia-Arroyo et al. 2011) is species and strain-dependent (Mühling et al. 2005). The mechanisms involved in the carbon uptake by microalgal cells are diffusion, active transportation, and phosphorylation of carrier proteins (Perez-Garcia et al. 2011). This ability of microalgae/cyanobacteria to utilize the organic compounds can be used for wastewater treatment (Markou et al. 2014).
Cyanobacterial cells are rich in protein content and therefore, demand for high nitrogen supply for growth (Becker 1994). Uptake of nitrogen takes place in inorganic (NO3, NO2−, NO, NH4+), molecular N2, and in organic form (urea and amino acids) (Flores and Herrero 2005) by energy-requiring active mechanisms. Nitrate salt, NaNO3, is mainly used, followed by KNO3 (Grobbelaar 2004), and microalgal cells can tolerate nitrate up to 100 mM, but further concentration higher showed toxic effects (Jeanfils et al. 1993). Nitric oxide (NO) from flue gas can also be provided as a nitrogen source as it gets oxidized to nitrite/nitrate in medium to be utilized by microalgae (Nagase et al. 2001), but tolerance level varies with species type (Brown 1996). Cyanobacteria also preferably use ammonia and require less energy for uptake and assimilation (Boussiba and Gibson 1991). Ammonia as a nitrogen source showed a similar growth rate with the one having nitrates (Boussiba 1989; Park et al. 2010), but cells are adversely affected even at low concentrations of free ammonia (Azov and Goldman 1982) while the tolerance varies from species to species. Many cyanobacteria can fix the atmospheric nitrogen and utilize it as their only nitrogen source (Gallon 2001); however, the reduction of N2 to ammonium by the nitrogenase complex is an energy-requiring process (Großkopf and LaRoche 2012; Peccia et al. 2013). Organic nitrogen, urea, and amino acids can also be utilized for cyanobacteria cultivation, but the type of organic nitrogenous compound utilization and the growth rate is species-dependent (Flores and Herrero 2005). However, studies showed the possibility of utilizing organic nitrogen-containing wastewater for microalgal cultivation to remove nutrients and biodiesel production (Li et al. 2011a, b). Phosphorous is another essential, but non-renewable nutrient required for microalgal/cyanobacterial cultivation media and is mostly in limiting conditions in natural conditions. Potassium, sodium, and ammonium salts of phosphorous and superphosphates derived from phosphate rocks are used for cultivation. Microalgae/cyanobacteria can store phosphorous intracellularly as polyphosphate granules (Powell et al. 2009), and so they were used to remove phosphate from wastewater (Powell et al. 2011). Intracellular phosphate storage by these microorganisms from the production medium will be a problem during mass cultivation with the aim of biomass accumulation and associated products. Therefore, the solution to this problem can be optimization of the cultivation media with limited phosphate or develop a strategy for slow/controlled release of phosphates during cell growth. Potassium, an important macronutrient required for the growth of microalgae/cyanobacteria and is provided as K2HPO4, KH2PO4, KNO3, KSO4, KCl salts in most cultivation mediums. At lower concentrations, potassium is actively transported by the cells while taken passively when present in higher concentrations (Malhotra and Glass 1995). Besides the above macronutrients, Mg, S, Ca, Na, Cl, Fe, Zn, Cu, Co, Mo, Mn, B, and Co are the micronutrients, and all of these have a significant role in cell growth and metabolism.
Most of the freshwater cyanobacterial strains are cultured in BG11 with/without minor modifications. The composition of BG11: 1.5 gL−1 NaNO3, 0.040 gL−1 K2HPO4, 0.075 gL−1 MgSO4·7H2O, 0.036 gL−1 CaCl2·2H2O, 0.006 gL−1 Citric acid, 0.006 gL−1 ferric ammonium citrate, 0.001 gL−1 Na2EDTA, 0.04 gL−1 Na2CO3, 1,000 × trace metals (S, Ca, Na, Cl, Fe, Zn, Cu, Co, Mo, Mn, B). For cyanobacterial biobutanol production, BG11 has been used without any optimization. However, a study showed the positive effect of nitrogen starvation on butanol production from Synechocystis sp. PCC 6803 mutant strain incapable of PHB synthesis (Anfelt et al. 2015). Therefore, a proper understanding of the role and effects of different nutrient components on the cyanobacterial cells is essential, which is to be followed by media optimization for increasing biomass and associated product.
One of the important hurdles to industrial-scale cyanobacterial cultivation is the increase in the production cost due to the requirement of freshwater for culture medium in huge amounts (Yashavanth et al. 2021). Microalgae/cyanobacteria are capable of growing in wastewater and during growth also release oxygen during photosynthesis, reducing the biological oxygen demand in these effluents. Cyanobacteria can remove excess nutrients and organic pollutants (Singh et al. 2016) and can also be used as chelating agents for heavy metal removal (Jiang et al. 2016; Shen et al. 2018). The use of wastewater for cyanobacterial cultivation can therefore be an economical and environment-friendly strategy (Mahesh et al. 2021). Nitrogen and phosphorus-rich wastewater are generated from households, agriculture, aquaculture, and livestock production. Optimization of the nutrient content in these wastewaters can help formulate cultivation media for cyanobacteria. Sewage wastewater optimized for growing microalgae favored both biomass and lipid accumulation (Gebremedhin et al. 2018). Cultivation of PHB-producing cyanobacterial strains in shrimp wastewater, aquaculture, and agricultural run-off using different strategies showed cell growth, nutrient removal, and polymer accumulation (Samantaray et al. 2011; Krasaesueb et al. 2019; Rueda et al. 2020). For cyanobacteria-based butanol production, such strategies can make the process economic while providing scope for reuse and effective wastewaters treatment.
2.4 Advances in genetic tool development
For genetic modification of any organism, the first requirement is the availability of an annotated genome sequence of the organism. The organism must allow easy uptake of foreign DNA with the generation of stable modified strains having the desired knock-ins or knock-outs. Some model and fast-growing non-model cyanobacterial strains (Table 1) are promising candidates for biochemical production (Gale et al. 2019; Mukherjee et al. 2020). The genetic constructs for strain modification can be transferred by favorable gene transfer methods (Table 1) and mainly depends on the strain type. In the case of cyanobacteria, limited genetic tools are available compared to the model heterotrophs like E. coli and S. cerevisiae (Sun et al. 2018), but presently for enhancing the industrial potential of cyanobacteria, a lot of work is done for developing and improving the genetic tools of these photosynthetic microorganisms.
2.4.1 Promoters
Promoters are key synthetic biology elements for gene expression, and for cyanobacteria, both native and foreign promoters have been explored (Table 1). These can be classified depending on their function as inducible and constitutive promoters. Continuous gene expression can be obtained using constitutive promoters, but endogenous cyanobacterial promoters are regulated by circadian rhythm. Further, in the case of inducible promoters, limiting factors like toxicity, leaky expression, and photoliability are obstacles for developing such promoters (Santos-Merino et al. 2019). Recently, many inducible promoters have been developed and implemented in the model and non-model cyanobacterial strains (Table 1). Among the heterologous promoter, Ptac/Ptrc promoter in E. coli has been widely used in model cyanobacteria for high-level gene expression (Geerts et al. 1995; Ng et al. 2000; Atsumi et al. 2009; Huang et al. 2010; Niederholtmeyer et al. 2010a; Lan and Liao 2011). A version of Ptac/Ptrc promoter (Ptrc1O) showed fourfold higher expression than the promoter of ribulose bisphosphate carboxylase/oxygenase (RuBisCO) large subunit, PrbcL in Synechocystis sp. PCC 6803. Plac, Ptet, λ PR are other E.coli promoters used for expression in the model cyanobacterium, but the previous study showed poor activities in them (Huang et al. 2010). Another E. coli derived inducible promoter, L03 promoter induced by anhydrotetracycline (aTc) were used in many cyanobacteria (Huang and Lindblad 2013), but aTc is light-sensitive, and so stable controlled expression is a problem in cyanobacteria (Zess et al. 2016). Recently, the metabolite-induced promoters of E. coli, such as L-arabinose-inducible araBAD promoter and rhamnose-inducible rhaBAD, have also been implemented in Synechococcus elongatus PCC 7942 (Cao et al. 2017) and Synechocystis sp. PCC 6803 (Immethun et al. 2017; Kelly et al. 2018). Another such promoter from Corynebacterium glutamicum, Pvan, which is induced by vanillate by binding to and removing the repressor VanR is used in Synechococcus elongatus PCC 7942 (Taton et al. 2017). A number of native inducible promoters such as arsB, ziaA, coat, nrsB, petE, nirA, etc., have also been successfully used in cyanobacterial gene expression (Table 1). Promoters such as PrbcL, Pcmp, Psbt belong to the CO2 fixation enzymes, PpsaA, PpsaD of photosystem I and PpsbA1, PpsbA2 of photosystem II and Pcpc of phycocyanin are native promoters of the genes required for photosynthesis. Commonly used native cyanobacterial constitutive promoters are PcpcB and PpsbA2 (Johnson et al. 1988; Mohamed and Jansson 1989). The gene expression by constitutive promoters may not always be consistent and change under varying conditions due to their inducible nature. The widely used native constitutive promoter, PpsbA2, is also a light-inducible promoter and under constant light functions as a constitutive expression system (Stensjö et al. 2018). For biobutanol production from cyanobacteria, both inducible and constitutive promoters are used (Liu et al. 2019). With the availability of cyanobacterial genomes along with transcriptomic and proteomic data related to a stress condition, there is a scope for finding more native inducible and constitutive promoters. Besides this, the development of synthetic promoters by tuning of the available promoters, for example, Pcpc560 based on PcpcB (Zhou et al. 2014) and truncated version of psbA2 (Englund et al. 2016), showed elevated gene expression in Synechocystis sp. PCC 6803.
2.4.2 Other control elements
For initiating proper translation, the ribosome binding site (RBS) has an important role and allows interaction between 16S rRNA and the Shine-Dalgarno (SD) sequence of RBS. The nucleotide sequence surrounding the RBS and spacing between SD and start codon can also influence the efficiency in translation (de Smit and Van Duin 1990; Chen et al. 1994; Pfleger et al. 2006). For predicting the efficiency of RBS, Salis et al. have developed a thermodynamic model for calculating the impact based on the above factors, which predicts the expression of proteins in E. coli (Salis et al. 2009), and optimization of RBS using such models can also be useful in cyanobacteria. Different RBS and their efficiencies have been studied by expressing the GFP in Synechocystis sp. PCC 6803 and also found that the efficiency of the RBS may vary in different species (Heidorn et al. 2011a, b). In Synechocystis sp. PCC 6803, 20 native RBS have recently been studied (Liu and Pakrasi 2018). In another study for cyanobacterial 2,3-butanediol production, different RBS from E. coli used for overexpression of heterologous genes showed an increase in solvent production (Oliver et al. 2013). A recent study demonstrated the effect of different RBS on the translation efficiency of heterologous proteins in Synechocystis sp. PCC 6803 and provided information regarding the selection of RBSs for overexpression of a gene or even in multicistronic constructs (Thiel et al. 2018).
Riboswitches are important regulatory elements present in the 5ʹ untranslated regions of mRNAs involved in controlling gene expression. Applications of riboswitches for gene regulation in cyanobacteria are not much explored. Firstly, in Synechococcus elongatus PCC 7942, tightly regulated protein expression was obtained by the use of theophylline-dependent synthetic riboswitch (Nakahira et al. 2013), following which this riboswitch was also used in other cyanobacterial strains, Synechocystis sp. PCC 6803, Leptolyngbya BL0902, Anabaena sp. PCC 7120, and Synechocystis sp. strain WHSyn (Ma et al. 2014; Armshaw et al. 2015; Ohbayashi et al. 2016). Recently, two native riboswitches, cobalamin and glutamine dependent, have been reported in Synechococcus PCC 7002 and Synechocystis sp. PCC 6803 respectively (Pérez et al. 2016; Klähn et al. 2018). Thus, there is a possibility of finding more native cyanobacterial riboswitches, which are yet to be identified. Another study showed that hybridization of theophylline-responsive riboswitch with the PrhaBAD could initiate the expression of CRISPR interference (CRISPRi) mechanism for targeting photosystem II in Synechocystis sp. PCC 6803 (Liu et al. 2020). Thus, a combination of different regulatory mechanisms can provide a scope for making improvements in expression of desired genes.
2.4.3 Reporter genes
Reporter genes coding for reporter proteins helps in the quantification of gene expression, protein interactions, and visualization of subcellular localizations (Berla et al. 2013) and for characterization of synthetic genetic tools. In cyanobacteria, autofluorescence of phycobilins and chlorophyll molecules causes a problem in the use of fluorophores (Ruffing et al. 2016). In cyanobacteria, reporter, a mutant of green fluorescent protein, GFPmut3B, and enhanced yellow fluorescent protein, EYFP are mostly used (Huang et al. 2010; Yang et al. 2010; Heidorn et al. 2011a, b; Huang and Lindblad 2013; Landry et al. 2013). Improved fluorophores, mOrange, mTurquiose, mNeonGreen, and Ypet with higher brightness, photostability, and quantum yield, are also used recently (Chen et al. 2012; Ruffing et al. 2016; Jordan et al. 2017). Reporter genes are mainly used to characterize the strength of promoters and other control elements, thereby contributing to the development of genetic tools for strain improvement.
2.4.4 Selectable markers
For genetic engineering and the selection of genetically modified organisms, selectable markers are the basic necessity. Antibiotics such as chloramphenicol (Rouhiainen et al. 2000), erythromycin (Vermaas 1998), gentamicin (Ng and Pakrasi 2001), kanamycin (Rouhiainen et al. 2000), neomycin (Wolk et al. 1984), spectinomycin (Golden et al. 1987), zeocin (Xu et al. 2004) are commonly used as selectable markers in cyanobacteria. For cyanobacterial biobutanol production, the antibiotic resistance cassettes are mostly used as selectable markers, and many genes at multiple sites have been inserted in a single strain by using combinations of antibiotic resistance cassettes (Liu et al. 2019). Selectable markers were also developed by the genetic insertions in the cyanobacteria leading to change in the cell phenotype. In Synechococcus elongatus UTEX 2973, the removal of nblA gene involved in phycobilisome degradation resulted in non-bleaching phenotype under nitrogen starvation and therefore can act as selectable markers (Wendt et al. 2016).
2.4.5 Vectors
Cyanobacterial vectors designed were specific to a particular strain, while in recent years, such vectors have been designed for working with diverse strains. Using BioBricks standard (Shetty et al. 2008), a shuttle vector that allowed the assembly of constructs as well as expression in several cyanobacteria was developed. pPMQAK1 is the first broad-host range shuttle vector for cyanobacteria (Huang et al. 2010). For genetic modification in cyanobacteria, replicative as well as integration vectors have been developed (Fig. 1). The replicative vectors can replicate inside the cyanobacterial host and are either broad-host range (Mermet-Bouvier and Chauvat 1994; Ng et al. 2000; Huang et al. 2010) or derived from endogenous plasmids (Wolk et al. 1984; Argueta et al. 2004; Iwaki et al. 2006). The integrative vectors integrate the foreign gene directly into the cyanobacterial genome by homologous recombination (Golden et al. 1987; Eaton-Rye 2004; Heidorn et al. 2011a, b), while these cannot replicate and removed from the cell if not integrated. A platform for constructing a broad-host range vector system with replicative and integrative modular plasmids was developed along with CYANO-VECTOR, a web server for in silico designing of plasmids with assembly strategies (Taton et al. 2014). Different chromosomal integration vectors targeting the neutral sites in the chromosomes have also been developed for Synechococcus elongatus PCC 7942 (Kim et al. 2017), Synechococcus PCC 7002 (Vogel et al. 2017), and Synechocystis sp. PCC 6803 (Englund et al. 2015). CyanoGate was developed by Vasudevan et al. based on Plant Golden Gate MoClo kit and the MoClo kit. This system was a powerful tool for cyanobacterial vector assembly tested in Synechocystis sp. PCC 6803 and Synechococcus elongatus UTEX 2973 (Vasudevan et al. 2019).
Schematic diagram showing different approaches for genetic modification and strain improvement of cyanobacterial strains. a genome modification using integration vector by homologous recombination at neutral site (NS) having upstream (US) and downstream (DS) of the NS present within the vector. b use of shuttle/expression vector for strain engineering. c CRISPR-based metabolic engineering for deletion and/or incorporation of gene of interest (GOIs) and pathways. Abbreviations: AbR, antibiotic resistance gene, Cas, CRISPR associated; CRISPR, clustered regularly interspaced short palindromic repeats; tracrRNA, trans-activating CRISPR RNA, crRNA, CRISPR RNA
2.4.6 CRISPR genome editing and gene regulation
In cyanobacteria, genome modification is carried out primarily using integrative plasmids through homologous recombination. Gene deletion in cyanobacteria by homologous recombination requires the use of antibiotic resistance markers for screening the mutant strain. There is always a need for a resistance marker for generating a strain with multiple deletions, thereby requiring more research on developing selectable markers. Moreover, the presence of oligoploid or polyploid genomes in many cyanobacteria (Griese et al. 2011) demands multiple segregation steps to ensure that the genetic modification is present in all its chromosomes and so is cumbersome. A solution to the above problems is the use of clustered regularly interspaced short palindromic repeats (CRISPR)-based technology for markerless genetic modification. The CRISPR-Cas system has been used for cyanobacterial genetic modification (Behler et al. 2018), and it can precisely modify multiple genes at different sites at a time without any scar and edit all the chromosomes in a single selection (Fig. 1). In Synechococcus elongatus PCC 7942, the CRISPR-Cas9 genome editing tool was used for redirecting carbon flux from glycogen to succinate pathway for increasing succinate production (Li et al. 2016). It was also used for deletion of nblA gene in Synechococcus elongatus UTEX 2973 involved in change in cell phenotype grown in the absence of nitrate (Wendt et al. 2016). However, this study also showed the toxicity of the cyanobacterial cells due to the accumulation of the Cas9 protein. The development of the CRISPR-Cas12a system has shown less toxic effects in cyanobacteria than Cas9 (Pattharaprachayakul et al. 2020). nifH and nblA gene deletion in Anabaena sp. PCC 7120 and Synechocystis sp. PCC 6803 respectively was done using CRISPR-Cas12a (Ungerer and Pakrasi 2016). This technology has been used to introduce heterologous genes, point mutation, and knock-outs in different cyanobacterial species such as Anabaena sp. PCC 7120 (Ungerer and Pakrasi 2016; Niu et al. 2018), Synechococcus elongatus PCC 7942 (Li et al. 2016; Ungerer et al. 2018), Synechocystis sp. PCC 6803 (Xiao et al. 2018), Synechococcus elongatus UTEX 2973 (Ungerer and Pakrasi 2016; Wendt et al. 2016). CRISPR-Cas systems have also been used to regulate gene expression by interfering with the process of transcription by using deactivated Cas proteins (dCas) (Zheng et al. 2019). CRISPRi with CRISPR-dCas9 and CRISPR-dCas12a has been used for overexpression/repression of a number of genes in cyanobacteria, such as Anabaena sp. PCC 7120 (Higo et al. 2018; Higo and Ehira 2019a, b), Synechococcus elongatus PCC 7942 (Choi and Woo 2020), Synechocystis sp. PCC 6803 (Yao et al. 2016; Kaczmarzyk et al. 2018), Synechococcus elongatus UTEX 2973 (Knoot et al. 2019). CRISPR-Cas-based genome editing and CRISPRi technologies have already been used for improving biofuel (fatty acids and fatty alcohol) and metabolites (amino acids, succinate, pyruvate) productivity, but it has not yet been used directly for cyanobacterial biobutanol production. The use of such technologies can help improve the butanol titer by solving the problems of improper segregation and the need for selectable markers for genetic modification.
3 Cyanobacterial biobutanol production
3.1 Third generation butanol production
The concept of utilizing microalgae/cyanobacterial biomass as an energy feedstock came in towards the end of the 1950s (Chen et al. 2010). The major work in this area started in 1970 with the oil crisis, following which extensive work is done on biofuel production from photosynthetic microbes (Borowitzka 2008). Many cyanobacteria contain a good amount of starch and glycogen, which serve as raw materials for biofuel production (Ueda et al. 1996). Direct conversion by pretreatment and fermentation of cyanobacterial biomass into carbohydrate-based biofuels has been explored (Arias et al. 2021). A cyanobacterial cell is simple with the peptidoglycan layer as compared to the eukaryotic microalgal cell wall, which is quite complicated (DS 2011). Further, the reserve carbohydrate in cyanobacteria is glycogen, which is not only water-soluble (Ball et al. 2011) but also can be quickly mobilized compared to starch from microalgae (Mamo et al. 2013). Cyanobacteria have been used as a feedstock for bioethanol production by yeast fermentation (John et al. 2011; Aikawa et al. 2013; Möllers et al. 2014). Butanol was produced by pretreated cyanobacterial biomass fermentation using immobilized Clostridium acetobutylicum cells (Efremenko et al. 2012). Subsequently, studies were done for proper optimization of conditions for pretreatment of cyanobacterial biomass and release of sugar (Kushwaha et al. 2017) and then fermentation of these hydrolysates to biobutanol (Kushwaha et al. 2020).
3.1.1 Pretreatment of cyanobacterial biomass
An essential step of upstream processing is a proper conversion of biomass into sugar, thus significantly improving the efficiency of fermentation (Dürre 2007). Physical, chemical, and physicochemical techniques have been used depending upon the type of feedstock used for fermentation. Second-generation feedstock requires extensive pretreatment to access fermentable sugar due to its complex structure comprising of cellulose, hemicellulose, and lignin (Moxley et al. 2008), while third-generation feedstock has a simpler cell structure making it a possible candidate for butanol production (Kushwaha et al. 2019). Chemical pretreatment using acids, alkali, peroxides, ozone, and organic solvents is very often used for the breakdown of complex feedstock (Sun and Cheng 2002; Zhao et al. 2008; Chen et al. 2015). Cyanobacterial/algal biomass containing substantial carbohydrate content with little lignin makes the pretreatment process simpler, thus serving as a suitable feedstock for the synthesis of biobutanol (Suutari et al. 2015). Carbohydrates from cyanobacteria can be converted to sugar with simple pretreatment strategies and converted to alcohols such as ethanol by fermentation (Möllers et al. 2014) and butanol (Efremenko et al. 2012; Ellis et al. 2012). The use of dilute acid/alkali with high temperature showed the release of sugar (33% of dry weight) from cyanobacterial biomass (Kushwaha et al. 2017).
3.1.2 Fermentation of cyanobacterial hydrolysates
Glycogen, a polysaccharide, is accumulated by cyanobacteria to around 50% of its biomass can serve as an essential substrate for the production of biofuel (John et al. 2011). Bioethanol has been produced by yeast fermentation from cyanobacterial biomass rich in glycogen (Aikawa et al. 2013; Möllers et al. 2014). Clostridium saccharoperbutylacetonicum N1–4 produced acetone, butanol, and ethanol (ABE) up to 2.74 gL−1 from wastewater algal biomass as a source of carbon (Ellis et al. 2012). Clostridium beijerinckii ATCC 35,702 produced butanol (8.873 gL−1) from cyanobacterial (Lyngbya limnetica) hydrolysates as a carbon source with 10 gL−1 glucose supplementations (Kushwaha et al. 2020). Therefore, using suitable Clostridium strain, butanol can be produced from the NPCM by fermentation of carbohydrates present in the hydrolysates. It can be concluded from Table 2 that cyanobacterial biomass can serve as a potential feedstock for butanol production even better than first-generation feedstock with many disadvantages.
3.2 Fourth-generation butanol production
The genetic tractability of cyanobacteria can be compared to Escherichia coli and yeast with a good number of genome sequences already or to be determined, making it more accessible for genetic modification. Genetic engineering in cyanobacteria has resulted in the synthetic of many value-added products such as ethanol (Deng and Coleman 1999), isobutyraldehyde (Atsumi et al. 2009), 1-butanol (Lan and Liao 2011), isoprene (Lindberg et al. 2010), ethylene (Sakai et al. 1997), hexoses (Niederholtmeyer et al. 2010a), cellulose (Nobles and Brown 2008), lactic acid (Niederholtmeyer et al. 2010a), fatty alcohols (Tan et al. 2011) and fatty acids (Yu et al. 2000). Although genetic information about cyanobacterial strains is available (Koksharova and Wolk 2002), a few strains are primarily used to research metabolic and genetic engineering (Flores et al. 2008; Heidorn et al. 2011a, b).
Third and fourth-generation biobutanol production utilize microalgae/cyanobacteria as photosynthetic feedstock. The former generally requires no strain improvement for butanol synthesis, where the biomass is utilized as a carbon and nitrogen source for growth and production by heterotrophic butanol producers. The latter, however, involves the use of genetically modified cyanobacteria capable of butanol synthesis from CO2 and light. The first demerit of third-generation biobutanol production comes with the dewatering of microalgal/cyanobacterial biomass before the pretreatment step (Uduman et al. 2010). Secondly, the pretreatment of biomass in itself is an energy demanding process and further produces inhibitors that are known to interfere with the fermentation process (Yoo et al. 2015). In the case of fourth-generation, biobutanol can be produced in a single step by cyanobacterial strains having a butanol biosynthetic pathway. Such cyanobacterial strains capable of direct butanol secretion in the cultivation media will decrease the butanol production cost. Recently a study evaluated the environmental impacts, and cumulation energy demand of third generation butanol production suggested the requirement of metabolic engineering of cyanobacteria for competing with fossil fuels and other biofuels (Nilsson et al. 2020).
3.2.1 Engineering cyanobacterial PHB pathway
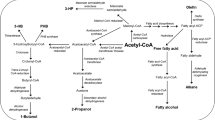
Direct butanol production from cyanobacteria is possible with the proper expression of the modified CoA-dependent pathway (Fig. 2). The first cyanobacterium to be genetically engineered for butanol production is Synechococcus elongatus PCC 7942. This strain containing the modified CoA-dependent 1-butanol pathway showed only a detectable amount (0.0145 gL−1) of butanol due to the oxygen sensitivity of genes of the Clostridial pathway (Lan and Liao 2011). Replacement of oxygen-sensitive genes with tolerant ones becomes essential as cyanobacteria perform oxygenic photosynthesis and evolve oxygen during the photolysis of water. Firstly, the replacement of oxygen-sensitive bifunctional aldehyde alcohol dehydrogenase (AdhE2) and the utilization of ATP as a driving force resulted in an almost twofold increase in the butanol yield (0.030 gL−1) (Lan and Liao 2012). Overexpression of acetyl-CoA carboxylase (ACCase) from Yarrowia lipolytica and improved coenzyme-a-acylating propionaldehyde dehydrogenase, PduP in S. elongatus PCC7942 gave 0.418 gL−1 butanol in 6 days (Fathima et al. 2018a, b). To eliminate the problem of oxygen sensitivity of the pathway genes, heterocyst of Anabaena sp. PCC7120 was targeted for enzyme expression, which yielded 0.0028 gL−1 butanol (Higo and Ehira 2019a, b). Recently, a study with the cyanobacterium Synechocystis sp. PCC 6803, with a remodeled Clostridial CoA-dependent 1-butanol pathway, showed a maximum yield of 0.836 gL−1 and a cumulative titer of 4.8 gL−1. In this work, a multi-level modular strategy was used for modification of the biosynthetic pathway with optimization of the expression units, rewiring of endogenous carbon flux, and finally recasting the central carbon metabolism (Liu et al. 2019). For n-butanol production, cyanobacterial strains modified with genes introduced/overexpressed and production vessels used along with butanol yield are mentioned in Table 3. All these studies were done on a small scale in either plug-sealed or screw cap flasks, demanding the need for large-scale study for commercial production. Many cyanobacteria can synthesize and store PHB inside their cells. An important intermediate of the native PHB pathway is 3-hydroxybutyryl-CoA which is synthesized from acetyl-CoA which can also be utilized for n-butanol production. Therefore, incorporation of a few more genes extending the native PHB pathway can also produce butanol from good PHB accumulating cyanobacterial strains.
3.2.2 Engineering of 2-keto acid pathway
Branched amino acids are synthesized through the 2-keto acid pathway where 2-ketoisovalerate, an intermediate of valine pathway, has been converted to isobutanol by enzymatic decarboxylation and reduction (Fig. 2). For the synthesis of isobutanol, the carbon fluxes are diverted into the synthetic isobutanol pathway from the valine synthesis pathway. In E.coli, overexpressed α-ketoisovalerate decarboxylase, Kivd, and alcohol dehydrogenase, ADH, resulted in 22 gL−1 of isobutanol through valine synthetic pathway (Atsumi et al. 2008), and additional optimizations coupled with in situ removal of the product increased the titer to 50 gL−1 (Baez et al. 2011). Isobutanol was successfully produced through the Keto-acid pathway in other microbes such as Saccharomyces cerevisiae (0.14–0.18 gL−1) (Chen et al. 2011; Kondo et al. 2012; Lee et al. 2012), Corynebacterium glutamicum (4.9–12.97 gL−1) (Smith et al. 2010; Blombach et al. 2011), Bacillus subtilis (2.62 gL−1) (Li et al. 2011a, b), Zymomonas mobilis (4.0 gL−1) (Qiu et al. 2020) from glucose as their main carbon source. The cyanobacterium Synechococcus elongatus PCC 7942 was first used for isobutanol production by overexpression of acetolactate synthase (AlsS), acetohydroxy acid isomeroreductase (IlvC), dihydroxy-acid dehydratase (IlvD), Kivd, and ADHs from different aerobic heterotrophic bacteria (Atsumi et al. 2009). This pathway has also been utilized for photosynthetic isobutanol production with the yield of 0.003–0.911 gL−1 in another model cyanobacteria Synechocystis sp. PCC 6803 (Varman et al. 2013a, b; Miao et al. 2017; Miao et al. 2018a, b; Miao et al. 2018a, b). Although isobutanol could be synthesized from cyanobacteria, the yield is low compared to the heterotrophs. Overexpression of kivd from L. lactis along with ADHs (adh from L. lactis, yqhD and yjgB from E.coli, and slr0942 and slr1192 from Synechocystis) produced isobutanol and 3-methyl-1-butanol (3M1B) in Synechocystis sp. PCC 6803 (Miao et al. 2017). 2-methyl-1-butanol was also produced autotrophically from genetically modified Synechococcus elongatus PCC7942 having Kivd, YqhD, and citramalate pathway genes (Fig. 2). The synthesis of other alcohols could be minimized here by designing the citramalate pathway so that the enzymes of the native isoleucine pathway could compete with the overexpressed Kivd and prevent direct 2-ketobutyrate conversion into 1-propanol (Shen and Liao 2012). All these studies show that Kivd is the key player for cyanobacterial butanol synthesis via the ketoacid pathway. Further, the modification of the substrate-binding pocket of Kivd (KivdS286T) showed its increased activity. It was also suggested that an elevated enzyme expression level will be required to enhance the isobutanol production (Miao et al. 2018a, b; Miao et al. 2018a, b). Protein engineering for fine-tuning of these two main enzymes can therefore increase the specificity and activity which will ultimately help in improving the isobutanol yield.
3.2.3 Engineering 2-oxoglutarate pathway
Butanol could be synthesized from α-ketoglutarate (2-oxoglutarate) of the TCA cycle and was done by expressing seven enzymes from different microorganisms in E. coli K12. This engineered E. coli strain accumulated 85 mgL−1 1-butanol from glucose in a bioreactor (Ferreira et al. 2019). In cyanobacteria, butanol production through this pathway will require the incorporation of seven genes involved in the enzymatic conversion of 2-oxoglutarate to 1-butanol. Since the modified Clostridial CoA-dependent pathway in cyanobacteria yielded low butanol, overexpression of the first four enzymes of the 2-oxoglutarate pathway in the engineered strains could possibly increase the yield. TCA cycle intermediates are always at constant levels during the cyanobacterial growth phase giving a constant flux through this pathway (Hendry et al. 2017). Unlike heterotrophic hosts, cyanobacteria will not depend on the availability of organic carbon sources, so continuous yield is possible with minimal nutrients.
3.2.4 Enhancing yield by genetic modulation
For increasing biofuel titer in cyanobacteria, rerouting the carbon flux can play a crucial role in improving the flow of carbon in the desired pathway (Dexter et al. 2015; Hendry et al. 2017). Competitive pathways of butanol production in cyanobacteria are those involved in the synthesis of storage compounds, glycogen and PHA, acting as important carbon sinks. Besides this, enzymes of acetate metabolism can convert acetyl CoA to acetate, and this can directly affect the overall carbon flux towards the butanol pathway. A study with ΔglgC strain of Synechocystis sp. PCC 6803, having no ADP-glucose pyrophosphorylase (AGPase), showed reduced photochemical efficiency and NADPH pool, late initiation of Calvin-Benson-Bassham (CBB) cycle, problems with cyclic and non-cyclic electron transport, less PQ pool, and inability to manage excess energy (Holland et al. 2016; Cano et al. 2018). The addition of a new sink in another strain, JU547, for ethylene synthesis showed stable photosynthetic metabolism with an increase in inorganic carbon demand indicated by the expression of more bicarbonate transporters, sbtA (Holland et al. 2016). Therefore, adverse changes in photosynthetic metabolism upon deletion of a native carbon sink actually make it difficult to predict the consequences on the cellular phenotype and product synthesis in such genetically engineered strains. For isobutanol production from Synechococcus elongatus PCC 7942, a glycogen mutant strain was developed to enhance the titer. Cell growth deficiency was observed in the ΔglgC strain as it lacked a major carbon sink. The glycogen mutant stains with the introduced isobutanol pathway showed increased isobutanol production from 22 to 52% due to the availability of surplus energy and carbon, while the new sink prevented the growth inhibition as the ΔglgC strain (Li et al. 2014).
In cyanobacteria, PHB synthase (PhaE and PhaC) catalyzes polyhydroxybutyrate (PHB) synthesis as a storage compound. Acetyl-CoA is required in both PHB and 1-butanol pathways, so disruption of PhaEC can increase the carbon flux towards the constructed butanol pathway. For 1-butanol production from Synechocystis sp. PCC 6803, PhaEC has to be disrupted and used successfully as genomic integration loci for heterologous gene expression (Liu et al. 2019). The acetyl-CoA can be converted to acetate, and the acetate metabolism requires Pta (phosphotransacetylase), AckA (acetate kinase), Ach (acetyl-CoA hydrolase), and Acs (acetyl-coenzymeA synthetase). For 1-butanol production from cyanobacteria, the strain with deleted ach site proved best compared to acs and pta sites, and retaining the native AckA-Acs pathway of acetate metabolism led to improvement in the titer due to increased acetyl-CoA from the conversion of acetate and acetyl-P (Liu et al. 2019). Disruption and utilization of the genes of acetate metabolism and PHB biosynthetic pathway as integration sites proved beneficial for strain modification and butanol synthesis.
For improvement of the carbon flow towards the synthetic pathway in the case of metabolic engineering, finding of rate-limiting step of the pathway is essential. Metabolomics strategy has been used widely for the identification of the rate-limiting steps. The aforesaid approach showed the reduction of butanoyl-CoA to butanal by the enzyme, CoA-acylating propionaldehyde dehydrogenase (PduP) of Salmonella enterica in the 1-butanol synthetic pathway of Synechococcus elongatus BUOHSE is a rate-limiting step (Noguchi et al. 2016). In an attempt to improve PduP activity in Synechococcus elongatus, replacement of original RBS with modified RBS proved more effective, and the enzyme activity increased to 1.4-fold in the new strain, DC7, thereby regenerating free CoA producing acetyl-CoA at elevated levels. Introduction of Acetyl-CoA carboxylase (ACCase) of Yarrowia lipolytica in DC7 increased butanol titer by utilizing the generated acetyl-CoA (Fathima et al. 2018a, b). Following the identification of rate-limiting steps, enzyme as well cofactor engineering strategies can definitely help increase the biobutanol in genetically modified cyanobacteria.
4 Present challenges and solutions for fourth-generation biobutanol production
4.1 Large-scale cell cultivation
The important factors for cyanobacterial growth are light, nutrients, and ambient temperature. For commercial-scale microalgal cultivation, open outdoor systems are mostly preferred, but this system is not suitable for volatile biochemical production. Closed systems are therefore required for cyanobacterial biobutanol/biofuel production. Low biomass density is the major problem faced with cyanobacterial cultivation, and self-shading is one of the main factors influencing cell growth as well as productivity (Myers et al. 1951; Qiang and Richmond 1994). One possible solution to this problem can be the dilution of cyanobacterial culture with simultaneous recovery of cells and biochemicals from the point of density/product-based inhibition. In a closed system, mixing is another factor that affects both nutrient and light availability. However, mixing is dependent on the cell type as some filamentous cyanobacteria are more prone to shear stress and cell disruption. This problem demands the designing of a specific photobioreactor depending on the cell morphology and desired product. Solubility of nutrients and gases is dependent on the temperature, and as microalgal growth requires adequate dissolved CO2 while demands efficient oxygen removal, the maintenance of optimal temperature becomes crucial (Borowitzka and Vonshak 2017). Photorespiration also causes problems during microalgal cultivation under low/high irradiance, elevated O2 levels, and reduced CO2 levels where the fixed carbon is converted to CO2, leading to low growth and productivity (Richmond 2004). Therefore, for mass cultivation, proper mixing becomes essential to reduce photorespiration and maximizing production.
4.2 Photobioreactor for Cultivation of cyanobacteria
The closed systems used for microalgal/cyanobacterial cultivation, photobioreactors (PBRs), are constructed from plates, tubes, or bags of glass or plastic (Javanmardian and Palsson 1991; Pulz 2001; Hsieh and Wu 2009a, b; Xu et al. 2009). This system takes care of water loss and contaminants as well as can prevent the escape of products such as bioethanol and biobutanol during the production process. PBRs of varying sizes, shapes, and types such as tubular, bubble column, stirred tank, bag-based, and flat-panel PBRs have been constructed for cyanobacterial cultivation (Liao et al. 2014; Mahesh et al. 2019). Considerable research has been done development and scaling-up of new PBRs based on the factors like light availability, culture mixing, CO2, and O2 mass transfer for economic production. Optimal light intensity is the foremost need for cyanobacterial cell growth, and for large-scale production, diurnal sun light is mostly used, but for providing constant light, LED would be required, which will increase the cost. Maintenance of optimum culture temperature in a PBR is also essential as the rise in culture temperature will not only affect the growth and productivity but may also cause product evaporation (Pembroke and Ryan 2020). In a PBR, proper aeration is required for mixing and mass transfer for maintaining nutrient availability and preventing sedimentation, clumping, and fouling (Huang et al. 2017). For large-scale photosynthetic biofuel production, designing an ideal PBR becomes essential for reducing the operational cost due to the above-mentioned factors. Although a good number of PBRs have been developed for growing microalgae/cyanobacteria having the intercellular product. To prevent cell growth inhibition of the biobutanol secreting cyanobacterial cells, product recovery is also needed once the optimal levels are reached. Therefore, there is a requirement for the addition of a butanol collection system to the PBR or by the use of membrane separation (pervaporation) (Wee et al. 2008). Presently only small-scale studies in flasks/bottles have been carried out for cyanobacterial biobutanol production (Table 3), and a lot of research is still required in this area for the construction of an ideal system for economic cultivation and recovery of biobutanol.
4.3 Genetic instability
A major obstacle with metabolic engineering of the cyanobacterial strains is related to changes in the heterologous genes or their regulation, leading to less or no product formation even after successful cloning and transformation. Such changes can be due to deleterious mutations, which result in no gene expression or affects its RNA/protein functionality inside the cell. Due to genetic instability, as suggested, productivity might be affected where it must be a general problem but is not frequently reported as failed outcomes are not investigated (Jones 2014). A study with Synechococcus elongatus PCC 7942, having a gene for ethylene forming enzyme (efe) and capable of ethylene formation, appeared unhealthy yellow-green. The healthy green colonies appeared from the original culture, which lost the capability of ethylene production and had a truncated efe gene (Takahama et al. 2003). In Synechocystis sp. PCC 6803, the efe gene-containing plasmid expression system, showed no genetic instability (Guerrero et al. 2012). Similar examples of genetic instability were observed with mannitol and lactic-acid-producing cyanobacterial strains. The former showed the inability of complete segregation and losing productivity, whereas the latter reverted to the growing rate of wild-type strain from slow-growing lactic-acid producing strain (Angermayr et al. 2012; Jacobsen and Frigaard 2014). In the isopropanol-producing strain of S. elongatus PCC 7942, a single mutation reduced atoB functionality and enzyme activity, affecting isopropanol production (Kusakabe et al. 2013). The reason for genetic instability in cyanobacteria is not yet understood, but an expression of the heterologous pathway involved in metabolite production might be posing stress causing the cells to mutate or lose the foreign genes and revert to wild-type for having normal metabolism. Polyploidy can be a major factor in genetic instability. Genetic engineering of cyanobacteria to synthesize suitable products is mostly carried out by integrating the heterologous genes into the chromosome via recombination (Dexter and Fu 2009; da Silva et al. 2018; Pembroke et al. 2019). Due to the polyploid genome, extensive selection is required to ensure the complete segregation of the desired construct to get the stable product synthesizing recombinant. Reduction of chromosome copy number during the later stages of growth can also lead to selection of non-biofuel producers and thus making the complete process of strain modification futile.
However, in the case of polyploid genomes in cyanobacteria, the chromosomal integration of expression cassette can provide an additional advantage of a gene dosage effect. More copies of the heterologous genes can result in elevated enzyme expression with a suitable promoter and increased biofuel synthesis (Pembroke and Ryan 2020). Integration of two copies of ethanol synthesis cassette in the Synechocystis sp. PCC 6803 yielded 5.50 gL−1 (Gao et al. 2012), which is higher than ethanol-producing cyanobacterial strain having only a single cassette (Woods et al. 2004). Considering bioethanol production from cyanobacteria, chromosomal integration and high dosage of cassette systems significantly increased the productivity. For biobutanol production in cyanobacteria, integration of the cassettes is generally done in the one or more neutral sites present in the chromosome (Lan and Liao 2011). Integration of the cassettes directly into the functional gene sites also aided gene disruption for blocking competitive pathways and increasing yield (Liu et al. 2019). Although there are no reports of genetic instability for cyanobacterial biobutanol production, further investigation is required in this direction, considering low butanol titers compared to heterotrophs despite having a complete synthetic pathway.
4.4 Cofactor discrepancy
Cofactor imbalance in the cells can cause low productivity as it causes metabolic pressure on the cells. The cofactor availability depends on the need for host cell metabolism, and overexpression of heterologous genes can cause a shortage of these cofactors. Therefore, cofactor engineering strategies have been developed for balancing the cofactor requirements for improving the growth and product yield of the host organisms (Chen et al. 2014a, b). ATP, FAD, CoA, NADPH/NADP, and various metals are essential cofactors of the enzymes of the biological pathway, which significantly affect the functioning of the enzymes and pathways (Wang et al. 2013; Akhtar and Jones 2014). NADPH and NADH are the mainly targeted cofactors in cyanobacteria for engineering as these photosynthetic microorganisms have more NADPH than NADH (Cooley and Vermaas 2001; Tamoi et al. 2005). As reducing equivalents, availability and balance of NADPH/NADH are needed for the functioning of the dependent heterologous enzymes of the synthetic pathway. Therefore, the genes of NADH-dependent enzymes from heterotrophic organisms show reduced activities in cyanobacteria, thereby affecting the yield. For biobutanol production, the approach taken for solving the issue of NADPH/NADH imbalance is the utilization of NADPH-dependent enzymes in the synthetic pathway (Atsumi et al. 2009; Lan and Liao 2012). Two other approaches such as increasing NADH inside the cell and enzyme modification for changing the co-factor preferences from NADH to NADPH, can also be considered for biobutanol production. For D-lactate and L-lactate production from cyanobacteria, the co-expression of soluble transhydrogenase (sth) from Pseudomonas aeruginosa for NADH production along with the NADH-dependent pathway enzymes improved product yield (Angermayr et al. 2012; Varman et al. 2013a, b). Enzyme modification in the case of lactate production by site-directed mutations led to a change of cofactor preferences from NADH to NADPH (Richter et al. 2011; Li et al. 2015). The mutated codon-optimized lactate dehydrogenase overexpression in cyanobacteria showed better NADPH utilization and increased lactate production (Angermayr et al. 2014; Li et al. 2015). Although for cyanobacterial butanol production, NADH-depended enzymes were replaced by NADPH-dependent ones due to the availability of the copious NADPH pool. But approaches of co-expression of transhydrogenases and enzyme engineering can also be explored to overcome the co-factor imbalance and increase butanol titer in cyanobacteria.
4.5 Butanol toxicity
Butanol is toxic to both native and non-native butanol producers, which becomes a limiting factor during the production process. The tolerance limit of non-native hosts is even lower than the native producers, such as Clostridium (Jin et al. 2014), and the development of a solvent tolerant strain is a challenging task (Behera et al. 2018). In the native butanol producer, Clostridium acetobutylicum, overexpression of the spo0A gene increased the tolerance to butanol (Alsaker et al. 2004). Expression of a global regulator, IrrE, with a random mutation in E. coli increased biofuel tolerance. In E. coli, an increased growth rate in the presence of 1.2% butanol was obtained with random mutagenesis of transcription factor cyclic AMP receptor protein (CRP) (Zhang et al. 2012). The low titer in cyanobacterial biobutanol production may be due to several factors related to gene expression, functioning and stability of pathway enzymes, and product recovery. However, butanol toxicity is a crucial barrier to overcome for increasing the butanol titer (Jin et al. 2014) (Nicolaou et al. 2010). Compared to other microbial strains used for biobutanol production, the level of tolerance observed in Synechocystis sp. PCC 6803 is 10 times lower (Nicolaou et al. 2010). Solvents are known to adverse effects on the microbial cell membrane and also alter membrane fluidity (Huffer et al. 2011), causing cellular metabolite loss and reactive oxygen species (ROS) generation during respiration to compensate for ATP loss (Trinh et al. 2010). In cyanobacteria, besides impairing membrane and the related processes, the electron transport chain of photosynthesis is also affected (Horváth et al. 2012). For understanding the mechanism of butanol stress on cyanobacteria, studies conducted at transcriptomic, proteomic, and metabolomic levels revealed that in Synechocystis, a combination of numerous cellular metabolic changes occur for preventing solvent toxicity (Anfelt et al. 2013; Tian et al. 2013; Zhu et al. 2013). Identification of butanol-responsive targets through transcriptomics followed by genetic engineering and overexpression of heat-shock protein and ROS-scavenging enzymes has been carried out for increasing the tolerance in cyanobacteria, Synechocystis sp. PCC 6803 (Anfelt et al. 2013). Another strategy for improving butanol tolerance targeted the overexpression and deletion of several regulatory genes in Synechocystis. Butanol tolerance was improved in the strains having overexpressed RNA polymerase sigma factor SigB compared to the wild-type strains. A reduction in ROS accumulation with the increase in temperature and butanol tolerance also improved cyanobacterial cell viability and growth in the presence of butanol (Kaczmarzyk et al. 2014). Another regulatory protein, Slr1037 of Synechocystis found to be related to the butanol tolerance mechanism. This slr1037 gene was found to be associated with multiple cellular functions, while in cyanobacteria, such regulatory protein can serve as crucial targets for solvent tolerance and titer improvement (Chen et al. 2014a, b). In Synechocystis, an attempt made for increasing butanol tolerance through experimental evolution under butanol selection pressure was successful. In the process of 395 days (94 passages), the tolerance to butanol increased to 150% compared to wild-type (Wang et al. 2014). Therefore, developing solvent tolerant high butanol yielding cyanobacterial strains demands more research as solvent stress brings about various critical cellular changes.
4.6 Product recovery
Distillation is mainly preferred in the industrial-scale recovery process due to its ease of scale-up, high efficiency, and high concentration factors. However, a low concentration of butanol during production and its high boiling point, along with the presence of other products of fermentation, make the recovery process an energy-intensive one (Hartmanis and Gatenbeck 1984; Kim et al. 1984; Seedorf et al. 2008). Compared to heterotrophs, butanol titers from cyanobacteria are very low to date, and further butanol concentrations of 0.75 gL−1 and above have also shown cell inhibition (Anfelt et al. 2013; Lan et al. 2013). Although efforts have been made to increase the butanol tolerance and discussed in previous section, product recovery at concentrations below the toxicity level can also be an alternative. The butanol recovery from cyanobacterial cultivation becomes an energy-requiring process mainly due to the low butanol titer in the medium, making recovery costlier than the production process (Wagner et al. 2019). Many relatively economical and feasible separation techniques have been developed over time for in-situ butanol removal, namely, gas stripping, vacuum stripping, pervaporation, liquid–liquid extraction, perstraction, and adsorption (Huang et al. 2015; Outram et al. 2017; Xue et al. 2017). In-situ product recovery can eliminate toxicity and aid productivity, glucose conversion, and product concentration (Sharif Rohani et al. 2015; Sarchami et al. 2016). Most of the comparison studies for product recovery techniques are done for heterotrophic ABE fermentation. But cyanobacterial biobutanol production is different from ABE fermentation as only butanol is produced by genetically engineered cyanobacteria instead of the solvent mixture as in ABE fermentation. The two major problems with cyanobacterial butanol production are the low yield along with high toxicity even at considerably lower concentrations. As a solution to these problems, continuous butanol separation is required during the cultivation process to prevent the accumulation of butanol in the culture broth. However, the high energy requirement for continuous butanol separation can easily exceed the energy content of the synthesized fuel and making the production process industrially unfeasible. A recent study where butanol recovery was modelled considering cyanobacterial biobutanol production using technologies such as gas-stripping, distillation, pervaporation and ionic liquid extraction calculated the culture butanol concentration for making the process economical. Ionic liquid extraction method was found to be the most effective at 3.7 gL−1 butanol which was followed by distillation (9.3 gL−1) (Wagner et al. 2019). More research is therefore needed in this area for selecting the most efficient recovery technique and utilization of a combined cultivation and recovery technology for economic butanol production.
5 The future of cyanobacteria-based butanol production
Butanol is considered one of the finest biofuels having extraordinary properties similar to gasoline. With the increasing demand for butanol in industries as a chemical substituent, more research is being carried out for economic biobutanol production from renewable and sustainable biomass. Some companies have developed butanol fermentation processes at a commercial scale (Mariano et al. 2009). The major hurdle for commercial biobutanol production is the low productivity due to solvent toxicity, thereby requiring distillation for recovery from dilute streams making the process uneconomical compared to synthesis through petrochemical routes. The cost of feedstock and its price fluctuation in the case of first-generation butanol production also affects the product cost (Green 2011). The utilization of lignocellulosic biomass as feedstock will be cheaper, but the inhibitor removal process also adds to the cost (Qureshi et al. 2007; Taylor 2008). However, in the case of third/fourth generation biofuel production, the abundantly available solar energy and greenhouse gas, CO2 can be directly converted to biofuel, which seems to be economical but is still in its infancy. The present studies on biobutanol production from cyanobacteria/microalgae have shown that titers are even lower than the heterotrophic butanol producers. Although the production cost will be lowered due to the requirement of cheap raw material for growth, the major obstacle with biobutanol synthesis using photoautotrophic microorganisms is related to their slow growth rate, low biomass and product yield, and higher product toxicity. Recent development in synthetic biology tools has helped in the domestication of potential cyanobacterial strains as well as development of the model strains for improved butanol synthesis. The problem of poor product yield has to be solved by the utilization of fast-growing, high biomass yielding strains which are to be followed by the optimization of process parameters for economic photosynthetic butanol production (Fig. 3). Further, cyanobacterial cultivation coupled with wastewater treatment will reduce the production cost together with the removal of organic/inorganic pollutants from water. Recently, studies have been carried out to utilize industrial flue gas for economically feasible cyanobacteria-based chemical synthesis (Choi et al. 2020; Chou et al. 2021). Photosynthetic butanol production by sequestration of CO2 from flue gas can also help to reduce production costs. Conversion of cyanobacterial biomass into useful products such as propylene and bio-oil through hydrothermal liquefaction (HTL) as byproduct or ethanol/butanol through fermentation using heterotrophs followed by enzymatic/acid pretreatment has also been done (Efremenko et al. 2012; Möllers et al. 2014; Wagner et al. 2016) after getting extracellular butanol. Utilization of co-culture in the production process can provide a scope for direct conversion of renewable sources into biobutanol. In a recent study with a synthetic photoautotrophic co-culture system showed that a mutant acetate secreting Synechococcus sp. PCC 7002 could support the growth and lipid accumulation in Chlamydomonas reinhardtii (Therien et al. 2014). Although researchers developed different butanol-producing cyanobacterial strains, but the yield is still very low compared to heterotrophs. Researchers can target cyanobacterial metabolic pathways for secretion of carbon sources into the cultivation media. Genetically modified cyanobacteria capable of secreting carbon sources autotrophically in large amounts can be co-cultured with high yielding heterotrophic butanol producers for an economic production process. On the contrary, co-cultivation of photoautotrophs and heterotrophs for large-scale butanol production will demand adequate sophisticated control algorithm and process optimization. However, this technology of mixed culture-based biofuel synthesis is still in its infancy, and more research is required for use in large-scale butanol production. From the present studies, it is clear that the production of biobutanol from cyanobacteria is not feasible at an industrial scale based on current available knowledge, but improvement in process parameters with the aforementioned strategies and integration with biorefinery can definitely help to make the whole process economical.
Schematic diagram representing butanol production process from cyanobacteria. The steps required for large-scale production of butanol are strain development/improvement, cultivation in closed systems (PBRs) with simultaneous recovery of butanol. Here, ‘OR’ signifies the choice of any of the routes for the butanol recovery during the downstream processing
6 Conclusion
Biobutanol production using cyanobacterial cells is an advanced approach toward sustainability. In this article, various aspects of photosynthetic butanol synthesis from cyanobacteria have been reviewed.
The summary of the key findings of the review are:
-
Butanol has applications in chemical manufacturing industries and can be used directly as fuel, but for this, large-scale and cost-effective butanol production is of the utmost need
-
Cyanobacteria can serve as an alternative attractive non-native host for biofuel production
-
Advances in synthetic biology of cyanobacteria with the development of toolkits and genetic modification approaches will provide new and more straightforward strategies for strain improvement
-
Fourth-generation biobutanol production is a one-stage process which is advantageous over the third-generation two-stage production process
-
Improvement of product yield demands further genetic modulations together with the incorporation of butanol biosynthetic pathway
-
As mentioned previously, additional factors such as product toxicity, PBR design, etc., influence the product synthesis from genetically modified strains and should be dealt with to maximize the biobutanol yield.
There are still many technical and biological challenges to be overcome before scaling up cyanobacterial biobutanol production. The amount of butanol obtained from genetically engineered cyanobacteria under photoautotrophic conditions is, however, very low compared to the traditional ABE fermentation process. Further research and developments in the tools and methodologies of synthetic biology in cyanobacteria can help the utilization of thermophilic, fast-growing strains and butanol tolerant cyanobacteria for constructing more robust strains needed for industrial-scale butanol production. Genome scale metabolic model can be used as tool for assisting cyanobacterial engineering by identifying the rate-limiting steps of the biosynthetic pathway responsible for the low yield. This knowledge can help for improving butanol yield by enzyme overexpression or protein and cofactor engineering. Minimization of the loss of photosynthetically fixed carbon can also help increase the yield. The native pathway genes which are involved in carbon loss can be suppressed or deleted for increasing the flow of carbon in biosynthetic pathway. In situ product recovery coupled with autotrophic production process can also improve the yield by reducing the product toxicity. Besides these, more research for optimization of process parameters and development of mass cultivations systems and recovery strategies is still required for industrial-scale cyanobacterial biobutanol production.
References
Aikawa S, Joseph A, Yamada R, Izumi Y, Yamagishi T, Matsuda F, Kawai H, Chang J-S, Hasunuma T, Kondo A (2013) Direct conversion of Spirulina to ethanol without pretreatment or enzymatic hydrolysis processes. Energy Environ Sci 6(6):1844–1849. https://doi.org/10.1039/C3EE40305J
Akhtar MK, Jones PR (2014) Cofactor engineering for enhancing the flux of metabolic pathways. Front Bioeng Biotechnol 2:30. https://doi.org/10.3389/fbioe.2014.00030
Alsaker KV, Spitzer TR, Papoutsakis ET (2004) Transcriptional analysis of spo0A overexpression in Clostridium acetobutylicum and its effect on the cell’s response to butanol stress. J Bacteriol 186(7):1959–1971. https://doi.org/10.1128/JB.186.7.1959-1971.2004
Anfelt J, Hallström B, Nielsen J, Uhlén M, Hudson EP (2013) Using transcriptomics to improve butanol tolerance of Synechocystis sp. strain PCC 6803. Appl Environ Microbiol 79(23):7419–7427. https://doi.org/10.1128/AEM.02694-13
Anfelt J, Kaczmarzyk D, Shabestary K, Renberg B, Rockberg J, Nielsen J, Uhlén M, Hudson EP (2015) Genetic and nutrient modulation of acetyl-CoA levels in Synechocystis for n-butanol production. Microb Cell Factor 14(1):1–12. https://doi.org/10.1186/s12934-015-0355-9
Angermayr SA, Paszota M, Hellingwerf KJ (2012) Engineering a cyanobacterial cell factory for production of lactic acid. Appl Environ Microbiol 78(19):7098–7106. https://doi.org/10.1128/AEM.01587-12
Angermayr SA, Van der Woude AD, Correddu D, Vreugdenhil A, Verrone V, Hellingwerf KJ (2014) Exploring metabolic engineering design principles for the photosynthetic production of lactic acid by Synechocystis sp. PCC6803. Biotechnol Biofuels 7(1):1–15. https://doi.org/10.1186/1754-6834-7-99
Argueta C, Yuksek K, Summers M (2004) Construction and use of GFP reporter vectors for analysis of cell-type-specific gene expression in Nostoc punctiforme. J Microbiol Methods 59(2):181–188. https://doi.org/10.1016/j.mimet.2004.06.009
Arias DM, Ortíz-Sánchez E, Okoye PU, Rodríguez-Rangel H, Ortega AB, Longoria A, Domínguez-Espíndola R, Sebastian P (2021) A review on cyanobacteria cultivation for carbohydrate-based biofuels: cultivation aspects, polysaccharides accumulation strategies, and biofuels production scenarios. Sci Total Environ 794:148636. https://doi.org/10.1016/j.scitotenv.2021.148636
Armshaw P, Carey D, Sheahan C, Pembroke JT (2015) Utilising the native plasmid, pCA2. 4, from the cyanobacterium Synechocystis sp. strain PCC6803 as a cloning site for enhanced product production. Biotechnol Biofuels 8(1):1–10. https://doi.org/10.1186/s13068-015-0385-x
Atsumi S, Hanai T, Liao JC (2008) Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451(7174):86–89. https://doi.org/10.1038/nature06450
Atsumi S, Higashide W, Liao JC (2009) Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat Biotechnol 27(12):1177–1180. https://doi.org/10.1038/nbt.1586
Azov Y, Goldman JC (1982) Free ammonia inhibition of algal photosynthesis in intensive cultures. Appl Environ Microbiol 43(4):735–739. https://doi.org/10.1128/aem.43.4.735-739.1982
Baez A, Cho K-M, Liao JC (2011) High-flux isobutanol production using engineered Escherichia coli: a bioreactor study with in situ product removal. Appl Microbiol Biotechnol 90(5):1681–1690. https://doi.org/10.1007/s00253-011-3173-y
Ball S, Colleoni C, Cenci U, Raj JN, Tirtiaux C (2011) The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J Exp Bot 62(6):1775–1801. https://doi.org/10.1093/jxb/erq411
Becker EW (1994) Microalgae: biotechnology and microbiology. Cambridge University Press, Cambridge
Behera S, Sharma NK, Kumar S (2018) Prospects of solvent tolerance in butanol fermenting bacteria. In: Kumar S, Sani RK (eds) Biorefining of biomass to biofuels. Springer, pp. 249–264
Behler J, Vijay D, Hess WR, Akhtar MK (2018) CRISPR-based technologies for metabolic engineering in cyanobacteria. Trends Biotechnol 36(10):996–1010. https://doi.org/10.1016/j.tibtech.2018.05.011
Berla BM, Saha R, Immethun CM, Maranas CD, Moon TS, Pakrasi H (2013) Synthetic biology of cyanobacteria: unique challenges and opportunities. Front Microbiol 4:246. https://doi.org/10.3389/fmicb.2013.00246
Berto P, D’Adamo S, Bergantino E, Vallese F, Giacometti GM, Costantini P (2011) The cyanobacterium Synechocystis sp. PCC 6803 is able to express an active [FeFe]-hydrogenase without additional maturation proteins. Biochem Biophys Res Commun 405(4):678–683. https://doi.org/10.1016/j.bbrc.2011.01.095
Blasi B, Peca L, Vass I, Kós PB (2012) Characterization of stress responses of heavy metal and metalloid inducible promoters in Synechocystis PCC6803. J Microbiol Biotechnol 22(2):166–169. https://doi.org/10.4014/jmb.1106.06050
Blombach B, Riester T, Wieschalka S, Ziert C, Youn J-W, Wendisch VF, Eikmanns BJ (2011) Corynebacterium glutamicum tailored for efficient isobutanol production. Appl Environ Microbiol 77(10):3300–3310. https://doi.org/10.1128/AEM.02972-10
Borowitzka M (2008) Marine and halophilic algae for the production of biofuels. J Biotechnol. https://doi.org/10.1016/j.jbiotec.2008.07.1793
Borowitzka MA, Vonshak A (2017) Scaling up microalgal cultures to commercial scale. Eur J Phycol 52(4):407–418. https://doi.org/10.1080/09670262.2017.1365177
Boussiba S (1989) Ammonia uptake in the alkalophilic cyanobacterium Spirulina platensis. Plant Cell Physiol 30(2):303–308. https://doi.org/10.1093/oxfordjournals.pcp.a077743
Boussiba S, Gibson J (1991) Ammonia translocation in cyanobacteria. FEMS Microbiol Rev 8(1):1–14. https://doi.org/10.1111/j.1574-6968.1991.tb04953.x
Boyanapalli R, Bullerjahn GS, Pohl C, Croot PL, Boyd PW, McKay RML (2007) Luminescent whole-cell cyanobacterial bioreporter for measuring Fe availability in diverse marine environments. Appl Environ Microbiol 73(3):1019–1024. https://doi.org/10.1128/AEM.01670-06
Brown LM (1996) Uptake of carbon dioxide from flue gas by microalgae. Energy Convers Manag 37(6–8):1363–1367. https://doi.org/10.1016/0196-8904(95)00347-9
Brownstein AM (2014) Renewable motor fuels: the past, the present and the uncertain future. Butterworth-Heinemann, Oxford
Camiro-Vargas TK, Hernández-Ayón JM, Valenzuela-Espinoza E, Delgadillo-Hinojosa F, Cajal-Medrano R (2005) Dissolved inorganic carbon uptake by Rhodomonas sp. and Isochrysis aff. galbana determined by a potentiometric technique. Aquac Eng 33(2):83–95. https://doi.org/10.1016/j.aquaeng.2004.10.001
Cano M, Holland SC, Artier J, Burnap RL, Ghirardi M, Morgan JA, Yu J (2018) Glycogen synthesis and metabolite overflow contribute to energy balancing in cyanobacteria. Cell Rep 23(3):667–672. https://doi.org/10.1016/j.celrep.2018.03.083
Cao Y-Q, Li Q, Xia P-F, Wei L-J, Guo N, Li J-W, Wang S-G (2017) AraBAD based toolkit for gene expression and metabolic robustness improvement in Synechococcus elongatus. Sci Rep 7(1):1–10. https://doi.org/10.1038/s41598-017-17035-4
Chen F, Zhang Y (1997) High cell density mixotrophic culture of Spirulina platensis on glucose for phycocyanin production using a fed-batch system. Enzyme Microb Technol 20(3):221–224. https://doi.org/10.1016/S0141-0229(96)00116-0
Chen H, Bjerknes M, Kumar R, Jay E (1994) Determination of the optimal aligned spacing between the Shine-Dalgarno sequence and the translation initiation codon of Escherichia coli m RNAs. Nucleic Acids Res 22(23):4953–4957. https://doi.org/10.1093/nar/22.23.4953
Chen P, Min M, Chen Y, Wang L, Li Y, Chen Q, Wang C, Wan Y, Wang X, Cheng Y (2010) Review of biological and engineering aspects of algae to fuels approach. Int J Agric Biol Eng 2(4):1–30. https://doi.org/10.3965/j.issn.1934-6344.2009.04.001-030
Chen X, Nielsen KF, Borodina I, Kielland-Brandt MC, Karhumaa K (2011) Increased isobutanol production in Saccharomyces cerevisiae by overexpression of genes in valine metabolism. Biotechnol Biofuels 4(1):21. https://doi.org/10.1186/1754-6834-4-21
Chen AH, Afonso B, Silver PA, Savage DF (2012) Spatial and temporal organization of chromosome duplication and segregation in the cyanobacterium Synechococcus elongatus PCC 7942. PLoS One 7(10):e47837. https://doi.org/10.1371/journal.pone.0047837
Chen L, Wu L, Wang J, Zhang W (2014) Butanol tolerance regulated by a two-component response regulator Slr1037 in photosynthetic Synechocystis sp. PCC 6803. Biotechnol. Biofuels 7(1):1–13. https://doi.org/10.1186/1754-6834-7-89
Chen X, Li S, Liu L (2014b) Engineering redox balance through cofactor systems. Trends Biotechnol 32(6):337–343. https://doi.org/10.1016/j.tibtech.2014.04.003
Chen H, Zhao J, Hu T, Zhao X, Liu D (2015) A comparison of several organosolv pretreatments for improving the enzymatic hydrolysis of wheat straw: substrate digestibility, fermentability and structural features. Appl Energy 150:224–232. https://doi.org/10.1016/j.apenergy.2015.04.030
Choi SY, Woo HM (2020) CRISPRi-dCas12a: a dCas12a-mediated CRISPR interference for repression of multiple genes and metabolic engineering in cyanobacteria. ACS Synth Biol 9(9):2351–2361. https://doi.org/10.1021/acssynbio.0c00091
Choi SY, Sim SJ, Ko SC, Son J, Lee JS, Lee HJ, Chang WS, Woo HM (2020) Scalable cultivation of engineered cyanobacteria for squalene production from industrial flue gas in a closed photobioreactor. J Agric Food Chem 68(37):10050–10055. https://doi.org/10.1021/acs.jafc.0c03133
Chojnacka K, Marquez-Rocha F-J (2004) Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 3(1):21–34. https://doi.org/10.3923/biotech.2004.21.34
Chou H-H, Su H-Y, Chow T-J, Lee T-M, Cheng W-H, Chang J-S, Chen H-J (2021) Engineering cyanobacteria with enhanced growth in simulated flue gases for high-yield bioethanol production. Biochem Eng J 165:107823. https://doi.org/10.1016/j.bej.2020.107823
Cooley JW, Vermaas WF (2001) Succinate dehydrogenase and other respiratory pathways in thylakoid membranes of Synechocystis sp. strain PCC 6803: capacity comparisons and physiological function. J Bacteriol 183(14):4251–4258. https://doi.org/10.1128/JB.183.14.4251-4258.2001
Cui J, Sun T, Li S, Xie Y, Song X, Wang F, Chen L, Zhang W (2020) Improved salt tolerance and metabolomics analysis of Synechococcus elongatus UTEX 2973 by overexpressing Mrp Antiporters. Front Bioeng Biotechnol 8:500. https://doi.org/10.3389/fbioe.2020.00500
da Silva TL, Passarinho PC, Galriça R, Zenóglio A, Armshaw P, Pembroke JT, Sheahan C, Reis A, Gírio F (2018) Evaluation of the ethanol tolerance for wild and mutant Synechocystis strains by flow cytometry. Biotechnol Rep 17:137–147. https://doi.org/10.1016/j.btre.2018.02.005
de Smit MH, Van Duin J (1990) Secondary structure of the ribosome binding site determines translational efficiency: a quantitative analysis. Proc Natl Acad Sci 87(19):7668–7672. https://doi.org/10.1073/pnas.87.19.7668
Deng M-D, Coleman JR (1999) Ethanol synthesis by genetic engineering in cyanobacteria. Appl Environ Microbiol 65(2):523–528. https://doi.org/10.1128/AEM.65.2.523-528.1999
Desplancq D, Bernard C, Sibler A-P, Kieffer B, Miguet L, Potier N, Van Dorsselaer A, Weiss E (2005) Combining inducible protein overexpression with NMR-grade triple isotope labeling in the cyanobacterium Anabaena sp. PCC 7120. Biotechniques 39(3):405–411. https://doi.org/10.2144/05393RR02
Dexter J, Fu P (2009) Metabolic engineering of cyanobacteria for ethanol production. Energy Environ Sci 2(8):857–864. https://doi.org/10.1039/B811937F
Dexter J, Armshaw P, Sheahan C, Pembroke J (2015) The state of autotrophic ethanol production in Cyanobacteria. J Appl Microbiol 119(1):11–24. https://doi.org/10.1111/jam.12821
Dismukes GC, Carrieri D, Bennette N, Ananyev GM, Posewitz MC (2008) Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr Opin Biotechnol 19(3):235–240. https://doi.org/10.1016/j.copbio.2008.05.007
DS D (2011) eLS. John Wiley & Sons, Chichester
Dürre P (2007) Biobutanol: an attractive biofuel. Biotechnol J Healthc Nutr Technol 2(12):1525–1534. https://doi.org/10.1002/biot.200700168
Eaton-Rye JJ (2004). The construction of gene knockouts in the cyanobacterium Synechocystis sp. PCC 6803. In: Photosynthesis research protocols. Springer, pp. 309–324
Efremenko E, Nikolskaya A, Lyagin I, Senko O, Makhlis T, Stepanov N, Maslova O, Mamedova F, Varfolomeev S (2012) Production of biofuels from pretreated microalgae biomass by anaerobic fermentation with immobilized Clostridium acetobutylicum cells. Bioresour Technol 114:342–348. https://doi.org/10.1016/j.biortech.2012.03.049
Ellis JT, Hengge NN, Sims RC, Miller CD (2012) Acetone, butanol, and ethanol production from wastewater algae. Biores Technol 111:491–495. https://doi.org/10.1016/j.biortech.2012.02.002
Englund E, Andersen-Ranberg J, Miao R, Hamberger B, Lindberg P (2015) Metabolic engineering of Synechocystis sp. PCC 6803 for production of the plant diterpenoid manoyl oxide. ACS Synth Biol 4(12):1270–1278. https://doi.org/10.1021/acssynbio.5b00070
Englund E, Liang F, Lindberg P (2016) Evaluation of promoters and ribosome binding sites for biotechnological applications in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 6(1):1–12. https://doi.org/10.1038/srep36640
Eriksson J, Salih GF, Ghebramedhin H, Jansson C (2000) Deletion mutagenesis of the 5′ psbA2 region in Synechocystis 6803: identification of a putative cis element involved in photoregulation. Molecular Cell Biology Research Communications 3(5):292–298. https://doi.org/10.1006/mcbr.2000.0227
Fathima AM, Chuang D, Laviña WA, Liao J, Putri SP, Fukusaki E (2018a) Iterative cycle of widely targeted metabolic profiling for the improvement of 1-butanol titer and productivity in Synechococcus elongatus. Biotechnol Biofuels 11(1):188. https://doi.org/10.1186/s13068-018-1187-8
Fathima AM, Chuang D, Laviña WA, Liao J, Putri SP, Fukusaki E (2018b) Iterative cycle of widely targeted metabolic profiling for the improvement of 1-butanol titer and productivity in Synechococcus elongatus. Biotechnol Biofuels 11(1):1–12
Ferreira S, Pereira R, Liu F, Vilaça P, Rocha I (2019) Discovery and implementation of a novel pathway for n-butanol production via 2-oxoglutarate. Biotechnol Biofuels 12(1):1–14. https://doi.org/10.1186/s13068-019-1565-x
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281(5374):237–240. https://doi.org/10.1126/science.281.5374.237
Flores E, Muro-Pastor AM, Meeks JC (2008) Gene transfer to cyanobacteria in the laboratory and in nature. In: The cyanobacteria: molecular biology, genomics and evolution, pp. 45–57.
Flores E, Herrero A (2005) Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem Soc Trans 33(1):164–167. https://doi.org/10.1042/BST0330164
Fogg GE, Thake B (1987) Algal cultures and phytoplankton ecology. Univ of Wisconsin Press, Madison
Gale GA, Schiavon Osorio AA, Mills LA, Wang B, Lea-Smith DJ, McCormick AJ (2019) Emerging species and genome editing tools: future prospects in cyanobacterial synthetic biology. Microorganisms 7(10):409. https://doi.org/10.3390/microorganisms7100409
Gallon J (2001) N2 fixation in phototrophs: adaptation to a specialized way of life. Plant Soil 230(1):39–48. https://doi.org/10.1023/A:1004640219659
Gao Z, Zhao H, Li Z, Tan X, Lu X (2012) Photosynthetic production of ethanol from carbon dioxide in genetically engineered cyanobacteria. Energy Environ Sci 5(12):9857–9865. https://doi.org/10.1039/C2EE22675H
Gebremedhin M, Mishra S, Mohanty K (2018) Augmentation of native microalgae based biofuel production through statistical optimization of campus sewage wastewater as low-cost growth media. J Environ Chem Eng 6(5):6623–6632. https://doi.org/10.1016/j.jece.2018.08.061
Geerts D, Bovy A, de Vrieze G, Borrias M, Weisbeek P (1995) Inducible expression of heterologous genes targeted to a chromosomal platform in the cyanobacterium Synechococcus sp. PCC 7942. Microbiology 141(4):831–841. https://doi.org/10.1099/13500872-141-4-831
Golden SS, Brusslan J, Haselkorn R (1987) [12] Genetic engineering of the cyanobacterial chromosome. Methods Enzymol 153:215–231. https://doi.org/10.1016/0076-6879(87)53055-5
González A, Bes MT, Barja F, Peleato ML, Fillat MF (2010) Overexpression of FurA in Anabaena sp. PCC 7120 reveals new targets for this regulator involved in photosynthesis, iron uptake and cellular morphology. Plant Cell Physiol 51(11):1900–1914. https://doi.org/10.1093/pcp/pcq148.
Green EM (2011) Fermentative production of butanol—the industrial perspective. Curr Opin Biotechnol 22(3):337–343. https://doi.org/10.1016/j.copbio.2011.02.004
Griese M, Lange C, Soppa J (2011) Ploidy in cyanobacteria. FEMS Microbiol Lett 323(2):124–131. https://doi.org/10.1111/j.1574-6968.2011.02368.x
Grobbelaar JU (2004) Algal nutrition: mineral nutrition. Handb Microalgal Culture Biotechnol Appl Phycol. https://doi.org/10.1002/9780470995280.ch6
Großkopf T, LaRoche J (2012) Direct and indirect costs of dinitrogen fixation in Crocosphaera watsonii WH8501 and possible implications for the nitrogen cycle. Front Microbiol 3:236. https://doi.org/10.3389/fmicb.2012.00236
Guerrero F, Carbonell V, Cossu M, Correddu D, Jones PR (2012) Ethylene synthesis and regulated expression of recombinant protein in Synechocystis sp. PCC 6803. PloS one 7(11):e50470. https://doi.org/10.1371/journal.pone.0050470
Hartmanis MG, Gatenbeck S (1984) Intermediary metabolism in Clostridium acetobutylicum: levels of enzymes involved in the formation of acetate and butyrate. Appl Environ Microbiol 47(6):1277–1283. https://doi.org/10.1128/aem.47.6.1277-1283.1984
Hedges SB, Chen H, Kumar S, Wang DY, Thompson AS, Watanabe H (2001) A genomic timescale for the origin of eukaryotes. BMC Evol Biol 1(1):4. https://doi.org/10.1186/1471-2148-1-4
Heidorn T, Camsund D, Huang H-H, Lindberg P, Oliveira P, Stensjö K, Lindblad P (2011a) Synthetic biology in cyanobacteria: engineering and analyzing novel functions. Methods Enzymol 497:539–579
Heidorn T, Camsund D, Huang H-H, Lindberg P, Oliveira P, Stensjö K, Lindblad P (2011b) Synthetic biology in cyanobacteria: engineering and analyzing novel functions. Methods Enzymol 497:539–579. https://doi.org/10.1016/B978-0-12-385075-1.00024-X
Hendry JI, Prasannan C, Ma F, Möllers KB, Jaiswal D, Digmurti M, Allen DK, Frigaard NU, Dasgupta S, Wangikar PP (2017) Rerouting of carbon flux in a glycogen mutant of cyanobacteria assessed via isotopically non-stationary 13C metabolic flux analysis. Biotechnol Bioeng 114(10):2298–2308. https://doi.org/10.1002/bit.26350
Heredia-Arroyo T, Wei W, Ruan R, Hu B (2011) Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenerg 35(5):2245–2253. https://doi.org/10.1016/j.biombioe.2011.02.036
Higo A, Ehira S (2019) Anaerobic butanol production driven by oxygen-evolving photosynthesis using the heterocyst-forming multicellular cyanobacterium Anabaena sp PCC 7120. Appl Microbiol Biotechnol 103(5):2441–2447. https://doi.org/10.1007/s00253-019-09635-z
Higo A, Ehira S (2019) Spatiotemporal gene repression system in the heterocyst-forming multicellular cyanobacterium Anabaena sp. PCC 7120. ACS Synth Biol 8(4):641–646. https://doi.org/10.1021/acssynbio.8b00496
Higo A, Isu A, Fukaya Y, Ehira S, Hisabori T (2018) Application of CRISPR interference for metabolic engineering of the heterocyst-forming multicellular cyanobacterium Anabaena sp. PCC 7120. Plant Cell Physiol 59(1):119–127. https://doi.org/10.1093/pcp/pcx166
Holland SC, Artier J, Miller NT, Cano M, Yu J, Ghirardi ML, Burnap RL (2016) Impacts of genetically engineered alterations in carbon sink pathways on photosynthetic performance. Algal Res 20:87–99. https://doi.org/10.1016/j.algal.2016.09.021
Hönig V, Kotek M, Mařík J (2014) Use of butanol as a fuel for internal combustion engines. Agron Res 12(2):333–340
Horváth I, Glatz A, Nakamoto H, Mishkind ML, Munnik T, Saidi Y, Goloubinoff P, Harwood JL, Vigh L (2012) Heat shock response in photosynthetic organisms: membrane and lipid connections. Prog Lipid Res 51(3):208–220. https://doi.org/10.1016/j.plipres.2012.02.002
Hsieh C-H, Wu W-T (2009a) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Biores Technol 100(17):3921–3926. https://doi.org/10.1016/j.biortech.2009.03.019
Hsieh CH, Wu WT (2009b) A novel photobioreactor with transparent rectangular chambers for cultivation of microalgae. Biochem Eng J 46(3):300–305. https://doi.org/10.1016/j.bej.2009.06.004
Huang H-H, Lindblad P (2013) Wide-dynamic-range promoters engineered for cyanobacteria. J Biol Eng 7(1):1–11. https://doi.org/10.1186/1754-1611-7-10
Huang H-H, Camsund D, Lindblad P, Heidorn T (2010) Design and characterization of molecular tools for a Synthetic Biology approach towards developing cyanobacterial biotechnology. Nucleic Acids Res 38(8):2577–2593. https://doi.org/10.1093/nar/gkq164
Huang H, Singh V, Qureshi N (2015) Butanol production from food waste: a novel process for producing sustainable energy and reducing environmental pollution. Biotechnol Biofuels 8(1):1–12. https://doi.org/10.1186/s13068-015-0332-x
Huang Q, Jiang F, Wang L, Yang C (2017) Design of photobioreactors for mass cultivation of photosynthetic organisms. Engineering 3(3):318–329. https://doi.org/10.1016/J.ENG.2017.03.020
Huffer S, Clark ME, Ning JC, Blanch HW, Clark DS (2011) Role of alcohols in growth, lipid composition, and membrane fluidity of yeasts, bacteria, and archaea. Appl Environ Microbiol 77(18):6400–6408. https://doi.org/10.1128/AEM.00694-11
Imamura S, Asayama M, Shirai M (2004) In vitro transcription analysis by reconstituted cyanobacterial RNA polymerase: roles of group 1 and 2 sigma factors and a core subunit, RpoC2. Genes Cells 9(12):1175–1187. https://doi.org/10.1111/j.1365-2443.2004.00808.x
Immethun CM, DeLorenzo DM, Focht CM, Gupta D, Johnson CB, Moon TS (2017) Physical, chemical, and metabolic state sensors expand the synthetic biology toolbox for Synechocystis sp. PCC 6803. Biotechnol Bioeng 114(7):1561–1569. https://doi.org/10.1002/bit.26275
Iwaki T, Haranoh K, Inoue N, Kojima K, Satoh R, Nishino T, Wada S, Ihara H, Tsuyama S, Kobayashi H (2006) Expression of foreign type I ribulose-1, 5-bisphosphate carboxylase/oxygenase (EC 4.1. 1.39) stimulates photosynthesis in cyanobacterium Synechococcus PCC7942 cells. Photosynth Res 88(3):287–297. https://doi.org/10.1007/s11120-006-9048-x
Jacobsen JH, Frigaard N-U (2014) Engineering of photosynthetic mannitol biosynthesis from CO2 in a cyanobacterium. Metab Eng 21:60–70. https://doi.org/10.1016/j.ymben.2013.11.004
Javanmardian M, Palsson BO (1991) High-density photoautotrophic algal cultures - design, construction, and operation of a novel photobioreactor system. Biotechnol Bioeng 38(10):1182–1189. https://doi.org/10.1002/bit.260381010
Jeanfils J, Canisius M, Burlion N (1993) Effect of high nitrate concentrations on growth and nitrate uptake by free-living and immobilized Chlorella vulgaris cells. J Appl Phycol 5(3):369–374. https://doi.org/10.1007/BF02186240
Jiang J, Zhang N, Yang X, Song L, Yang S (2016) Toxic metal biosorption by macrocolonies of cyanobacterium Nostoc sphaeroides Kützing. J Appl Phycol 28(4):2265–2277. https://doi.org/10.1007/s10811-015-0753-8
Jin H, Chen L, Wang J, Zhang W (2014) Engineering biofuel tolerance in non-native producing microorganisms. Biotechnol Adv 32(2):541–548. https://doi.org/10.1016/j.biotechadv.2014.02.001
John RP, Anisha G, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: a renewable source for bioethanol. Biores Technol 102(1):186–193. https://doi.org/10.1016/j.biortech.2010.06.139
Johnson TR, Haynes J 2nd, Wealand J, Yarbrough LR, Hirschberg R (1988) Structure and regulation of genes encoding phycocyanin and allophycocyanin from Anabaena variabilis ATCC 29413. J Bacteriol 170(4):1858. https://doi.org/10.1128/jb.170.4.1858-1865.1988
Jones PR (2014) Genetic instability in cyanobacteria–an elephant in the room? Front Bioeng Biotechnol 2:12. https://doi.org/10.3389/fbioe.2014.00012
Jordan A, Chandler J, MacCready JS, Huang J, Osteryoung KW, Ducat DC (2017) Engineering cyanobacterial cell morphology for enhanced recovery and processing of biomass. Appl Environ Microbiol. https://doi.org/10.1128/AEM.00053-17
Kaczmarzyk D, Anfelt J, Särnegrim A, Hudson EP (2014) Overexpression of sigma factor SigB improves temperature and butanol tolerance of Synechocystis sp. PCC6803. J Biotechnol 182:54–60. https://doi.org/10.1016/j.jbiotec.2014.04.017
Kaczmarzyk D, Cengic I, Yao L, Hudson EP (2018) Diversion of the long-chain acyl-ACP pool in Synechocystis to fatty alcohols through CRISPRi repression of the essential phosphate acyltransferase PlsX. Metab Eng 45:59–66
Katayama N, Iijima H, Osanai T (2018) Production of bioplastic compounds by genetically manipulated and metabolic engineered cyanobacteria. Synth Biol Cyanobact 1080:155–169. https://doi.org/10.1007/978-981-13-0854-3_7
Kelly CL, Taylor GM, Hitchcock A, Torres-Mendez A, Heap JT (2018) A rhamnose-inducible system for precise and temporal control of gene expression in cyanobacteria. ACS Synth Biol 7(4):1056–1066. https://doi.org/10.1021/acssynbio.7b00435
Kim BH, Bellows P, Datta R, Zeikus J (1984) Control of carbon and electron flow in Clostridium acetobutylicum fermentations: utilization of carbon monoxide to inhibit hydrogen production and to enhance butanol yields. Appl Environ Microbiol 48(4):764–770. https://doi.org/10.1128/aem.48.4.764-770.1984
Kim WJ, Lee S-M, Um Y, Sim SJ, Woo HM (2017) Development of SyneBrick vectors as a synthetic biology platform for gene expression in Synechococcus elongatus PCC 7942. Front Plant Sci 8:293. https://doi.org/10.3389/fpls.2017.00293
Kirschner M (2006) n‐Butanol. Chemical Market Reporter January 30–February 5, ABI/INFORM Global, p42
Klähn S, Bolay P, Wright PR, Atilho RM, Brewer KI, Hagemann M, Breaker RR, Hess WR (2018) A glutamine riboswitch is a key element for the regulation of glutamine synthetase in cyanobacteria. Nucleic Acids Res 46(19):10082–10094. https://doi.org/10.1093/nar/gky709
Knoot CJ, Biswas S, Pakrasi HB (2019) Tunable repression of key photosynthetic processes using Cas12a CRISPR interference in the fast-growing Cyanobacterium Synechococcus sp UTEX 2973. ACS Synth Biol 9(1):132–143. https://doi.org/10.1021/acssynbio.9b00417
Koksharova O, Wolk C (2002) Genetic tools for cyanobacteria. Appl Microbiol Biotechnol 58(2):123–137. https://doi.org/10.1007/s00253-001-0864-9
Kolesinska B, Fraczyk J, Binczarski M, Modelska M, Berlowska J, Dziugan P, Antolak H, Kaminski ZJ, Witonska IA, Kregiel D (2019) Butanol synthesis routes for biofuel production: trends and perspectives. Materials 12(3):350. https://doi.org/10.3390/ma12030350
Kondo T, Tezuka H, Ishii J, Matsuda F, Ogino C, Kondo A (2012) Genetic engineering to enhance the Ehrlich pathway and alter carbon flux for increased isobutanol production from glucose by Saccharomyces cerevisiae. J Biotechnol 159(1–2):32–37. https://doi.org/10.1016/j.jbiotec.2012.01.022
Kopka J, Schmidt S, Dethloff F, Pade N, Berendt S, Schottkowski M, Martin N, Dühring U, Kuchmina E, Enke H (2017) Systems analysis of ethanol production in the genetically engineered cyanobacterium Synechococcus sp. PCC 7002. Biotechnol Biofuels 10(1):1–21. https://doi.org/10.1186/s13068-017-0741-0
Krasaesueb N, Incharoensakdi A, Khetkorn W (2019) Utilization of shrimp wastewater for poly-β-hydroxybutyrate production by Synechocystis sp PCC 6803 strain ΔSphU cultivated in photobioreactor. Biotechnol Rep 23:e00345. https://doi.org/10.1016/j.btre.2019.e00345
Kumar S, Gujjala LK, Banerjee R (2017) Simultaneous pretreatment and saccharification of bamboo for biobutanol production. Industrial Crops and Products 101:21–28. https://doi.org/10.1016/j.indcrop.2017.02.028
Kunert A, Vinnemeier J, Erdmann N, Hagemann M (2003) Repression by Fur is not the main mechanism controlling the iron-inducible isiAB operon in the cyanobacterium Synechocystis sp. PCC 6803. FEMS Microbiol Lett 227(2):255–262. https://doi.org/10.1016/S0378-1097(03)00689-X
Kusakabe T, Tatsuke T, Tsuruno K, Hirokawa Y, Atsumi S, Liao JC, Hanai T (2013) Engineering a synthetic pathway in cyanobacteria for isopropanol production directly from carbon dioxide and light. Metab Eng 20:101–108. https://doi.org/10.1016/j.ymben.2013.09.007
Kushwaha D, Mishra I, Srivastava N, Mishra PK (2017) Optimization of pretreatment conditions for enhanced sugar release. Int J Green Energy 14(13):1110–1118. https://doi.org/10.1080/15435075.2017.1359784
Kushwaha D, Srivastava N, Mishra I, Upadhyay SN, Mishra PK (2019) Recent trends in biobutanol production. Rev Chem Eng 35(4):475–504. https://doi.org/10.1515/revce-2017-0041
Kushwaha D, Srivastava N, Prasad D, Mishra P, Upadhyay S (2020) Biobutanol production from hydrolysates of cyanobacteria Lyngbya limnetica and Oscillatoria obscura. Fuel 271:117583. https://doi.org/10.1016/j.fuel.2020.117583
Lai MC, Lan EI (2015) Advances in metabolic engineering of cyanobacteria for photosynthetic biochemical production. Metabolites 5(4):636–658. https://doi.org/10.3390/metabo5040636
Lan EI, Liao JC (2011) Metabolic engineering of cyanobacteria for 1-butanol production from carbon dioxide. Metab Eng 13(4):353–363. https://doi.org/10.1016/j.ymben.2011.04.004
Lan EI, Liao JC (2012) ATP drives direct photosynthetic production of 1-butanol in cyanobacteria. Proc Natl Acad Sci 109(16):6018–6023. https://doi.org/10.1073/pnas.1200074109
Lan EI, Ro SY, Liao JC (2013) Oxygen-tolerant coenzyme A-acylating aldehyde dehydrogenase facilitates efficient photosynthetic n-butanol biosynthesis in cyanobacteria. Energy Environ Sci 6(9):2672–2681. https://doi.org/10.1039/C3EE41405A
Landry BP, Stöckel J, Pakrasi HB (2013) Use of degradation tags to control protein levels in the cyanobacterium Synechocystis sp. strain PCC 6803. Appl Environ Microbiol 79(8):2833. https://doi.org/10.1128/AEM.03741-12
Lau N-S, Matsui M, Abdullah AA-A (2015) Cyanobacteria: photoautotrophic microbial factories for the sustainable synthesis of industrial products. Biomed Res Int 2015:754934. https://doi.org/10.1155/2015/754934
Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, Jung KS (2008) Fermentative butanol production by Clostridia. Biotechnol Bioeng 101(2):209–228. https://doi.org/10.1002/bit.22003
Lee W-H, Seo S-O, Bae Y-H, Nan H, Jin Y-S, Seo J-H (2012) Isobutanol production in engineered Saccharomyces cerevisiae by overexpression of 2-ketoisovalerate decarboxylase and valine biosynthetic enzymes. Bioprocess Biosyst Eng 35(9):1467–1475. https://doi.org/10.1007/s00449-012-0736-y
Li S, Wen J, Jia X (2011a) Engineering Bacillus subtilis for isobutanol production by heterologous Ehrlich pathway construction and the biosynthetic 2-ketoisovalerate precursor pathway overexpression. Appl Microbiol Biotechnol 91(3):577–589. https://doi.org/10.1007/s00253-011-3280-9
Li Y, Chen Y-F, Chen P, Min M, Zhou W, Martinez B, Zhu J, Ruan R (2011) Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour Technol 102(8):5138–5144. https://doi.org/10.1016/j.biortech.2011b.01.091
Li X, Shen CR, Liao JC (2014) Isobutanol production as an alternative metabolic sink to rescue the growth deficiency of the glycogen mutant of Synechococcus elongatus PCC 7942. Photosynth Res 120(3):301–310. https://doi.org/10.1007/s11120-014-9987-6
Li C, Tao F, Ni J, Wang Y, Yao F, Xu P (2015) Enhancing the light-driven production of d-lactate by engineering cyanobacterium using a combinational strategy. Sci Rep 5(1):1–11. https://doi.org/10.1038/srep09777
Li H, Shen CR, Huang C-H, Sung L-Y, Wu M-Y, Hu Y-C (2016) CRISPR-Cas9 for the genome engineering of cyanobacteria and succinate production. Metab Eng 38:293–302. https://doi.org/10.1016/j.ymben.2016.09.006
Liao Q, Li L, Chen R, Zhu X (2014) A novel photobioreactor generating the light/dark cycle to improve microalgae cultivation. Biores Technol 161:186–191. https://doi.org/10.1016/j.biortech.2014.02.119
Lindberg P, Park S, Melis A (2010) Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab Eng 12(1):70–79. https://doi.org/10.1016/j.ymben.2009.10.001
Liu D, Pakrasi HB (2018) Exploring native genetic elements as plug-in tools for synthetic biology in the cyanobacterium Synechocystis sp. PCC 6803. Microb Cell Factor 17(1):1–8. https://doi.org/10.1186/s12934-018-0897-8
Liu X, Miao R, Lindberg P, Lindblad P (2019) Modular engineering for efficient photosynthetic biosynthesis of 1-butanol from CO 2 in cyanobacteria. Energy Environ Sci 12(9):2765–2777. https://doi.org/10.1039/C9EE01214A
Liu D, Johnson VM, Pakrasi HB (2020) A reversibly induced CRISPRi system targeting Photosystem II in the cyanobacterium Synechocystis sp. PCC 6803. ACS Synth Biol 9(6):1441–1449. https://doi.org/10.1021/acssynbio.0c00106
Ma AT, Schmidt CM, Golden JW (2014) Regulation of gene expression in diverse cyanobacterial species by using theophylline-responsive riboswitches. Appl Environ Microbiol 80(21):6704. https://doi.org/10.1128/AEM.01697-14
Machado IM, Atsumi S (2012) Cyanobacterial biofuel production. J Biotechnol 162(1):50–56. https://doi.org/10.1016/j.jbiotec.2012.03.005
Mahesh R, Naira VR, Maiti SK (2019) Concomitant production of fatty acid methyl ester (biodiesel) and exopolysaccharides using efficient harvesting technology in flat panel photobioreactor with special sparging system via Scenedesmus abundans. Biores Technol 278:231–241. https://doi.org/10.1016/j.biortech.2019.01.091
Mahesh R, Panda SK, Das M, Yashavanth P, Dhull S, Negi BB, Jakhwal P, Maiti SK (2021) Advances in biotechnological tools for bioremediation of wastewater using bacterial–algal symbiotic system. In: Shah MP, Sarkar A, Mandal S (eds) Wastewater Treatment. Elsevier, pp. 385–411
Malhotra B, Glass AD (1995) Potassium fluxes in Chlamydomonas reinhardtii (I. Kinetics and electrical potentials). Plant Physiol 108(4):1527–1536. https://doi.org/10.1104/pp.108.4.1527
Mamo G, Faryar R, Karlsson EN (2013) Microbial glycoside hydrolases for biomass utilization in biofuels applications. In: Gupta VK, Tuhoy MG (eds) Biofuel technologies. Springer, pp. 171–188
Mansur MC, O'Donnell MK, Rehmann MS, Zohaib M (2010) ABE fermentation of sugar in Brazil, Senior Design Reports, Dept. of Chem. and Biomol. Eng., Univ. of Pennsylvania, Philadelphia, USA
Mariano AP, Costa CBB, de Angelis DdF, Maugeri Filho F, Atala DIP, Maciel MRW, Maciel Filho RJAB, Biotechnology, (2009) Optimization strategies based on sequential quadratic programming applied for a fermentation process for butanol production. Appl Biochem Biotechnol 159(2):366–381. https://doi.org/10.1007/s12010-008-8450-6
Markou G, Vandamme D, Muylaert K (2014) Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res 65:186–202. https://doi.org/10.1016/j.watres.2014.07.025
Mermet-Bouvier P, Chauvat F (1994) A conditional expression vector for the cyanobacteria Synechocystis sp. strains PCC6803 and PCC6714 or Synechococcus sp. strains PCC7942 and PCC6301. Curr Microbiol 28(3):145–148. https://doi.org/10.1007/BF01571055
Miao R, Liu X, Englund E, Lindberg P, Lindblad P (2017) Isobutanol production in Synechocystis PCC 6803 using heterologous and endogenous alcohol dehydrogenases. Metab Eng Commun 5:45–53. https://doi.org/10.1016/j.meteno.2017.07.003
Miao R, Xie H, Ho FM, Lindblad P (2018a) Protein engineering of α-ketoisovalerate decarboxylase for improved isobutanol production in Synechocystis PCC 6803. Metab Eng 47:42–48. https://doi.org/10.1016/j.ymben.2018.02.014
Miao R, Xie H, Lindblad P (2018b) Enhancement of photosynthetic isobutanol production in engineered cells of Synechocystis PCC 6803. Biotechnol Biofuels 11(1):1–9. https://doi.org/10.1186/s13068-018-1268-8
Mikhodyuk O, Zavarzin G, Ivanovsky R (2008) Transport systems for carbonate in the extremely natronophilic cyanobacterium Euhalothece sp. Microbiology 77(4):412–418. https://doi.org/10.1134/S002626170804005X
Mohamed A, Jansson C (1989) Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol Biol 13(6):693–700. https://doi.org/10.1007/BF00016024
Möllers KB, Cannella D, Jørgensen H, Frigaard N-U (2014) Cyanobacterial biomass as carbohydrate and nutrient feedstock for bioethanol production by yeast fermentation. Biotechnol Biofuels 7(1):64. https://doi.org/10.1186/1754-6834-7-64
Moon HG, Jang Y-S, Cho C, Lee J, Binkley R, Lee SY (2016) One hundred years of clostridial butanol fermentation. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnw001
Moxley G, Zhu Z, Zhang Y-HP (2008) Efficient sugar release by the cellulose solvent-based lignocellulose fractionation technology and enzymatic cellulose hydrolysis. J Agric Food Chem 56(17):7885–7890. https://doi.org/10.1021/jf801303f
Mühling M, Belay A, Whitton BA (2005) Screening Arthrospira (Spirulina) strains for heterotrophy. J Appl Phycol 17(2):129–135. https://doi.org/10.1007/s10811-005-7214-8
Mukherjee B, Madhu S, Wangikar PP (2020) The role of systems biology in developing non-model cyanobacteria as hosts for chemical production. Curr Opin Biotechnol 64:62–69. https://doi.org/10.1016/j.copbio.2019.10.003
Myers J, Phillips JN Jr, Graham J-R (1951) On the mass culture of algae. Plant Physiol 26(3):539. https://doi.org/10.1104/pp.26.3.539
Nagase H, Yoshihara K-i, Eguchi K, Okamoto Y, Murasaki S, Yamashita R, Hirata K, Miyamoto K (2001) Uptake pathway and continuous removal of nitric oxide from flue gas using microalgae. Biochem Eng J 7(3):241–246. https://doi.org/10.1016/S1369-703X(00)00122-4
Nakahira Y, Ogawa A, Asano H, Oyama T, Tozawa Y (2013) Theophylline-dependent riboswitch as a novel genetic tool for strict regulation of protein expression in cyanobacterium Synechococcus elongatus PCC 7942. Plant Cell Physiol 54(10):1724–1735. https://doi.org/10.1093/pcp/pct115
Nanda S, Golemi-Kotra D, McDermott JC, Dalai AK, Gökalp I, Kozinski JA (2017) Fermentative production of butanol: perspectives on synthetic biology. New Biotechnol 37(Pt B):210–221. https://doi.org/10.1016/j.nbt.2017.02.006
Nawab S, Wang N, Ma X, Huo Y-X (2020) Genetic engineering of non-native hosts for 1-butanol production and its challenges: a review. Microb Cell Fact 19(1):1–16. https://doi.org/10.1186/s12934-020-01337-w
Ndaba B, Chiyanzu I, Marx S (2015) n-Butanol derived from biochemical and chemical routes: a review. Biotechnol Rep 8:1–9. https://doi.org/10.1016/j.btre.2015.08.001
Ng W-O, Pakrasi H (2001) DNA photolyase homologs are the major UV resistance factors in the cyanobacterium Synechocystis sp PCC 6803. Mol Gener Genet MGG 264(6):924–931. https://doi.org/10.1007/s004380000383
Ng W-O, Zentella R, Wang Y, Taylor J-SA, Pakrasi HB (2000) PhrA, the major photoreactivating factor in the cyanobacterium Synechocystis sp. strain PCC 6803 codes for a cyclobutane-pyrimidine-dimer-specific DNA photolyase. Arch Microbiol 173(5):412–417. https://doi.org/10.1007/s002030000164
Nicolaou SA, Gaida SM, Papoutsakis ET (2010) A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: from biofuels and chemicals, to biocatalysis and bioremediation. Metab Eng 12(4):307–331. https://doi.org/10.1016/j.ymben.2010.03.004
Niederholtmeyer H, Wolfstädter BT, Savage DF, Silver PA, Way JC (2010a) Engineering cyanobacteria to synthesize and export hydrophilic products. Appl Environ Microbiol 76(11):3462. https://doi.org/10.1128/AEM.00202-10
Nilsson A, Shabestary K, Brandão M, Hudson EP (2020) Environmental impacts and limitations of third-generation biobutanol: life cycle assessment of n-butanol produced by genetically engineered cyanobacteria. J Ind Ecol 24(1):205–216. https://doi.org/10.1111/jiec.12843
Niu T-C, Lin G-M, Xie L-R, Wang Z-Q, Xing W-Y, Zhang J-Y, Zhang C-C (2018) Expanding the potential of CRISPR-Cpf1-based genome editing technology in the cyanobacterium Anabaena PCC 7120. ACS Synth Biol 8(1):170–180. https://doi.org/10.1021/acssynbio.8b00437
Nobles DR, Brown RM (2008) Transgenic expression of Gluconacetobacter xylinus strain ATCC 53582 cellulose synthase genes in the cyanobacterium Synechococcus leopoliensis strain UTCC 100. Cellulose 15(5):691–701. https://doi.org/10.1007/s10570-008-9217-5
Noguchi S, Putri SP, Lan EI, Lavina WA, Dempo Y, Bamba T, Liao JC, Fukusaki E (2016) Quantitative target analysis and kinetic profiling of acyl-CoAs reveal the rate-limiting step in cyanobacterial 1-butanol production. Metabolomics 12(2):26. https://doi.org/10.1007/s11306-015-0940-2
Ohbayashi R, Akai H, Yoshikawa H, Hess WR, Watanabe S (2016) A tightly inducible riboswitch system in Synechocystis sp. PCC 6803. J Gen Appl Microbiol 62(3):154–159. https://doi.org/10.2323/jgam.2016.02.002
Oliver JW, Machado IM, Yoneda H, Atsumi S (2013) Cyanobacterial conversion of carbon dioxide to 2, 3-butanediol. Proc Natl Acad Sci 110(4):1249–1254. https://doi.org/10.1073/pnas.1213024110
Ou J, Ma C, Xu N, Du Y, Liu XM (2015) High butanol production by regulating carbon, redox and energy in Clostridia. Front Chem Sci Eng 9(3):317–323. https://doi.org/10.1007/s11705-015-1522-6
Outram V, Lalander CA, Lee JG, Davies ET, Harvey AP (2017) Applied in situ product recovery in ABE fermentation. Biotechnol Prog 33(3):563–579. https://doi.org/10.1002/btpr.2446
Pang Z-W, Lu W, Zhang H, Liang Z-W, Liang J-J, Du L-W, Duan C-J, Feng J-X (2016) Butanol production employing fed-batch fermentation by Clostridium acetobutylicum GX01 using alkali-pretreated sugarcane bagasse hydrolysed by enzymes from Thermoascus aurantiacus QS 7-2-4. Bioresource Technol 212:82–91. https://doi.org/10.1016/j.biortech.2016.04.013
Park J, Jin H-F, Lim B-R, Park K-Y, Lee K (2010) Ammonia removal from anaerobic digestion effluent of livestock waste using green alga Scenedesmus sp. Biores Technol 101(22):8649–8657. https://doi.org/10.1016/j.biortech.2010.06.142
Pasteur L (1862) Quelques résultats nouveaux relatifs aux fermentations acétique et butyrique. Bull Soc Chim Paris pp. 52–53
Pate R, Klise G, Wu B (2011) Resource demand implications for US algae biofuels production scale-up. Appl Energy 88(10):3377–3388. https://doi.org/10.1016/j.apenergy.2011.04.023
Pattharaprachayakul N, Lee M, Incharoensakdi A, Woo HM (2020) Current understanding of the cyanobacterial CRISPR-Cas systems and development of the synthetic CRISPR-Cas systems for cyanobacteria. Enzym Microb Technol 140:109619. https://doi.org/10.1016/j.enzmictec.2020.109619
Peccia J, Haznedaroglu B, Gutierrez J, Zimmerman JB (2013) Nitrogen supply is an important driver of sustainable microalgae biofuel production. Trends Biotechnol 31(3):134–138. https://doi.org/10.1016/j.tibtech.2013.01.010
Pembroke JT, Ryan MP (2020). Cyanobacterial biofuel production: current development, challenges and future needs. In: Yadav AN, Rastegari AA, Yadav N, Gaur R (eds) Biofuels production–sustainability and advances in microbial bioresources. Springer, pp 35–62
Pembroke JT, Armshaw P, Ryan MP (2019) Metabolic engineering of the model photoautotrophic cyanobacterium synechocystis for ethanol production: optimization strategies and challenges. In: Basso, TP, Basso, LC (eds) Fuel ethanol production from sugarcane. pp. 199–219. Doi: https://doi.org/10.5772/intechopen.77271
Pérez AA, Rodionov DA, Bryant DA (2016) Identification and regulation of genes for cobalamin transport in the cyanobacterium Synechococcus sp. strain PCC 7002. J Bacteriol 198(19):2753. https://doi.org/10.1128/JB.00476-16
Perez-Garcia O, Escalante FM, De-Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45(1):11–36. https://doi.org/10.1016/j.watres.2010.08.037
Pfleger BF, Pitera DJ, Smolke CD, Keasling JD (2006) Combinatorial engineering of intergenic regions in operons tunes expression of multiple genes. Nat Biotechnol 24(8):1027–1032. https://doi.org/10.1038/nbt1226
Potts T, Du J, Paul M, May P, Beitle R, Hestekin J (2012) The production of butanol from Jamaica bay macro algae. Environ Progress Sustain Energy 31(1):29–36. https://doi.org/10.1002/ep.10606
Powell N, Shilton A, Chisti Y, Pratt S (2009) Towards a luxury uptake process via microalgae–defining the polyphosphate dynamics. Water Res 43(17):4207–4213. https://doi.org/10.1016/j.watres.2009.06.011
Powell N, Shilton A, Pratt S, Chisti Y (2011) Luxury uptake of phosphorus by microalgae in full-scale waste stabilisation ponds. Water Sci Technol 63(4):704–709. https://doi.org/10.2166/wst.2011.116
Price GD, Badger MR, Woodger FJ, Long BM (2008) Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J Exp Bot 59(7):1441–1461. https://doi.org/10.1093/jxb/erm112
Pringsheim EG (2016) Pure cultures of algae. Cambridge University Press, Cambridge
Pulz O (2001) Photobioreactors: production systems for phototrophic microorganisms. Appl Microbiol Biotechnol 57(3):287–293. https://doi.org/10.1007/s002530100702
Qi Q, Hao M, Ng W-o, Slater SC, Baszis SR, Weiss JD, Valentin HE (2005) Application of the Synechococcus nirA promoter to establish an inducible expression system for engineering the Synechocystis tocopherol pathway. Appl Environ Microbiol 71(10):5678–5684. https://doi.org/10.1128/AEM.71.10.5678-5684.2005
Qiang H, Richmond A (1994) Optimizing the population density in Isochrysis galbana grown outdoors in a glass column photobioreactor. J Appl Phycol 6(4):391–396. https://doi.org/10.1007/BF02182155
Qiu M, Shen W, Yan X, He Q, Cai D, Chen S, Wei H, Knoshaug EP, Zhang M, Himmel ME (2020) Metabolic engineering of Zymomonas mobilis for anaerobic isobutanol production. Biotechnol Biofuels 13(1):15. https://doi.org/10.1186/s13068-020-1654-x
Qureshi N, Saha BC, Cotta MA (2007) Butanol production from wheat straw hydrolysate using Clostridium beijerinckii. Bioprocess Biosyst Eng 30(6):419–427. https://doi.org/10.1007/s00449-007-0137-9
Raja R, Hemaiswarya S, Kumar NA, Sridhar S, Rengasamy R (2008) A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol 34(2):77–88. https://doi.org/10.1080/10408410802086783
Ranjan A, Khanna S, Moholkar V (2013) Feasibility of rice straw as alternate substrate for biobutanol production. Appl Energy 103:32–38. https://doi.org/10.1016/j.apenergy.2012.10.035
Richmond A (2004) Handbook of microalgal culture: biotechnology and applied phycology. Wiley Online Library, Hoboken
Richter N, Zienert A, Hummel W (2011) A single-point mutation enables lactate dehydrogenase from Bacillus subtilis to utilize NAD+ and NADP+ as cofactor. Eng Life Sci 11(1):26–36. https://doi.org/10.1002/elsc.201000151
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111(1):1–61. https://doi.org/10.1099/00221287-111-1-1
Roberts K, Granum E, Leegood RC, Raven JA (2007) C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control. Plant Physiol 145(1):230–235. https://doi.org/10.1104/pp.107.102616
Rouhiainen L, Paulin L, Suomalainen S, Hyytiäinen H, Buikema W, Haselkorn R, Sivonen K (2000) Genes encoding synthetases of cyclic depsipeptides, anabaenopeptilides, in Anabaena strain 90. Mol Microbiol 37(1):156–167. https://doi.org/10.1046/j.1365-2958.2000.01982.x
Rueda E, García-Galán MJ, Ortiz A, Uggetti E, Carretero J, García J, Díez-Montero R (2020) Bioremediation of agricultural runoff and biopolymers production from cyanobacteria cultured in demonstrative full-scale photobioreactors. Process Saf Environ Prot 139:241–250. https://doi.org/10.1016/j.psep.2020.03.035
Ruffing AM (2014) Improved free fatty acid production in cyanobacteria with Synechococcus sp. PCC 7002 as host. Front Bioeng Biotechnol 2:17. https://doi.org/10.3389/fbioe.2014.00017
Ruffing AM, Jensen TJ, Strickland LM (2016) Genetic tools for advancement of Synechococcus sp. PCC 7002 as a cyanobacterial chassis. Microbial Cell Factor 15(1):1–14. https://doi.org/10.1186/s12934-016-0584-6
Sakai M, Ogawa T, Matsuoka M, Fukuda H (1997) Photosynthetic conversion of carbon dioxide to ethylene by the recombinant cyanobacterium, Synechococcus sp. PCC 7942, which harbors a gene for the ethylene-forming enzyme of Pseudomonas syringae. J Ferment Bioeng 84(5):434–443. https://doi.org/10.1016/S0922-338X(97)82004-1
Salis HM, Mirsky EA, Voigt CA (2009) Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol 27(10):946–950. https://doi.org/10.1038/nbt.1568
Samantaray S, Nayak JK, Mallick N (2011) Wastewater utilization for poly-β-hydroxybutyrate production by the cyanobacterium Aulosira fertilissima in a recirculatory aquaculture system. Appl Environ Microbiol 77(24):8735–8743. https://doi.org/10.1128/AEM.05275-11
Santos-Merino M, Singh AK, Ducat DC (2019) New applications of synthetic biology tools for cyanobacterial metabolic engineering. Front Bioeng Biotech 7:33. https://doi.org/10.3389/fbioe.2019.00033
Sarathy SM, Vranckx S, Yasunaga K, Mehl M, Oßwald P, Metcalfe WK, Westbrook CK, Pitz WJ, Kohse-Höinghaus K, Fernandes RX (2012) A comprehensive chemical kinetic combustion model for the four butanol isomers. Combust Flame 159(6):2028–2055. https://doi.org/10.1016/j.combustflame.2011.12.017
Sarchami T, Munch G, Johnson E, Kießlich S, Rehmann L (2016) A review of process-design challenges for industrial fermentation of butanol from crude glycerol by non-biphasic Clostridium pasteurianum. Fermentation 2(2):13. https://doi.org/10.3390/fermentation2020013
Sauer M (2016) Industrial production of acetone and butanol by fermentation—100 years later. FEMS Microbiol Lett 363(13):fnw134. https://doi.org/10.1093/femsle/fnw134
Seedorf H, Fricke WF, Veith B, Brüggemann H, Liesegang H, Strittmatter A, Miethke M, Buckel W, Hinderberger J, Li F (2008) The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features. Proc Natl Acad Sci 105(6):2128–2133. https://doi.org/10.1073/pnas.0711093105
Sharif Rohani A, Mehrani P, Thibault J (2015) Comparison of in-situ recovery methods of gas stripping, pervaporation, and vacuum separation by multi-objective optimization for producing biobutanol via fermentation process. Can J Chem Eng 93(6):986–997. https://doi.org/10.1002/cjce.22186
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the US Department of Energy’s aquatic species program: biodiesel from algae. Natl Renew Energy Lab 328:1–294
Shen CR, Liao JC (2012) Photosynthetic production of 2-methyl-1-butanol from CO 2 in cyanobacterium Synechococcus elongatus PCC7942 and characterization of the native acetohydroxyacid synthase. Energy Environ Sci 5(11):9574–9583. https://doi.org/10.1039/C2EE23148D
Shen L, Li Z, Wang J, Liu A, Li Z, Yu R, Wu X, Liu Y, Li J, Zeng W (2018) Characterization of extracellular polysaccharide/protein contents during the adsorption of Cd (II) by Synechocystis sp. PCC6803. Environ Sci Pollut Res 25(21):20713–20722. https://doi.org/10.1007/s11356-018-2163-3
Shetty RP, Endy D, Knight TF (2008) Engineering BioBrick vectors from BioBrick parts. J Biol Eng 2(1):1–12. https://doi.org/10.1186/1754-1611-2-5
Shukor H, Abdeshahian P, Al-Shorgani NKN, Hamid AA, Rahman NA, Kalil MS (2016) Enhanced mannan-derived fermentable sugars of palm kernel cake by mannanase-catalyzed hydrolysis for production of biobutanol. Bioresource Technol 218:257–264. https://doi.org/10.1016/j.biortech.2016.06.084
Singh JS, Kumar A, Rai AN, Singh DP (2016) Cyanobacteria: a precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front Microbiol 7:529. https://doi.org/10.3389/fmicb.2016.00529
Singh R, Parihar P, Singh M, Bajguz A, Kumar J, Singh S, Singh VP, Prasad SM (2017) Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: current status and future prospects. Front Microbiol 8:515. https://doi.org/10.3389/fmicb.2017.00515
Smith KM, Cho K-M, Liao JC (2010) Engineering Corynebacterium glutamicum for isobutanol production. Appl Microbiol Biotechnol 87(3):1045–1055. https://doi.org/10.1007/s00253-010-2522-6
Stensjö K, Vavitsas K, Tyystjärvi T (2018) Harnessing transcription for bioproduction in cyanobacteria. Physiol Plant 162(2):148–155. https://doi.org/10.1111/ppl.12606
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Biores Technol 83(1):1–11. https://doi.org/10.1016/s0960-8524(01)00212-7
Sun T, Li S, Song X, Diao J, Chen L, Zhang W (2018) Toolboxes for cyanobacteria: recent advances and future direction. Biotechnol Adv 36(4):1293–1307. https://doi.org/10.1016/j.biotechadv.2018.04.007
Suutari M, Leskinen E, Fagerstedt K, Kuparinen J, Kuuppo P, Blomster J (2015) Macroalgae in biofuel production. Phycol Res 63(1):1–18. https://doi.org/10.1111/pre.12078
Takahama K, Matsuoka M, Nagahama K, Ogawa T (2003) Construction and analysis of a recombinant cyanobacterium expressing a chromosomally inserted gene for an ethylene-forming enzyme at the psbAI locus. J Biosci Bioeng 95(3):302–305. https://doi.org/10.1016/s1389-1723(03)80034-8
Tamoi M, Miyazaki T, Fukamizo T, Shigeoka S (2005) The Calvin cycle in cyanobacteria is regulated by CP12 via the NAD (H)/NADP (H) ratio under light/dark conditions. Plant J 42(4):504–513. https://doi.org/10.1111/j.1365-313X.2005.02391.x
Tan X, Yao L, Gao Q, Wang W, Qi F, Lu X (2011) Photosynthesis driven conversion of carbon dioxide to fatty alcohols and hydrocarbons in cyanobacteria. Metab Eng 13(2):169–176. https://doi.org/10.1016/j.ymben.2011.01.001
Taton A, Lis E, Adin DM, Dong G, Cookson S, Kay SA, Golden SS, Golden JW (2012) Gene transfer in Leptolyngbya sp. strain BL0902, a cyanobacterium suitable for production of biomass and bioproducts. PloS one 7(1): e30901. https://doi.org/10.1371/journal.pone.0030901
Taton A, Unglaub F, Wright NE, Zeng WY, Paz-Yepes J, Brahamsha B, Palenik B, Peterson TC, Haerizadeh F, Golden SS (2014) Broad-host-range vector system for synthetic biology and biotechnology in cyanobacteria. Nucleic Acids Res 42(17):e136–e136. https://doi.org/10.1093/nar/gku673
Taton A, Ma AT, Ota M, Golden SS, Golden JW (2017) NOT gate genetic circuits to control gene expression in cyanobacteria. ACS Synth Biol 6(12):2175–2182. https://doi.org/10.1021/acssynbio.7b00203
Taylor G (2008) Biofuels and the biorefinery concept. Energy Policy 36(12):4406–4409. https://doi.org/10.1016/j.enpol.2008.09.069
Therien JB, Zadvornyy OA, Posewitz MC, Bryant DA, Peters JW (2014) Growth of Chlamydomonas reinhardtii in acetate-free medium when co-cultured with alginate-encapsulated, acetate-producing strains of Synechococcus sp. PCC 7002. Biotechnol Biofuels 7(1):1–8. https://doi.org/10.1186/s13068-014-0154-2
Thiel K, Mulaku E, Dandapani H, Nagy C, Aro E-M, Kallio P (2018) Translation efficiency of heterologous proteins is significantly affected by the genetic context of RBS sequences in engineered cyanobacterium Synechocystis sp PCC 6803. Microbial Cell Factor 17(1):1–12. https://doi.org/10.1186/s12934-018-0882-2
Tian X, Chen L, Wang J, Qiao J, Zhang W (2013) Quantitative proteomics reveals dynamic responses of Synechocystis sp. PCC 6803 to next-generation biofuel butanol. J Proteomics 78:326–345. https://doi.org/10.1016/j.jprot.2012.10.002
Trinh CT, Huffer S, Clark ME, Blanch HW, Clark DS (2010) Elucidating mechanisms of solvent toxicity in ethanologenic Escherichia coli. Biotechnol Bioeng 106(5):721–730. https://doi.org/10.1002/bit.22743
Uduman N, Qi Y, Danquah MK, Forde GM, Hoadley A (2010) Dewatering of microalgal cultures: a major bottleneck to algae-based fuels. J Renew Sustain Energy 2(1):012701. https://doi.org/10.1063/1.3294480
Ueda R, Hirayama S, Sugata K, Nakayama H (1996) Process for the production of ethanol from microalgae, Google Patents
Ungerer J, Pakrasi HB (2016) Cpf1 is a versatile tool for CRISPR genome editing across diverse species of cyanobacteria. Sci Rep 6(1):1–9
Ungerer J, Wendt KE, Hendry JI, Maranas CD, Pakrasi HB (2018) Comparative genomics reveals the molecular determinants of rapid growth of the cyanobacterium Synechococcus elongatus UTEX 2973. Proc Natl Acad Sci 115(50):E11761–E11770. https://doi.org/10.1073/pnas.1814912115
Varman AM, Xiao Y, Pakrasi HB, Tang YJ (2013) Metabolic engineering of Synechocystis sp. strain PCC 6803 for isobutanol production. Appl Environ Microbiol 79(3):908–914. https://doi.org/10.1128/AEM.02827-12
Varman AM, Yu Y, You L, Tang YJ (2013b) Photoautotrophic production of D-lactic acid in an engineered cyanobacterium. Microb Cell Factor 12(1):1–8. https://doi.org/10.1186/1475-2859-12-117
Vasudevan R, Gale GA, Schiavon AA, Puzorjov A, Malin J, Gillespie MD, Vavitsas K, Zulkower V, Wang B, Howe CJ (2019) CyanoGate: a modular cloning suite for engineering cyanobacteria based on the plant MoClo syntax. Plant Physiol 180(1):39–55. https://doi.org/10.1104/pp.18.01401
Vermaas WF (1998) [20] Gene modifications and mutation mapping to study the function of photosystem II. Methods Enzymol 297:293–310. https://doi.org/10.1016/S0076-6879(98)97022-7
Vogel AIM, Lale R, Hohmann-Marriott MF (2017) Streamlining recombination-mediated genetic engineering by validating three neutral integration sites in Synechococcus sp PCC 7002. J Biol Eng 11(1):1–9. https://doi.org/10.1186/s13036-017-0061-8
Wagner J, Bransgrove R, Beacham TA, Allen MJ, Meixner K, Drosg B, Ting VP, Chuck CJ (2016) Co-production of bio-oil and propylene through the hydrothermal liquefaction of polyhydroxybutyrate producing cyanobacteria. Biores Technol 207:166–174. https://doi.org/10.1016/j.biortech.2016.01.114
Wagner JL, Lee-Lane D, Monaghan M, Sharifzadeh M, Hellgardt K (2019) Recovery of excreted n-butanol from genetically engineered cyanobacteria cultures: process modelling to quantify energy and economic costs of different separation technologies. Algal Res 37:92–102. https://doi.org/10.1016/j.algal.2018.11.008
Wang Y, San K-Y, Bennett GN (2013) Cofactor engineering for advancing chemical biotechnology. Curr Opin Biotechnol 24(6):994–999. https://doi.org/10.1016/j.copbio.2013.03.022
Wang Y, Shi M, Niu X, Zhang X, Gao L, Chen L, Wang J, Zhang W (2014) Metabolomic basis of laboratory evolution of butanol tolerance in photosynthetic Synechocystis sp. PCC 6803. Microbial Cell Factor 13(1):1–12. https://doi.org/10.1186/s12934-014-0151-y
Wang Y, Guo W, Cheng C-L, Ho S-H, Chang J-S, Ren N (2016) Enhancing bio-butanol production from biomass of Chlorella vulgaris JSC-6 with sequential alkali pretreatment and acid hydrolysis. Bioresource Technol 200:557–564. https://doi.org/10.1016/j.biortech.2015.10.056
Wee S-L, Tye C-T, Bhatia S (2008) Membrane separation process—Pervaporation through zeolite membrane. Sep Purif Technol 63(3):500–516. https://doi.org/10.1016/j.seppur.2008.07.010
Wendt KE, Ungerer J, Cobb RE, Zhao H, Pakrasi HB (2016) CRISPR/Cas9 mediated targeted mutagenesis of the fast growing cyanobacterium Synechococcus elongatus UTEX 2973. Microb Cell Factor 15(1):1–8. https://doi.org/10.1186/s12934-016-0514-7
Wolk CP, Vonshak A, Kehoe P, Elhai J (1984) Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci 81(5):1561–1565. https://doi.org/10.1073/pnas.81.5.1561
Woods RP, Coleman JR, De Deng M (2004) Genetically modified cyanobacteria for the production of ethanol, the constructs and method thereof, Google Patents
Xiao Y, Wang S, Rommelfanger S, Balassy A, Barba-Ostria C, Gu P, Galazka JM, Zhang F (2018) Developing a Cas9-based tool to engineer native plasmids in Synechocystis sp. PCC 6803. Biotechnol Bioeng 115(9):2305–2314. https://doi.org/10.1002/bit.26747
Xu H, Vavilin D, Funk C, Vermaas W (2004) Multiple deletions of small Cab-like proteins in the cyanobacterium Synechocystis sp. PCC 6803: consequences for pigment biosynthesis and accumulation. J Biol Chem 279(27):27971–27979. https://doi.org/10.1074/jbc.M403307200
Xu L, Weathers PJ, Xiong XR, Liu CZ (2009) Microalgal bioreactors: challenges and opportunities. Eng Life Sci 9(3):178–189. https://doi.org/10.1002/elsc.200800111
Xu J, Fan X, Zhang X, Xu D, Mou S, Cao S, Zheng Z, Miao J, Ye N (2012) Evidence of coexistence of C 3 and C 4 photosynthetic pathways in a green-tide-forming alga, Ulva prolifera. Plos One 7(5):e37438. https://doi.org/10.1371/journal.pone.0037438
Xue C, Zhao J, Chen L, Yang S-T, Bai F (2017) Recent advances and state-of-the-art strategies in strain and process engineering for biobutanol production by Clostridium acetobutylicum. Biotechnol Adv 35(2):310–322. https://doi.org/10.1016/j.biotechadv.2017.01.007
Yang Q, Pando BF, Dong G, Golden SS, van Oudenaarden A (2010) Circadian gating of the cell cycle revealed in single cyanobacterial cells. Science 327(5972):1522–1526. https://doi.org/10.1126/science.1181759
Yang M, Zhang J, Kuittinen S, Vepsäläinen J, Soininen P, Keinänen M, Pappinen A (2015) Enhanced sugar production from pretreated barley straw by additive xylanase and surfactants in enzymatic hydrolysis for acetone–butanol–ethanol fermentation. Bioresource technology 189: 131–137. https://doi.org/10.1016/j.biortech.2015.04.008
Yao L, Cengic I, Anfelt J, Hudson EP (2016) Multiple gene repression in cyanobacteria using CRISPRi. ACS Synth Biol 5(3):207–212. https://doi.org/10.1021/acssynbio.5b00264
Yashavanth P, Das M, Maiti SK (2021) Recent progress and challenges in cyanobacterial autotrophic production of polyhydroxybutyrate (PHB), a bioplastic. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.105379
Yoo G, Park MS, Yang JW (2015) Chemical pretreatment of algal biomass. In: Pandey A, Negi S, Binod P, Larroche C (eds) Pretreatment of Biomass. Elsevier, pp 227–258
Yu R, Yamada A, Watanabe K, Yazawa K, Takeyama H, Matsunaga T, Kurane R (2000) Production of eicosapentaenoic acid by a recombinant marine cyanobacterium Synechococcus sp. Lipids 35(10):1061–1064. https://doi.org/10.1007/s11745-000-0619-6
Yu J, Liberton M, Cliften PF, Head RD, Jacobs JM, Smith RD, Koppenaal DW, Brand JJ, Pakrasi HB (2015) Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO 2. Scientific Reports 5(1):1–10. https://doi.org/10.1038/srep08132
Zang X, Liu B, Liu S, Arunakumara K, Zhang X (2007) Optimum conditions for transformation of Synechocystis sp. PCC 6803. J Microbiol 45(3):241–245
Zess EK, Begemann MB, Pfleger BF (2016) Construction of new synthetic biology tools for the control of gene expression in the cyanobacterium Synechococcus sp. strain PCC 7002. Biotechnol Bioeng 113(2):424–432. https://doi.org/10.1002/bit.25713
Zhang H, Chong H, Ching CB, Song H, Jiang R (2012) Engineering global transcription factor cyclic AMP receptor protein of Escherichia coli for improved 1-butanol tolerance. Appl Microbiol Biotechnol 94(4):1107–1117. https://doi.org/10.1007/s00253-012-4012-5
Zhao Y, Wang Y, Zhu J, Ragauskas A, Deng Y (2008) Enhanced enzymatic hydrolysis of spruce by alkaline pretreatment at low temperature. Biotechnol Bioeng 99(6):1320–1328. https://doi.org/10.1002/bit.21712
Zheng Y-N, Li L-Z, Xian M, Ma Y-J, Yang J-M, Xu X, He D-Z (2009) Problems with the microbial production of butanol. J Ind Microbiol Biotechnol 36(9):1127–1138. https://doi.org/10.1007/s10295-009-0609-9
Zheng Y, Su T, Qi Q (2019) Microbial CRISPRi and CRISPRa systems for metabolic engineering. Biotechnol Bioprocess Eng 24(4):579–591. https://doi.org/10.1007/s12257-019-0107-5
Zhou J, Zhang H, Meng H, Zhu Y, Bao G, Zhang Y, Li Y, Ma Y (2014) Discovery of a super-strong promoter enables efficient production of heterologous proteins in cyanobacteria. Sci Rep 4(1):1–6. https://doi.org/10.1038/srep04500
Zhu H, Ren X, Wang J, Song Z, Shi M, Qiao J, Tian X, Liu J, Chen L, Zhang W (2013) Integrated OMICS guided engineering of biofuel butanol-tolerance in photosynthetic Synechocystis sp. PCC 6803. Biotechnol Biofuels 6(1):1–14. https://doi.org/10.1186/1754-6834-6-106
Acknowledgements
The authors would like to thank the Indian Institute of Technology Guwahati for providing the necessary facilities for research work.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Das, M., Maiti, S.K. Current knowledge on cyanobacterial biobutanol production: advances, challenges, and prospects. Rev Environ Sci Biotechnol 21, 483–516 (2022). https://doi.org/10.1007/s11157-022-09618-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-022-09618-z