Abstract
In this work, the mathematical optimization of a continuous flash fermentation process for the production of biobutanol was studied. The process consists of three interconnected units, as follows: fermentor, cell-retention system (tangential microfiltration), and vacuum flash vessel (responsible for the continuous recovery of butanol from the broth). The objective of the optimization was to maximize butanol productivity for a desired substrate conversion. Two strategies were compared for the optimization of the process. In one of them, the process was represented by a deterministic model with kinetic parameters determined experimentally and, in the other, by a statistical model obtained using the factorial design technique combined with simulation. For both strategies, the problem was written as a nonlinear programming problem and was solved with the sequential quadratic programming technique. The results showed that despite the very similar solutions obtained with both strategies, the problems found with the strategy using the deterministic model, such as lack of convergence and high computational time, make the use of the optimization strategy with the statistical model, which showed to be robust and fast, more suitable for the flash fermentation process, being recommended for real-time applications coupling optimization and control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The optimized operation of butanol fermentation processes is essential to run a biobutanol industry that can compete effectively with the current butanol derived from the petrochemical route, once the acetone, butanol, and ethanol (ABE) fermentation, as normally is called the fermentation to produce butanol, is characterized by its low productivity. Product toxicity results in low butanol concentration in the reactor. In addition, the use of dilute sugar solution results in large process volumes. Mainly because of these problems and due to high costs related to the distillation of dilute product streams, the production of biobutanol on a commercial scale has been considered to be uneconomical [1, 2], when compared to the conventional petrochemical route, which is currently responsible for all butanol produced worldwide. In order to turn the butanol fermentation viable, experts suggest that the production cost must be less than US$0.44 kg−1 [3]. Recently, DuPont (US) and BP (UK) announced their plans to produce biobutanol. It is anticipated that the first plants would operate on sugar or corn starch; however, it is likely that agricultural waste will become a potential substrate [2].
During the past two decades, a significant amount of research has been performed on the development of alternative technologies designed to remove the butanol continuously from the fermentation broth (e.g., adsorption, gas stripping, ionic liquids, liquid–liquid extraction, pervaporation, aqueous two-phase separation, supercritical extraction, perstraction, etc.) [2]. These recovery techniques reduce the effect of product inhibition, allowing an increase in the substrate concentration which results in a reduction in the process streams, higher productivity, and lower distillation costs [4]. According to Qureshi and Blaschek [3], pervaporative recovery significantly reduces the price of butanol production from corn. From US$0.55 kg−1 when employing the batch fermentation and distillative recovery, the production cost can drop to US$0.11–0.36 kg−1. In the process presented in this study, the continuous recovery of butanol is carried out by the flash fermentation technology [5–9] where the fermentor remains at atmospheric pressure while the broth is circulated to a vacuum chamber where butanol is continuously boiled off.
A key factor for the optimization of a process is the understanding of the system’s dynamics which can be obtained using an accurate mathematical model of the process [9]. This approach has been employed for the optimization of different ethanol fermentation processes, including flash fermentation [6, 8–12]. However, the use of mathematical modeling for the performance evaluation of ABE processes is scarce. Volesky and Votruba [13] developed mathematical models for a fermentor operating in different modes and more recently, Shi et al. [14] evaluated the performance of a continuous flash extractive process in terms of productivity, energy requirement, and product purity. Other examples can be found in Honda et al. [15] and Shukla et al. [16]. Furthermore, in the literature revised by the authors of the present work, no study about the use of mathematical optimizers employed to the ABE fermentation was found. For this reason, it is necessary to evaluate the performance of mathematical optimization techniques for the ABE processes, which can be a very useful tool in the search for economic feasibility of the biobutanol plants by operating them in optimized conditions.
In the present study, the optimization problem is written as a nonlinear programming problem and is solved with the sequential quadratic programming (SQP) technique. Two approaches to model the system are evaluated when the process is optimized using the SQP technique. The first one is a deterministic model with the kinetic parameters determined experimentally by Mulchandani and Volesky [17], and the second one is a statistical model obtained using the factorial design technique combined with simulation.
Materials and Methods
Process Description
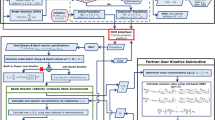
Figure 1 depicts the flash fermentation process, which consists of three interconnected units, as follows: fermentor, cell-retention system (tangential microfiltration), and vacuum flash vessel. The process starts as a conventional continuous fermentation until steady state is reached, then the flash tank separation system is turned on where a partial separation of the solvents and water mixture occurs. The liquid fraction returns to the fermentor and the vapor fraction (after condensation) plus the purge and permeate streams will compose the final stream that is sent to distillation.
Originally, this process was developed for ethanol fermentation in laboratory scale at the Laboratory of Bioprocess Engineering (FEA/UNICAMP). This laboratory has wide experience in the study of ethanol fermentation processes. Among them, the process developed using mathematical modeling by Andrietta and Maugeri [10] stands out. Later this process was implemented in several large-scale Brazilian distilleries (Guarani, Costa Pinto and others).
The efficiency of the flash fermentation process was experimentally validated for ethanol fermentation [18] and these experimental results are in agreement with previous works based on mathematical modeling and computer simulation that demonstrated the technical feasibility of the continuous flash fermentation for ethanol fermentation [6–9].
In this study, the use of the flash fermentation technology is proposed for the ABE fermentation. The study is carried out through computer simulation of a mathematical model based on experimental kinetic parameters [17], thus ensuring the physical meaning of the simulations.
Deterministic Model of the Process
Assuming constant volume, the mass balance equations for the fermentor are given by Eqs. 1, 2, 3, 4, and 5 where the kinetic parameters were determined experimentally by Mulchandani and Volesky [17], whose model was developed on the basis of the following assumptions:
-
1.
Carbon substrate (glucose) limitation only.
-
2.
No nitrogen and nutrient limitation.
-
3.
Product inhibition.
-
4.
Acetic acid and butyric acid are intermediate metabolites and are reduced to acetone and butanol, respectively.
-
5.
Acetone and butanol are also synthesized directly from carbon substrate.
-
6.
Ethanol is synthesized from carbon substrate only.
-
7.
Fermentation is performed at (a) optimal temperature of 37 °C, (b) optimal pH of 4.5, and (c) under anaerobic conditions.
-
8.
All the cells (Clostridium acetobutylicum) are metabolically active and viable.
These experiments were carried out in steady-state mode in a cell-retention culture apparatus (laboratory scale) which consisted of an in situ solid–liquid separation mechanism [17]. Inlet substrate concentration (glucose) was 35.0 g/L and the dilution rate was 0.089 h−1.
where “i” stands for butanol, acetone, ethanol, butyric acid, and acetic acid.
The dynamics of the flash tank are much faster than that of the fermentation process, so a “pseudo” steady state was assumed for the flash tank. The mass balance over the flash tank is given by Eq. 6:
The modeling of the flash tank was based on the isothermal and isobaric evaporation model proposed by Sandler [19] and a multicomponent system (water, butanol, acetone, ethanol, acetic acid, and butyric acid) was considered. The vapor–liquid equilibrium of the mixture was calculated by Eq. 7; the value of \(P_{\text{i}}^{{\text{sat}}} \) was calculated by Antoine’s equation and the value of the activity coefficient (γ ι) was calculated by the universal quasichemical (UNIQUAC) model. The results generated by the model of the flash tank were validated by the HYSYS® simulator.
Eqs. 1, 2, 3, 4, 5, 6, and 7 were solved using a Fortran program with integration with an algorithm based on the fourth-order Runge–Kutta method.
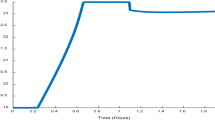
Figures 2 and 3 show the dynamic behavior of the flash continuous fermentation process. At the time when the flash system is switched on, significant changes in the process parameters are observed. The concentration of butanol in the fermentor lowers, what represents a significant reduction in the inhibitory effect, and as a consequence, biomass concentration increases, resulting in a higher conversion of substrate.
The operating conditions considered for the ABE fermentation are listed in Table 1. An industrial process scale was taken into account for the design.
Statistical Model of the Process
Two statistical models of the process were obtained by simulating the deterministic model (Eqs. 1, 2, 3, 4, 5, 6, and 7) following the factorial design of 24 plus star configuration (25 simulations) with the most relevant variables: inlet substrate concentration (S 0), residence time (t r), purge flow (F PU), and the inlet flash tank flow (F c), determined by a previous parametric analysis. The observed responses were steady-state butanol productivity and yield and substrate conversion.
Table 2 shows the coded factor levels and real values for the variables considered in the factorial design. Different operating ranges were considered for each statistical model in order to evaluate the influence of the ranges on the performance of a statistical model in the optimization problem. As the most attractive characteristic of the technologies for continuous butanol recovery from the fermentation broth is the increase of productivity by processing a concentrated feed stream, the range of the substrate concentration chosen (120–160/170 g/L) was considerably higher than the typical maximum concentration found in batch processes (60 g/L) [2].
In the simulations, the fresh medium flow rate (F 0) was considered constant (100 m3/h), so that variations in residence time led to variations in the fermentor volume.
The performance of the SQP optimizer was evaluated for both statistical models. After the solution of the optimization problem, the optimal values of S 0, t r, F PU, and F c were used in the deterministic model to determine if the statistical predictions for optimal productivity present deviations from the values calculated by the deterministic model.
Optimization of the Process
Two strategies were compared for the optimization of the process. In the first one, the process was represented by the statistical model 1 or 2, and in the second, by the deterministic model. For all cases, the optimization problem was solved with the SQP technique.
The variables considered for optimization were the same as those considered for the formulation of the statistical models: inlet substrate concentration (S 0), residence time (t r), purge flow (F PU), and the inlet flash tank flow (F c).
The objective of the optimization problem is to maximize butanol productivity for a desired conversion. Productivity and conversion were defined as:
The optimization problem can be written as follows:
subject to:
For the optimization of the statistical models, the constraints for the operating variables (S 0, t r, F PU, and F c) are given by the upper and lower levels considered in the factorial design (Table 2).
The optimization problem was solved with the subroutine DNCONF of the IMSL math library of FORTRAN in a computer with an Intel Pentium 4 (3.0 GHz) processor and 1.0 GB RAM memory.
The SQP method is a known deterministic optimization method, based on iterative formulation and on the solution of quadratic programming subproblems. The subproblems are obtained using quadratic approximation of the Lagrangian and by linearizing the constraints. The augmented Lagrangian is the objective function less the sum of the active constraints multiplied by their respective estimated Lagrange multipliers. The Hessian of the augmented Lagrangian and the Jacobian of the active constraints compose a linear system, whose solution determines the search direction (line search) and then the new point (butanol productivity) [20].
Results and Discussion
Two statistical models (Eqs. 17, 18, 19, 20, 21, and 22) were obtained from the factorial designs (Table 2) using the software Statistica (Statsoft, v. 7.0). They consist of second-order algebraic equations (Eqs. 17, 18, 19, 20, 21, and 22) representing butanol yield and productivity and sugar conversion where only the significant effects were considered (significance level of 95%). The equations represent in a predictive way the process performance as functions of S 0, t r, F PU, and F c, which are the variables to be manipulated. Tables 3 and 4 show the analysis of variance (ANOVA) for statistical models 1 and 2, respectively. They present high correlation coefficients (higher than 0.91) and the models can be considered statistically significant according to the F test with 95% of confidence, since the calculated values were at least more than six times greater than the listed value. As a rule of thumb, a model has statistical significance if the calculated F value is at least three to five times greater than the listed value [11, 21]. The very good prediction accuracy of the statistical models can be visualized with the comparison between the yield, productivity, and conversion predicted by the statistical models and that calculated by the deterministic model (Fig. 4).
It is important to explain that the statistical models are in coded form for the factors, i.e., when yield, productivity, and conversion are to be calculated, coded values of S 0, t r, F PU, and F c (values inside the interval [−2.0, 2.0]) must be used, and not the real (decoded) values for these variables.
Statistical Model 1
Statistical Model 2
Solutions of the optimization problem for both strategies: (1) SQP+statistical model 1 or 2 and (2) SQP+deterministic model, are presented in Table 5.
For strategy 1, the optimization problem was solved for each desired minimal conversion (values between 93% and 98%) being the solutions extremely fast, demanding up to 1 s for convergence. For the great majority of cases, the optimal values calculated were the same, independently of the initial guesses tested. The unique exception occurred with statistical model 2 for a conversion of 98%. For this case, Table 5 shows the results when the initial guess was the lower bound (S 0 = 120 g/L; t r = 3.0 h; F PU = 25 m3/h; F c = 400 m3/h). For the other initial guesses, the results were: S 0 = 144.2 g/L; t r = 3.9 h; F PU = 25 m3/h; F c = 500 m3/h; Prod But = 7.39 g/(L h); Rend But = 20.0%; Conv = 98%. However, these results present a higher deviation from the values determined by the deterministic model (Prod But = 7.31 g/(L h); Rend But = 19.8%; Conv = 96.9%.) when fed with the values of the optimized variables determined by the statistical model.
The dependence of the solution on the initial guess shows one of the disadvantages of the SQP technique: the optimization algorithm can reach a local maximum [9, 22]. Nevertheless, the local optimizer SQP, benefited from the fact that the statistical models are simple algebraic equations, had an excellent performance in finding optimal solutions independently of the initial estimates for most of the cases evaluated.
For the sake of comparison, Table 5 also shows, in parenthesis, the process performance calculated by the deterministic model when using as input values the optimized variables (S 0, t r, F PU, and F c) determined by the optimization of the statistical models. The results for productivity, yield, and conversion are very close, confirming the statistical significance of the models.
For every solution, the value of the purge flow (F PU) was 25 m3/h. F PU is the variable that directly influences the biomass concentration. Simulations of the deterministic model show that for the operating ranges given by Eqs. 12, 13, 14, and 15, a maximum biomass concentration of around 30 g/L (value recommended by Tashiro et al. [23] in order to avoid bubbling and broth outflow) is achieved with a purge flow of 25 m3/h. Then, the purge flow is decisive to keep the system running with an optimized biomass concentration, and once the product formation is proportional to the number of cells present, the lower the purge flow, the higher the volumetric productivity of the fermentor. Similarly, for most of the cases, the values calculated for the feed flow rate of the flash tank (F c) corresponds to the upper bound of 500 m3/h. According to simulations of the deterministic model, the extraction of butanol from the fermentor is maximized with this value of F c. Varying it, for example, to 450 m3/h, the concentration of butanol in the fermentor increases from 7.5 to 8.0 g/L (for an inlet sugar concentration of 155 g/L), thus intensifying the inhibitory effect to the cells and consequently lowering productivity. It is important to stress that values of F c higher than 500 m3/h can cause the fermentor to empty, since for these operating conditions, the resulting vapor flow plus the other outlet flows (F p and F PU) can be greater than the system feed flow (F 0 = 100 m3/h).
Another point to be discussed is the difference between the solutions obtained with statistical models 1 and 2 (Table 5). Although both models had statistical significance, the solutions with model 2 are practically the same as those obtained with the deterministic model (strategy 2), while the optimization with model 1 led to different values and lower productivity. It demonstrates that the performance of a statistical model in an optimization problem can be influenced by the choice of the bounds of the operating variables used in the factorial design. Thus, the model built with the narrower bounds (model 2) had a clear better performance.
The results of the optimization using the SQP technique with the deterministic model (strategy 2) are presented in Table 5. Solutions were only obtained for minimal conversions of 93% and 95%, and for these cases, convergence only occurred when the initial guesses were normalized to the interval [0, 1] where 0 and 1 stand for the lower and upper bounds, respectively. In relation to computational effort, the time elapsed for solution was around 20 min.
With two different initial guesses: [S 0 = 156.7 g/L; t r = 3.76 h; F PU = 25 m3/h; F c = 500 m3/h] and [S 0 = 176.0 g/L; t r = 3.60 h; F PU = 28.2 m3/h; F c = 380 m3/h], the same solution for the minimal conversion of 95% was achieved. This can indicate that the solution corresponds to a global maximum. It is interesting to note that the first initial guess corresponds to the solution obtained with statistical model 1. In the same way, the initial guess used in the solution for the case of minimal conversion of 93% was the solution obtained with statistical model 2. Unfortunately, this conjunction between the two strategies of optimization did not work out for the other cases (Conv ≥ 94%, 96%, 97%, and 98%), which would be very interesting once the optimizer only accomplished convergence with the initial guesses listed in this paragraph. For the other 30 initial guesses tested for each case and for attempts considering constraints with narrower bounds (Eqs. 12, 13, 14, and 15), the following fatal error occurred in the calculations of the subroutine DNCONF: “Error 2: The line search used more than five function calls,” thus stopping the optimization without any solution. An explanation to this error is that the optimization strategy 2 is a problem of high dimension since its equality constraints is composed of differential equations (deterministic model). The use of differential equations as constraints in an optimization problem makes its solution by the SQP optimizer difficult and increases the incidence of convergence problems [9, 22, 24].
The performance of the optimized process shows that the flash fermentation technology is characterized by its high productivity when compared to traditional continuous processes, as verified for ethanol fermentation via computational simulation and experimentally [6–9, 18]. For the case of the minimal conversion of 95%, simulation with the deterministic model with optimized variables shows that the total solvents productivity (butanol plus acetone and ethanol) is 15.5 g/L h, which is expressively higher than that obtained in other processes such as batch ones with solvents productivity limited to less than 0.50 g/L h and continuous ones with cell recycle, whose value is up to 6.5 g/L h [2]. Moreover, with the final butanol concentration (sum of the outlet streams F v, F p, and F PU) achieved (27.6 g/L), the energy costs in the distillation stage can be halved according to Phillips and Humphrey [25]. However, for a higher degree of conversion such as 98%, the solvents productivity drops to 9.36 g/L h (decrease of 39.6%), as determined by optimization strategy 1. This significant decrease in productivity in order to guarantee a more restricted level of conversion also occurred when the continuous flash fermentation was employed for ethanol fermentation [9]. The decision on which operating conditions the process is more profitable must be based on an economic analysis covering the raw material cost and the selling price of the solvents, but this is beyond the scope of the current paper. In relation to the solvents yield, the result was 31.8% (value obtained with the deterministic model for a minimal conversion of 95%), which is inside the typical range of 29% to 34% for industrial-scale processes found throughout the world in the last century [13, 26, 27]. These results indicate that the flash fermentation process can be a promising technology for the biobutanol industry.
Comparing the solutions obtained with the optimization strategies (1) SQP+statistical model 2 and (2) SQP+deterministic model (Table 5), for the two cases (Conv ≥ 93 and 95%) that strategy 2 achieved convergence, the results were practically the same. The performance of the strategies were also compared for another optimization problem where the operating variables found optimize productivity for a given inlet sugar concentration (Table 6). Again, strategy 2 had problems of convergence, but for the only two cases whose solutions were obtained, very similar optimized operating conditions were obtained with both strategies, thus corroborating the capability of strategy 1 to solve the optimization problem trustfully.
Once both optimization strategies presented very similar solutions, the problems found with strategy 2 (SQP+deterministic model), such as lack of convergence and high computational time, make the use of optimization strategy 1 (SQP+statistical model), which showed to be robust and fast, more suitable for the flash fermentation process.
Fast optimization strategies with good prediction capabilities such as strategy 1 are always welcome especially for real-time applications coupling optimization and control. The time required to predict the values of the variables was extremely short with the statistical model (since it is a simple algebraic equation), so that it may be used in algorithms which require thousands of evaluations in a very limited time with low computational burden. In this way, the real-time process integration could be carried out, for example, with the two-layer approach where the optimization layer is responsible for the calculations of the set points to the controller.
Finally, due to the renewed interest in the biobutanol fermentation caused by the “green chemistry” movement and by the oil price rise, lately, this process has experienced significant improvements, among them the development of new in situ product removal technologies. Thus, in this context, mathematical optimizers can be an important tool to extract the maximum performance of these new processes, summing up the efforts to turn the biobutanol industry commercially viable.
- F 0 :
-
fresh broth flow rate, m3/h
- F :
-
fermentor outflow rate, m3/h
- F c :
-
flash tank inlet flow rate, m3/h
- F p :
-
permeate flow rate, m3/h
- F PU :
-
fermentor purge flow rate, m3/h
- F r :
-
flash tank liquid outlet flow rate, m3/h
- F re :
-
return stream flow rate, m3/h
- F v :
-
flash tank vapor outlet flow rate, m3/h
- K i :
-
equilibrium constant
- P flash :
-
flash tank pressure, kPa
- \(P_{\text{i}}^{{\text{sat}}} \) :
-
vapor pressure of component i, kPa
- P 0 :
-
inlet product concentration, g/L
- P i :
-
fermentor product concentration, g/L
- P r :
-
product concentration in the flash tank liquid outlet flow, g/L
- P v :
-
product concentration in the flash tank vapor outlet flow, g/L
- r x :
-
rate of cell growth, g/L h
- r s :
-
rate of substrate utilization, g/L h
- \(r_{P_{\text{i}} } \) :
-
rate of products production, g/L h
- S 0 :
-
inlet substrate concentration, g/L
- S :
-
fermentor substrate concentration, g/L
- S r :
-
substrate concentration in the flash tank liquid outlet flow, g/L
- S v :
-
substrate concentration in the flash tank vapor outlet flow, g/L
- T ferm :
-
fermentor temperature, °C
- T flash :
-
flash tank temperature, °C
- t r :
-
residence time, h
- V :
-
volume of the fermentor, m3
- x i :
-
liquid molar fraction of component i
- X 0 :
-
inlet biomass concentration, g/L
- X :
-
fermentor biomass concentration, g/L
- X c :
-
biomass concentration in the flash tank inlet flow, g/L
- X p :
-
biomass concentration in the permeate, g/L
- X v :
-
biomass concentration in the flash tank vapor outlet flow, g/L
- y i :
-
vapor molar fraction of component i
- γ i :
-
activity coefficient
References
Ishizaki, A., Michiwaki, S., Crabbe, E., Kobayashi, G., Sonomoto, K., & Yoshino, S. (1999). Journal of Bioscience and Bioengineering, 87, 352–356. doi:10.1016/S1389-1723(99)80044-9.
Ezeji, T. C., Qureshi, N., & Blaschek, H. P. (2007). Current Opinion in Biotechnology, 18, 220–227. doi:10.1016/j.copbio.2007.04.002.
Qureshi, N., & Blaschek, H. P. (2001). Bioprocess and Biosystems Engineering, 24, 219–226. doi:10.1007/s004490100257.
Groot, W. J., van der Lans, R. G. J. M., & Luyben, C. A. M. (1992). Process Biochemistry, 27, 61–75. doi:10.1016/0032-9592(92)80012-R.
Roffler, S. R., Blanch, H. W., & Wilke, C. R. (1984). Trends in Biotechnology, 2, 129–136. doi:10.1016/0167-7799(84)90022-2.
Silva, F. L. H., Rodrigues, M. I., & Maugeri Filho, F. (1999). Journal of Chemical Technology and Biotechnology (Oxford, Oxfordshire), 74, 176–182. doi:10.1002/(SICI)1097-4660(199902)74:2<176::AID-JCTB995>3.0.CO;2-C.
Costa, A. C., Dechechi, E. C., Silva, F. L. H., Maugeri Filho, F., & Maciel Filho, R. (2000). Applied Biochemistry and Biotechnology, 84, 577–593. doi:10.1385/ABAB:84-86:1-9:577.
Costa, A. C., Atala, D. I. P., Maugeri Filho, F., & Maciel Filho, R. (2001). Process Biochemistry, 37, 125–137. doi:10.1016/S0032-9592(01)00188-1.
Costa, A. C., & Maciel Filho, R. (2004). Applied Biochemistry and Biotechnology, 114, 485–496. doi:10.1385/ABAB:114:1-3:485.
Andrietta, S. R., & Maugeri Filho, F. (1994). In E. Galindo, & O. T. Ramirez (Eds.), Advances in bioprocess engineering pp. 47–52. The Netherlands: Kluwer.
Kalil, S. J., Maugeri Filho, F., & Rodrigues, M. I. (2000). Process Biochemistry, 35, 539–550. doi:10.1016/S0032-9592(99)00101-6.
Rivera, E. C., Costa, A. C., Atala, D. I. P., Maugeri Filho, F., Wolf Maciel, M. R., & Maciel Filho, R. (2006). Process Biochemistry, 41, 1682–1687. doi:10.1016/j.procbio.2006.02.009.
Volesky, B., & Votruba, J. (1992). Modeling and optimization of fermentation process. Amsterdam: Elsevier.
Shi, Z., Zhang, C., Chen, J., & Mao, Z. (2005). Bioprocess and Biosystems Engineering, 27, 175–183. doi:10.1007/s00449-004-0396-7.
Honda, H., Mano, T., Taya, M., Shimizu, K., Matsubara, M., & Kobayashi, T. (1987). Chemical Engineering Science, 42, 493–498. doi:10.1016/0009-2509(87)80011-8.
Shukla, R., Kang, W., & Sirkar, K. K. (1989). Biotechnology and Bioengineering, 34, 1158–1166. doi:10.1002/bit.260340906.
Mulchandani, A., & Volesky, B. (1986). Modelling of the acetone–butanol fermentation with cell retention. Canadian Journal of Chemical Engineering, 64, 625–631.
Atala, D. I. P. (2004). Ph.D. thesis, School of Food Engineering, University of Campinas, Campinas, Brazil.
Sandler, S. I. (1999). Chemical & engineering thermodynamics (3rd ed.). New York: Wiley.
Costa, C. B. B., & Maciel Filho, R. (2005). Chemical Engineering Science, 60, 5312–5322. doi:10.1016/j.ces.2005.04.068.
Barros Neto, B., Scariminio, I. S., & Bruns, R. E. (2001). Planejamento e Otimização de experimentos (3rd ed.). Campinas: Editora da Unicamp.
Costa, C. B. B., Costa, A. C., & Maciel Filho, R. (2005). Chemical Engineering Progress, 44, 737–753. doi:10.1016/j.cep.2004.08.004.
Tashiro, Y., Takeda, K., & Kobayashi, G. (2005). Journal of Biotechnology, 120, 197–206. doi:10.1016/j.jbiotec.2005.05.031.
Rezende, M. C., Costa, A. C., & Maciel Filho, R. (2004). International Journal of Chemical Reactor Engineering, 2, A21.
Phillips, J. A., & Humphrey, A. E. (1983). In E. J. Soltes (Ed.), Wood and agricultural residues: research on use for feed, fuels and chemicals. New York: Academic.
Jones, D. T., & Woods, D. R. (1986). Microbiological Reviews, 50, 484–524.
Gapes, J. R. (2000). Journal of Molecular Microbiology and Biotechnology, 2, 27–32.
Acknowledgements
The authors gratefully acknowledge the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the financial support (process numbers 2007/00341-1 and 2006/55177-9).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pinto Mariano, A., Bastos Borba Costa, C., de Franceschi de Angelis, D. et al. Optimization Strategies Based on Sequential Quadratic Programming Applied for a Fermentation Process for Butanol Production. Appl Biochem Biotechnol 159, 366–381 (2009). https://doi.org/10.1007/s12010-008-8450-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8450-6