Abstract
The past decades have witnessed the discovery of several gaseous signaling molecules in plants, including nitric oxide (NO), carbon monoxide (CO), hydrogen sulfide (H2S), and methane (CH4). These gasotransmitters (GTs) are endogenously synthesized in plant cells and participate in a variety of developmental processes and stress responses. Heavy metals (HMs) are one of the most widespread environmental cues that cause hazardous effects in plants and raise wide safety concerns. The involvement of GTs in the plant responses to HM has been demonstrated; however, our understanding of their exact roles and mechanisms in this area remains very fragmented. In this review, we provide an overview of the recent advances in the biosynthesis and regulation of each of the four GTs, their roles and mechanisms in plant HM uptake, accumulation, and detoxification, and the crosstalk between various GTs related to HM. Some reports on the negative roles of GTs on plant HM responses are also discussed. Certain important future directions for more in-depth studies are proposed based on the current understanding. Overall, this review provides a collection of rare information that helps to elucidate the regulation and functions of GTs in plant HM responses. This subject is of significant importance in the strategies of the agricultural efforts to reduce the risks associated with HMs by manipulating GTs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The term ‘heavy metal’ (HM) generally refers to metals with a density higher than 4–5 g cm− 3, such as cadmium (Cd), copper (Cu), zinc (Zn), nickel (Ni), cobalt (Co), chromium (Cr), lead (Pb), and mercury (Hg) (Arao et al. 2010). Due to the wide use of chemical fertilizers and sewage sludge in agriculture, inadequate irrigation, deployment of mining industry, and poor industrial waste disposal, agricultural lands in many areas of the world are contaminated by HMs (Mahar et al. 2016; Toth et al. 2016). Some of the HMs, including Co, Cu, iron (Fe), manganese (Mn), molybdenum (Mo), Ni, and Zn, are necessary for the normal growth of plants; however, HM concentrations must be strictly maintained below the threshold values to avoid toxicity. Other HMs, namely, Cd, Hg, and Pb, are nonessential and highly toxic to plants (Rascio and Izzo 2011). The cellular mechanisms of HM toxicity to plants include oxidative stress inducing free radicals and/or functional disruption of key enzymes due to the replacement of essential metals; HM stress typically causes attenuated plant growth and lower yield (Guala et al. 2010). In addition to harming plant growth, HMs accumulated in plant tissues can enter the food chain and threaten human health due to bioaccumulation.
To cope with the harmful HM concentrations in the soil, the activation and coordination of a complex signaling network in plants is required. Players of this network include reactive oxygen species (ROS), calcium–calmodulin system, phosphorylation cascades, hormones, and other emerging signal transmitters, such as gasotransmitters (GTs) (Dalcorso et al. 2010; Lamattina and García-Mata 2016). GTs are a class of signal molecules that meet the following criteria: (i) they are small gas molecules; (ii) they can freely cross biological membranes independent of cognate membrane receptors; (iii) they are endogenously generated by specific enzymes; (iv) their functions can be mimicked by exogenous application of donors; and (v) they have specific cellular and molecular targets, but their effects do not necessarily rely on secondary messengers (Wang 2002; García-Mata and Lamattina 2013). Four GTs, namely, nitric oxide (NO), carbon monoxide (CO), hydrogen sulfide (H2S), and methane (CH4), have been recently reported to be regulated by HM stimuli, and exogenous application of their donors alters cellular HM accumulation, redox status, ion homeostasis and photosynthesis (Han et al. 2008; Gill et al. 2013; Han et al. 2014; Kaur et al. 2015; Mostofa et al. 2015; Cui et al. 2017; Samma et al. 2017; Yu et al. 2019). Numerous review articles have described the biology of plant abiotic stress related to HM; however, there are no comprehensive reviews on the GT-HM relationship in plants. To address this gap in knowledge, we summarize the recent research progress on the biosynthesis, regulation, biochemical function and signal transduction of GTs in responses to HM stress in plants. Specifically, we provide a systematic view on the mechanisms that underpin GTs-regulated HM stress tolerance and point out their unique features. Furthermore, the crosstalk between different GTs and their roles in the interaction with HM are analyzed and discussed. Therefore, our review provides insight into the interplay between plant HM tolerance and GTs and proposes important future directions for additional in-depth studies to understand the functions of GTs in HM stress tolerance.
2 Functions of gasotransmitters in response to heavy metals in plants
2.1 Nitric oxide
2.1.1 General information, biochemical characteristics and biosynthesis
NO is naturally released as a toxic compound from industrial waste, vehicle exhaust and cigarette smoke. NO has been recognized as a GT mediating the vasodilatory effect of a number of factors in animal cells since the 1980s (Furchgott and Zawadzki 1980; Garthwaite et al. 1988; Schmidt and Walter 1994). The signaling functions of NO in plants were defined almost two decades later. In higher plants, NO can be produced by the reductive pathways or the oxidative pathways (Table 1, Cooney et al. 1994; Rockel 2002; Tun et al. 2006; Corpas et al. 2009). Cytosol-localized Nitrate reductase (NR), plasma membrane-bound nitrite-NO-reductase (NiNOR), and xanthine oxidoreductase (XOR) that is associated with peroxisomes are the key enzymes involved in the reductive pathways (Rockel 2002; Corpas et al. 2008; Neill et al. 2008). In addition, cytochrome-c-oxidase involved in the electron-transport chain of mitochondria also can reduce nitrite to produce NO (Farnese et al. 2016). The polyamine, L-arginine and hydroxylamine have been considered as the substrates for the synthesis of NO in the oxidative routes (Tun et al. 2006; Corpas et al. 2009; Rumer et al. 2009). It is assumed that polyamine oxidase and NO synthase (NOS) play roles in these oxidative transformations (Corpas et al. 2009; Farnese et al. 2016). However, the mechanisms underlying these oxidation reactions remain largely unclear (Shivaraj et al. 2020). Nonenzymatic systems for NO synthesis have also been reported, which include the ascorbic acid-, carotenoid- and nitrification-denitrification cycle-mediated pathways (Cooney et al. 1994; Wojtaszek 2000; Tun et al. 2006)
.
2.1.2 Regulation
Endogenous NO levels in plants can rapidly respond to many hormonal and stress stimuli implying fundamental functions of this GT in plants (Gupta et al. 2011; Shi et al. 2012; Gill et al. 2013). Pharmacological studies using an inhibitor of mammalian NO synthase (NOS) [L-NG-nitro arginine methyl ester (L-NAME)], NR inhibitor (tungsten), NO donors [S-nitrosoglutathione (GSNO), sodium nitroprusside (SNP) and S-nitroso-N-acetyl-D-penicillamine (SNAP)], and NO scavenger [2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxy-3-oxide (cPTIO)] (Shi et al. 2012; Cerana and Malerba 2015; Yang et al. 2016; Kaya et al. 2020), together with several lines of genetic evidence, have further supported the role of NO as an important signaling element in diverse physiological processes including HM response. However, the regulation of in vivo NO content by HMs appears to be concentration and species dependent (Table 2; Fig. 1, Bartha et al. 2005; Rodríguez-Serrano et al. 2009; Leterrier et al. 2012; Cerana and Malerba 2015
).
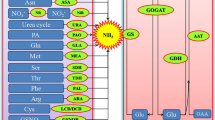
Illustration of the stimulatory or inhibitory effects of different heavy metals on endogenous gastransmitters release in plants. In general, the generation of CO and CH4 was stimulated by heavy metal treatments, and the accumulation of NO and H2S could be triggered or inhibited by different heavy metal stimuli. In details: despite the general stimulatory effect on all four gastransmitters, Cd treatment at specific conditions inhibited the generation of NO and H2S; Pb could induce H2S synthesis and either inhibit or activate NO production, depending on treatment scenarios; Al could trigger H2S and CH4 synthesis, and it exerted a dual function on NO; Cu enhanced the synthesis of NO and CH4; Fe could activate NO burst; Zn had a positive role on synthesis of NO and H2S; Cr and Ni were proved to trigger H2S generation; Hg was observed to induce gene expression of the CO synthesis enzyme, but whether it triggered CO release needs experimental confirmation. Arrowhead: activation; blunt end: inactivation
In wheat (Triticum aestivum) roots, Pb treatment at a concentration of 250 µM dramatically decreased the endogenous NO level, and exposure to 50 µM Pb slightly decreased the root NO level (Kaur et al. 2015). Treatment of Pogonatherum crinitum root cells with Pb at a concentration of 100 µM immediately induced the NR-dependent NO burst (Yu et al. 2012). NR-mediated early NO production was also observed in the shoots of hulless barley (Hordeum vulgare) upon treatment with a high concentration of Cu (450 µM) (Hu et al. 2015). In soybean (Glycine max) roots, Cd-induced generation of NO was also accompanied with the induction of NR activity (Perez-Chaca et al. 2014). In Arabidopsis (Arabidopsis thaliana), Cd-induced NO synthesis was suggested to be catalyzed by an as yet unidentified NOS-like enzyme (De Michele et al. 2009).While Cd treatment induces NO in the majority of investigated plant species, including Arabidopsis, rice (Oryza sativa), Barassica juncea, wheat, bermudagrass (Cynodon dactylon), and soybean (Besson-Bard et al. 2009; De Michele et al. 2009; Verma et al. 2013; Perez-Chaca et al. 2014; Shi et al. 2014; Yang et al. 2016; Kaya et al. 2020), inhibition of NO generation was reported in pea (Pisum sativum) in both root and leaf tissues after exposure to 50 µM Cd for 14 days (Rodríguez-Serrano et al. 2006, 2009). In addition, Akinyemi et al. (2017) reported a significant decrease of NO level in maize (Zea mays) plants accompanied by lowered content of total phenolics and glutathione (GSH) after 5 ppm Cd treatment. Al treatment at the concentration of 100 µM could inhibit NOS-mediated endogenous NO generation in Hibiscus moscheutos (Tian et al. 2007), while a release of NR-dependent NO was detected in Phaseolus vulgaris root after exposure to 50 µM Al for 24 h (Wang et al. 2010a). Excessive NO accumulation induced by Zn in Solanum nigrum roots was almost completely inhibited by a NOS inhibitor named L-NAME (Xu et al. 2010b). Taken together, these results indicate a complex regulation and function of NO in HM tolerance in plants.
2.1.3 Cytotoxic and cytoprotective roles
NO is a signal molecule and a member of the reactive nitrogen species (RNS); however, whether NO plays a cytotoxic or cytoprotective role under HM stress is controversial (Fig. 2a). On the one hand, excessive accumulation of NO or NO-derived RNS, including peroxynitrite (ONOO−), dinitrogen trioxide (N2O3) and GSNO, may induce nitrosative stress that can damage DNA, lipids, proteins, and carbohydrates leading to impaired cellular functions (Corpas et al. 2007; Liu et al. 2018). For example, NO contributes to Zn toxicity in the primary root tips of Solanum nigrum by increasing ROS accumulation and promoting programmed cell death (PCD) (Xu et al. 2010b). NO aggravates Cd-induced PCD by promoting mitogen-activated protein kinase 6 (MPK6)-mediated caspase-like activation, modulating the concentration and function of phytochelatin (PC) through protein S-nitrosylation or regulation of iron deprivation response in Arabidopsis (Besson-Bard et al. 2009; De Michele et al. 2009; Ye et al. 2013). Arabidopsis mutants with lower NO content in the roots were more resistant to Cd stress than WT plants due to better maintenance of the antioxidant systems (Terron-Camero et al. 2020). In pea plants, NO promoted Cd stress by inhibiting the expression and activity of S-nitrosoglutathione reductase (GSNOR) (Barroso et al. 2006). Collectively, these data demonstrate cytotoxic roles of the excessive NO triggered by heavy metal stress in plant cells.
Generalized model of gastransmitter signaling in response to heavy metal stresses in plants. a Cytotoxic and protective roles of NO in response to HMs. HMs trigger or inhibit in vivo NO release through up- or down-regulation of NO synthesis enzymes including NOS and NR. GSNO and SNP supplement can enhance the accumulation of NO under HM conditions. NO contributes to HM toxicity via two pathways. First, NO can induce nitrosative stress, activate MPK6-mediated Capase-3-like activation of ROS generation, reduce antioxidant capability through inhibition of APX and CAT activities, or enhance GSNOR-inhibited S-nitrosylation on PCs and MTs followed by a decrease of chelated HM level to enhance oxidative damage. Second, NO can promote uptake and further metal accumulation such as Cd and Pb. Beneficial effects can also be triggered by NO. NO can block HM accumulation through inhibiting HM uptake and root-to-shoot movement mediated by PEM-activated pectin demethylation. Photosynthetic, iron homeostasis, mineral nutrients and ATPase activity can be enhanced by NO signal in response to HM stress. In addition, NO can crosstalk with hormonal and other signals to alleviate HM toxicity. b The protective role of CO in response to HM. CO release triggered by HM or through supplementation of its donors including β-CD-hemin, heme and hemin alleviates HM toxicity through regulating HM accumulation and redox homeostasis. In relation to inhibition of HM accumulation, CO improves iron homeostasis by down-regulating IRT1, IRT2 and FRO2, blocks HM uptake by down-regulating ZIPs and NRMPs and enhances HM exclusion by up-regulating HMA2/4. c The protective role of H2S in response to HM. HM-regulated H2S generation is mediated by DCD activity and CDPKs-triggered LCD activity. H2S can activate antioxidant system to scavenge excessive ROS, leading to alleviated oxidative damage by HM. The inhibition of ROS by H2S also lowers HM uptake and accumulation as the plasma membrane calcium channels controlling HM influx is blocked. H2S can also promote HMs sequestration by enhancing their movement from cytosol to vacuolar, trigger PME-mediated HMs fixation to reduce their shootward movement, and improve photosynthesis. d The protective role of CH4 in response to HM. CH4 can scavenge ROS by activating antioxidant system or NADPH-dependent H2O2 signal to alleviate HM-induced oxidative damage. CH4 regulates expression of miRNA genes, whereby affect their mRNA targets to inhibit HM accumulation. Besides, CH4 can enhance organic acid secretion in roots by up-regulating expression of genes including MDH1/2, ALMT1, AACT and CS, leading to HM chelation. Solid line: protective pathway activated by HM. Hollow line: cytotoxic pathway triggered by HM. Dotted line: pathways need confirmation. Arrowhead: activation; blunt end: inhibition
On the other hand, NO can exert cellular protective functions in some cases. NO can directly or indirectly reduce ROS levels in plant cells (Saxena and Shekhawat 2013; Kaur et al. 2015). This effect is due to the antioxidant properties of NO, which can scavenge ROS, such as superoxide anion (O2−), to form ONOO−, which is less toxic than peroxides. SNP (100 µM) supplementation counteracted Pb-induced oxidative stress by modulating malondialdehyde, conjugated dienes, hydroxyl ions, and superoxide anion but not the antioxidant enzyme activities in wheat roots thereby suggesting its role as an antioxidant (Kaur et al. 2015). Additionally, NO can indirectly reduce ROS through activation of the antioxidant systems (Saxena and Shekhawat 2013). NO supplementation enhanced the activities of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) in tomato under Cu stress (Wang et al. 2010b) and increased the levels of polyamine and proline in Medicago truncatula under Cd stress (Filippou et al. 2013). Verma et al. (2013) showed that NO alleviated Cd-induced cytotoxicity by scavenging ROS through regulation of antioxidant responses in Brassica juncea. GSH is known to play a role in metal chelation and sequestration and stimulates the antioxidant system by regulating the redox signals under HM stress (Hasan et al. 2016). In Medicago truncatula, NO induced expression of the genes regulating GSH (Xu et al. 2011). Metallothioneins (MTs) are low molecular weight cysteine-rich metal-binding proteins that detoxify plant cells under HM stress. Exogenous NO can increase the content of MTs and enhance the resistance of tomato to Cu stress. In antisense-MT transgenic tomato plants, exogenous NO failed to significantly enhance plant resistance to Cu stress indicating that MTs play a role in NO-dependent alleviation of HM toxicity (Wang et al. 2010b).
2.1.4 Regulation of metal uptake and accumulation
A burst in NO is involved in Pb uptake and Cd accumulation (Besson-Bard et al. 2009; Yu et al. 2012); however, exogenous NO application can reduce the absorption of Cd by alfalfa (Xu et al. 2010a), Al by Cassia tora. (Wang and Yang 2005), argentum (Ag) by pea (Tripathi et al. 2017) and Zn accumulation in wheat and peas (Abdel-Kader 2007). The cell wall is the first barrier blocking HM entrance into protoplast and an important site of HM storage in plants; hence, deposition of the cell wall is considered a crucial mechanism of HM tolerance (Xiong et al. 2009). Treatment with 0.1 mM SNP significantly increased pectin and hemicellulose contents but decreased cellulose content in the cell walls of rice root, suggesting a mechanism of Cd reduction in soluble fraction of leaves by promoting Cd retention in root cell walls (Xiong et al. 2009). Wheat plants treated with the NO scavenger cPTIO had decreased activity of pectin methyl esterase (PME) in the roots resulting in an increase in pectin methylation in the cell wall and a reduction in the affinity of Al binding to pectin. Consequently, cPTIO boosted root growth and reduced Al accumulation in the roots (Sun et al. 2015).
2.1.5 Interaction of nitric oxide with hormonal signals
NO can interact with the hormonal signals in plants. This interaction can occur at different points of hormone actions, such as biosynthesis, degradation, conjugation, distribution or signaling, at the transcriptional or posttranslational level (Freschi 2013). For example, Cd stress inhibited the growth of the primary root and induced the formation of the lateral root (LR) and adventitious root (AR). Supplementation with the SNP donor GSNO increased the number of LR and AR under Cd stress, and use of cPTIO reversed this effect (Xu et al. 2011). The authors proposed that NO acts as a messenger partly by interacting with auxin to influence LR and AR development (Xu et al. 2011). NO interaction with plant hormones may play a role in Cd-mediated inhibition of root meristem growth (Yuan and Huang 2016). Cd stabilized the indole-3-acetic acid inducible 17 (AXR3/IAA17) protein to suppress auxin signaling in Cd-mediated root meristem growth inhibition, and the role of NO was confirmed by using the NO scavenger cPTIO or NOS inhibitor L-NAME (Yuan and Huang 2016). Salicylic acid (SA) and NO were coordinately involved in protective reactions of wheat under HM stress (Gilvanova et al. 2012). NO signaling was shown to participate in gibberellin (GA)-alleviated Cd toxicity in Arabidopsis (Zhu et al. 2012).
Moreover, NO can help to maintain cellular ion homeostasis, balance the mineral nutrients, reestablish ATPase activity, and hold the photosynthetic efficiency of plants (Gong et al. 2017; Kabala et al. 2019). The mechanisms of NO-dependent alleviation of HM toxicity also include modulation of protein kinase activities and elevation of cytosolic Ca2+ concentration through regulating of Ca2+ channels/transporters. The mobilization of other secondary messengers involved in the signaling cascade of gene expression regulation, such as is the case for cyclic guanosine monophosphate (cGMP) and cyclic adenosine diphosphate ribose (cADPR), was reported (Besson-Bard et al. 2009; Shi et al. 2014; Amooaghaie et al. 2015). The crosstalk between NO and other GTs, including CO and H2S, is discussed in detail in the third section of this review (3. Crosstalk of GTs in response to HMs).
2.2 Carbon monoxide
2.2.1 General information, biochemical characteristics and biosynthesis
CO is a colorless and odorless gaseous molecule that is ubiquitous in nature. CO is known as a “silent killer” due to high toxicity to humans and animals. The physiological functions of CO depend on its ability to bind to heme proteins. Similar to animals, the main source of endogenous CO production in plants is heme oxygenase (HO), which catalyzes degradation of heme to produce free iron, biliverdin Ixα, and CO (Table 1, Kikuchi et al. 2005; Ryter et al. 2006; Xuan et al. 2008a). In Arabidopsis, the HO family comprises four members that fall into two subfamilies as opposed to three subfamilies in animals (Dulak and Jozkowicz 2003; Bauer et al. 2008). The HO1 subfamily includes HO-1 (also known as HY1), HO-3, and HO-4, and the HO2 subfamily consists of only HO-2 (Emborg et al. 2006). In plants, the generation of CO is closely linked to the HO-1 enzymatic source (Xuan et al. 2008a; He aand He 2014). CO release in plants was reported as early as 1959 (Wilks 1959). So far, various roles of CO in plant physiology and stress tolerance have been revealed by using CO fumigation, aqueous CO solution, the artificial CO donors hematin and hemin, and combination of these agents with genetic approaches, e.g. transgenic overexpression of HO-1 or knockdown mutants (Han et al. 2008, 2014; Shen et al. 2011; Wei et al. 2011). However, CO has been only recently recognized as the second GT by the order of discovery after nitric oxide (Sukmansky and Reutov 2016).
2.2.2 Regulation
The determination of CO emission and increase in the activity of the rate-limiting enzyme HO-1 in plants upon HM treatment suggests the involvement of CO signaling in the HM stress response (Table 2; Fig. 1). Treatment with 100 µM or 200 µM Cd increased CO release in the root tissues of alfalfa as a result of upregulation of HO-1 expression (Han et al. 2008). GSH depletion is considered a part of the mechanisms involved in the transcriptional regulation (Cui et al. 2011). Furthermore, enhanced HO-1 activity combined with an increase in the relative expression level of HO-1 was characterized in Hg stress (Wei et al. 2011). Pretreatment of alfalfa plants with 50% CO-saturated aqueous solution for 6 h followed by Cd exposure resulted in an increase in CO and alleviation of Cd-induced oxidative damage (Han et al. 2008). Similarly, plants treated with exogenous CO and subsequently exposed to HgCl2 accumulated lower amount of Hg and displayed mitigated root inhibition (Meng et al. 2011). The induction of the HO-1 transcript in the root tissue of alfalfa by hematin and β-CD-hemin protected the plants from HM-induced oxidative damage. Overexpression of BnHO-1 in Brassica napus enhanced plant tolerance to Hg toxicity, indicating that HO-1-mediated CO signaling plays a cytoprotective role under HM stress (Shen et al. 2011). Moreover, HO-1-mediated CO release may function downstream of SA to alleviate Cd-induced oxidative stress in the root tissues of alfalfa seedlings (Cui et al. 2012). Collectively, the CO signal triggered by HM can protect the plants from the negative effect caused by HM.
2.2.3 Roles in suppressing heavy metal accumulation
The transcription and activity of HO-1 have been extensively shown to be markedly upregulated by hemin and are inducible by its own substrate, heme (Maines 1988; Xuan et al. 2008a; Hyvelin et al. 2010). Zhu et al. (2019) suggested that heme-induced amelioration of Cd toxicity in Chinese cabbage seedlings may be associated with inhibition of Cd uptake. In alfalfa seedlings, the induction of HO-1 by β-CD-hemin and hemin pretreatment reduced Cd toxicity partly by lowering Cd accumulation (Fu et al. 2011). A similar observation was reported in BnHO1-overexpressing transgenic rapeseed (Brassica napus) plants, which accumulated less Hg per unit of biomass compared to that in wild type plants (Shen et al. 2011). The inhibitory effect of CO on HM accumulation was confirmed in algae because overexpression of HO-1 in Chlamydomonas reinhardtii decreased Hg accumulation (Wei et al. 2011). Pharmacological experiments indicated that protection of the plants by CO against other HMs, including Al, Zn, Pb, and Cr, is related to suppressed metal accumulation in the tissues (Cui et al. 2013; Chen et al. 2017a, 2018).
The mechanism of CO inhibition of HM accumulation in plants remains poorly understood (Fig. 2b). It is known that Cd2+ uptake by the roots is mediated by low-specificity metal transporters or channels for Fe2+, Zn2+, and Ca2+ (Clemens 2006). Previous data showed that Cd2+ competed with Fe2+ in the process of absorption by inducing the transcripts of iron-regulated transporter 1 (IRT1) gene and activating a Fe-starvation signal (Vert et al. 2002). Therefore, homeostasis of essential metals, especially iron, can contribute to Cd uptake and transportation. In Arabidopsis, overexpression of HY1 (encodes HO-1) could down-regulate the genes related to Fe uptake including ferric reduction oxidase 2 (FRO2) and IRT1/2, thus contributing to Fe homeostasis and Cd2+ exclusion (Han et al. 2014). The result suggests that CO regulates nonessential HM uptake partly by controlling iron homeostasis. Additionally, downregulation of the genes related to HM uptake, such as members of the zinc-regulated transporter (ZIP) family and natural resistance-associated macrophage protein (NRAMP) family, may be another mechanism of CO -dependent reduction in HM accumulation (Chen et al. 2018; Zhu et al. 2019).
2.2.4 Functions in cytoprotection
In addition to suppression of metal accumulation, cytoprotection is an important mechanism involved in CO-mediated HM stress tolerance (Fig. 2b). First, CO can regulate the activities of antioxidant enzymes, including glutathione reductase (GR), monodehydroascorbate reductase (MDHAR), POD and SOD, leading to the scavenging of overproduced ROS induced by HM (Han et al. 2007; Shen et al. 2011). Treatment of HM-stressed rice plants with hemin, a HO-1 inducer, dramatically stimulated SOD, ascorbate peroxidase (APX) and GR activities and increased the content of ascorbic acid (AsA) and GSH, as opposed to the non-treated control (Chen et al. 2017a). Second, endogenous release of CO or upregulation of HO-1 can alleviate HM-induced oxidative damage by reestablishing GSH homeostasis via regulation of the enzymes responsible for GSH metabolism (Han et al. 2008; Fu et al. 2011). Third, nonenzymatic antioxidants, such as proline, have been shown to be involved in CO-dependent HM detoxification (Meng et al. 2011). Moreover, the HO-1/CO system can protect plants from HM-induced oxidative injury by improving accumulation of pigments including chlorophyll and carotenoid. Chen et al. (2017a) reported that application of hemin improved the accumulation of chlorophylla/b in the leaves of rice plants subjected to Zn, Pb and Cr stress. In Chinese cabbage, hemin-induced elevation in the chlorophyll and carotenoid content and photosynthetic enhancement were related to alleviation of Cd toxicity (Zhu et al. 2019).
2.3 Hydrogen sulfide
2.3.1 General information, biochemical characteristics and biosynthesis
H2S, with its typical odor of rotten eggs, has long been considered a phytotoxic substance (Jiang et al. 2016). The endogenous production and signaling role of H2S as a secondary messenger was established back in 1996 (Abe and Kimura 1996), and it was accepted as a GT a few year later (Wang 2002).
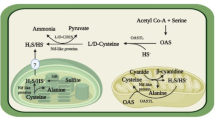
In higher plants, H2S release can occur mostly through the enzymatic pathways (Table 1), and a part of H2S is generated by the nonenzymatic pathways (Aroca et al. 2018). In the enzymatic pathways, L-cysteine desulfhydrase (L-DES/LCD) and D-cysteine desulfhydrase (D-DES/DCD) use L-cysteine and D-cysteine, respectively, as a substrate to generate H2S (Riemenschneider et al. 2005; Álvarez et al. 2010; Gotor et al. 2010). Cyanoalanine synthase (CAS) converts L-cysteine to cyanide that in turn leads to H2S synthesis (Akopyan et al. 1975), and cysteine synthase (CS) catalyzes the reversible conversion of L-cysteine and acetate to O-acetyl-L-serine (OAS) and H2S (Wirtz and Hell 2006). Carbonic anhydrase (CA) uses carbonyl sulfide taken up through stomata to generate H2S and carbon dioxide (CO2) (Notni et al. 2007; Rudenko et al. 2015). Sulfite reductase (SiR) catalyzes the reduction of sulfite to H2S in plant cells in the presence of ferredoxin (Da-Silva and Modolo 2018). Considerable evidence has proven the involvement of H2S in multiple physiological, developmental and metabolic processes (Xuan et al. 2020), including the modulation of stomatal movement (Jin et al. 2013), tissue maturation and senescence (Zhang et al. 2011), lateral root formation (Fang et al. 2014), and tolerance to various environmental stimuli (Ma et al. 2016; Zhou et al. 2018). In the following several paragraphs, the latest progress on the relationship between H2S and HM stimuli is summarized.
2.3.2 Gene expression and enzymatic activity regulation
Endogenous H2S levels in plants are inducible by various types of HM (Table 2; Fig. 1), including Cd (in bermudagrass, Shi et al. 2014; Brassica rapa, Hu et al. 2018; Salix matsudana, Yang et al. 2018 and rice, Mostofa et al. 2015), Zn (in Solanum nigrum, Liu et al. 2016), Cr (in Setaria italica, Fang et al. 2016) and Ni (in rice, Rizwan et al. 2019), Al (in barley, Chen et al. 2013), and Pb (in Capsicum annuum, Kaya 2020). However, a decrease of in vivo H2S in Brassica napus was observed after exposure of the plants to 20 µM CdCl2 for 7 days (Yu et al. 2019). Pretreatment of bermudagrass plants with sodium hydrosulfide hydrate (NaHS), an H2S donor, led to a strong induction of endogenous H2S upon Cd treatment compared to that in the non-pretreated controls indicating a positive feedback relationship (Shi et al. 2013). Cd-stimulated endogenous H2S level was found to be accompanied by elevated mRNA expression levels and enhanced enzymatic activities of both LCD and DCD in the roots of CdCl2-treated Brassica rapa (Hu et al. 2018). In Arabidopsis, Cd induction of LCD activity was further revealed to be mediated by calcium-dependent protein kinases (CDPKs) (Qiao et al. 2015). ZnCl2 treatment at the concentration of 400 µM, which is another type of HM stress, caused a similar enhancement of LCD activity and H2S content in the leaf and root tissues of Solanum nigrum, a known Cd/Zn hyperaccumulator (Liu et al. 2016). These results collectively suggest that LCD and DCD are important enzymes involved in H2S biosynthesis in response to HM stress.
2.3.3 Cytoprotection
The induction of H2S in vivo by HM is thought to play a cytoprotective role based on pharmacological experiments (Fig. 2c). Exogenous application of NaHS, an H2S donor, alleviated the growth inhibition induced by Cd stress in alfalfa, whereas the use of H2S synthesis inhibitors eliminated NaHS-induced Cd resistance (Cui et al. 2014). The mitigating effect of NaHS on Cd stress was also observed in other plants, including alfalfa, rape (Brassica napus), rice, and cucumber (Cucumis sativus) (Zhang et al. 2015; Fang et al. 2019; Kabala et al. 2019; Yu et al. 2019). In addition to reducing Cd toxicity, the application of NaHS can alleviate damage caused by Cr, Cu, Zn, Al, Ni, and Hg in a number of plant species partly by activating cellular protective pathways (Zhang et al. 2008, 2010a, b; Liu et al. 2016; Chen et al. 2017b; Rizwan et al. 2019). Maintenance and activation of the antioxidant system are considered one of the major pathways of cytoprotective function of H2S. H2S effectively blocked elevation of ROS upon Cd treatment by activation of protective enzymes (SOD, CAT, POD and APX) and GSH signaling cassettes to remove ROS in the roots of Brassica rapa (Zhang et al. 2015; Hu et al. 2018). In addition to the roots, application of NaHS activated a series of ROS-detoxifying enzymes (SOD, APX, CAT, MDHAR, dehydroascorbate reductase (DHAR), GR and glutathione peroxidase (GPX) and increased the levels of the nonenzymatic antioxidants AsA and GSH in the leaves of rice plants (Mostofa et al. 2015). In Arabidopsis, Cd-triggered H2S production increased cysteine level by upregulating the cysteine biosynthesis genes serine acetyltransferase 1 (SAT1) and cysteine synthase 1 (OASA1), which subsequently led to an increase in the expression of the MT and PC genes that are known ROS scavengers and HM chelators (Cobbett 2002; Jia et al. 2016). Additionally, proline content adjustment has been indicated as an important aspect of the maintenance of ROS homeostasis by H2S. In Cd-treated foxtail millet seedlings, inhibition of endogenous H2S blocked Cd-induced endogenous proline and aggravated cellular lipid peroxidation (Tian et al. 2016).
2.3.4 Photosynthetic enhancement
H2S was shown to modulate photosynthesis by promoting chloroplast biogenesis, photosynthetic enzyme expression, thiol redox modification and the activity of ribulose-1,5-bisphosphate carboxylase (Fig. 2c, Garcia-Mata and Lamattina 2010; Chen et al. 2011). In agreement with these findings, supplementation with H2S of Cd-stressed plants was found to alleviate the trend of chlorophyll and carotenoid decrease and to rescue the decrease in photosynthetic parameters, such as net photosynthetic rate, stomatal conductance, internal CO2 concentration, and transpiration rate (Ali et al. 2014; Mostofa et al. 2015). Therefore, enhancement of photosynthetic activity is considered to be an additional mechanism of H2S-triggered protective effects in response to HM. However, certain interesting questions remain. For instance, how does H2S regulate stomatal closure to affect its conductance? It was reported that H2S signaling induced stomatal closure under drought by impacting the activities of the inward K+ ion and anion channels (Papanatsiou et al. 2015; Wang et al. 2016; Jin et al. 2017; Du et al. 2018), whether the same mechanism holds true under HM stress is not known and may be an interesting subject of further studies.
2.3.5 Roles in regulating heavy metal uptake, movement and accumulation
Additionally, H2S plays a role in regulating HM uptake, movement and accumulation in plants (Fig. 2c, Mostofa et al. 2015; Fang et al. 2019). Pretreatment with NaHS significantly inhibited accumulation of Al and Cr in Hordeum vulgare (Ail et al. 2013; Chen et al. 2013), Cd in Brassica napus, rice, Arabidopsis and Salix matsudana (Ali et al. 2014; Mostofa et al. 2015; Yang et al. 2018), and Zn in Solanum nigrum (Liu et al. 2016). However, Sun et al. (2013) found that mitigation of Cd-induced oxidative damage by H2S in Populus euphratica cells was not only due to decreased Cd influx through hydrogen peroxide (H2O2)-activated plasma membrane calcium channels but also to increased partitioning of Cd to the vacuole through activation of tonoplast Cd2+/H+ antiporters. In Brassica napus, NaHS application markedly reduced Cd content in the stems and leaves and increased Cd retention in the root cell walls (Yu et al. 2019). Therefore, H2S plays a role in the root-shoot Cd translocation in plants, which is an important factor to consider with regard to the food safety and bioremediation of crops. However, our understanding of the mechanism of H2S regulation of HM intake to the roots and the subsequent translocation is very limited.
2.3.6 S-sulfhydration
S-sulfhydration is a posttranslational modification of proteins that converts cysteinyl thiolates (Cys-S(-)) to persulfides (Cys-S-S(-)) and is a specific mechanism of action of H2S (Aroca et al. 2015). S-sulfhydration can either activate, as described for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), parkin E3 ligase and respiratory burst oxidase homologue D (RBOHD) (Mustafa et al. 2009; Vandivder et al. 2013) or inactivate enzyme activities, as described for protein tyrosine phosphatases 1B (PTP1B) (Krishnan et al. 2011). S-sulfhydration has also been shown to modify protein-protein interactions, e.g. in the case of Kelch-like ECH-associated protein 1 (KEAP1), which acts as a negative regulator of nuclear factor (erythroid-derived 2)-like 2 (NRF2), a master regulator of the antioxidant response in mice (Yang et al. 2013). In plants, H2S-dependent S-sulfhydration has been involved in responses to heat, low temperature, ultraviolet radiation (UV), salinity, and osmotic stress (Hancock 2018; Gotor et al. 2019). Cysteines in proteins are the primary sites of S-sulfhydration (Aroca et al. 2015), and the GSSH content can be an indicator of the protein S-sulfhydration level (Qiao et al. 2015). Since GSSH content under HM stress is affected by H2S, S-sulfhydration is likely to occur in cysteine-rich proteins that are accumulated under HM stress, such as PCs and MTs.
2.4 Methane
2.4.1 Property and source
CH4 is the second most prevalent greenhouse gas on earth with the characteristics of being colorless, odorless, nontoxic, volatile and slightly soluble in water (Keppler et al. 2009; Li et al. 2019). CH4 was recognized as a GT only recently after NO, CO, and H2S (Wang 2014). Biological production of CH4 is predominantly related to anoxic environments and the activity of anaerobic prokaryotes (Archaea) (Conrad 2009; Kirschke et al. 2013); however, endogenous release of CH4 has also been observed in eukaryotes even in the absence of microbes and in the presence of oxygen (Keppler et al. 2006; Wang et al. 2011; Lenhart et al. 2012; Althoff et al. 2014). Enzymes involved in endogenous production of CH4 in plants are largely unknown (Table 1, Li et al. 2019). Numerous studies in plants have confirmed direct endogenous generation of CH4 under both normal growth and stress conditions, including cutting injuries, UV-B radiation, hypoxia, high temperature, water stress, low light, and salinity (Keppler et al. 2006; McLeod et al. 2008; Vigano et al. 2008; Bruggemann et al. 2009; Qaderi and Reid 2009; Wang et al. 2011; Abdulmajeed et al. 2017; Martel and Qaderi 2017).
2.4.2 Biological roles in plant heavy metal responses
A number of studies have revealed the role of CH4 in regulation of plant physiology. For example, CH4 acted as an upstream signaling molecule of NO to control lateral and adventitious rooting, redox homeostasis and starch metabolism (Qi et al. 2017; Zhang et al. 2018; Jin et al. 2020). Several recent lines of evidence have established a relationship between CH4 and HM response in plants (Table 2; Fig. 1, Samma et al. 2017; Cui et al. 2017; Gu et al. 2018). Aerobic nonmicrobial CH4 emission was determined in Cu-stressed alfalfa seeds, and the function of the emission can be mimicked by 0.39 mM CH4 in fresh saturation solution (Samma et al. 2017). Exogenous CH4 application mitigated Cu-inhibited seed germination and seedling growth and alleviated oxidative stress by decreasing lipid peroxidation and increasing plasma membrane integrity (Samma et al. 2017). The protective role of CH4 against Al stress was also established in alfalfa seedlings as demonstrated by the remission of Al-induced inhibition of root elongation, nutrient disorder, and relative electrolyte leakage (Cui et al. 2017). The release of CH4 in alfalfa root tissues was further confirmed under Cd stress, and pretreatment with exogenous CH4 mitigated Cd-induced inhibition of seedling growth (Gu et al. 2018). Taken together, these results suggest a generally advantageous effect of CH4 on HM tolerance in plants. Thus, regulation of cellular redox homeostasis and inhibition of metal accumulation have been linked to the mechanisms of the protective effects of CH4 in HM toxicity (Fig. 2d).
2.4.3 Roles in maintaining redox homeostasis
CH4 ameliorates HM-induced redox imbalance by regulating antioxidant enzymes, proline metabolism and GSH homeostasis. Reduction in SOD, APX and CAT activities and/or their gene expression levels under Cd, Al, and Cu stress was significantly suppressed by CH4 pretreatment (Cui et al. 2017; Samma et al. 2017; Gu et al. 2018). POD activity induced by Cu, Al, and Cd stress was further enhanced by CH4 (Cui et al. 2017; Samma et al. 2017; Gu et al. 2018). CH4 pretreatment blocked the oxidation of the GSH pool in alfalfa induced by Cd stress, which was accompanied by an increase in the expression levels of γ-glutamylcysteinyl synthetase (ECS), homoglutathione synthetase (hGS), GR1/2, and glutathione-S-transferase (GST); all these enzymes are related to the GSH synthesis and/or metabolism (Gu et al. 2018).
Despite the fact that overproduction of ROS under HM stress can be blocked by CH4, ROS are considered inducers of nonmicrobial CH4 emission. Conclusive evidence has shown that aerobic nonmicrobial CH4 production is limited by ROS removal and enhanced by the enzymes inhibiting this removal (Messenger et al. 2010). Moreover, certain types of ROS, e.g. nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase-dependent H2O2 production, can be triggered by CH4 to induce LR formation in tomato seedlings (Zhao et al. 2019). These lines of evidence indicate complex interactions between CH4 and ROS, and the biological significance of these interactions during HM stress remains to be explored in detail.
2.4.4 Roles in suppressing heavy metal accumulation
CH4 was shown to impede Cd accumulation in the roots of alfalfa seedlings by regulating HM transporters. Specific microRNAs, namely, miR159 and miR167 that participated in the modulation of the ATP-binding cassette (ABC) and NRAMP6 transporters, were down- and up-regulated by CH4, respectively (Gu et al. 2018). In CH4-pretreated alfalfa seedlings under Al stress, a reduction in Al intake was accompanied by upregulation of genes involved in organic acid metabolism and transport, i.e. citrate synthase, malate dehydrogenase 1/2 (MDH1/2), Al-activated malate transporter 1 (ALMT1), and Al-activated citrate transporter (AACT) were upregulated suggesting that CH4 prevents HM accumulation likely in an organic acid-dependent fashion (Cui et al. 2017).
3 Crosstalk of gasotransmitters in response to heavy metals
In addition to individual GTs that are indispensable for plant growth, development and environmental responses, the interactions of GTs have also been observed in the regulation of various physiological processes. For example, the HO-1/CO system was proposed to be a component or a signaling partner of the H2S-induced cytoprotection against PCD in wheat aleurone layers treated by GA (Xie et al. 2014). Cucumber HO-1 is also involved in NaHS-induced AR formation (Lin et al. 2012). CH4 functions upstream of NO in both LR and AR formation (Qi et al. 2017; Jin et al. 2020). In terms of root development, CO and H2S may be the second messengers downstream of CH4 (Cui et al. 2015; Kou et al. 2018; Mei et al. 2019). Similar results were demonstrated under the stress conditions coupled with redox imbalance thus confirming that CO, NO, and H2S are interlinked via the molecular signaling mechanisms involved in the CH4-induced stress tolerance in plant tissues (Zhu et al. 2016; Han et al. 2017; Samma et al. 2017; Zhang et al. 2018). However, to the best of our knowledge, there is no evidence showing crosstalk between CO and H2S or CH4 and other GTs in plant tolerance against HMs. The relationship between NO and CO under HM stress is discussed in the next section. The crosstalk between NO and H2S in HM detoxification is also discussed (Fig. 3).
Crosstalk of gastransmitters under heavy metal stresses. NO generation was inhibited by CO under Cd stress in Arabidopsis. However, CO has proved to be triggered by NO under Cd stress in soybean. Under Cd stress, NO was upstream of H2S signal in bermudagrass but downstream in wheat. Under Al stress, H2S inhibited NO synthesis in rice root. There is no evidence yet showing crosstalk between CO and H2S or CH4 and other GTs in plant tolerance against HMs. Arrowhead: activation; blunt end: inactivation. Question mark: undetected
3.1 Crosstalk between nitric oxide and carbon monoxide
It is becoming clear that NO and CO can modulate the actions of each other (Li et al. 2011; Xuan et al. 2012). For example, in Vicia guard cells, CO generation mediated by HO-1 causes stomatal closure in a NO- and cGMP-dependent manner (Cao et al. 2007). HO-1 is the enzymatic source of CO involved in the modulation of cucumber AR formation (Xuan et al. 2008b) and LR formation related with auxin and NO signal in tomato (Guo et al. 2008) via NO signaling. In response to osmotic stress, CO alleviated inhibition of seed germination and induction of lipid peroxidation, and this effect required participation of NO (Liu et al. 2010). Moreover, CO induced salt tolerance through maintenance of ion homeostasis and upregulation of antioxidant defense in wheat seedlings, and the effect was also mediated by NO (Xie et al. 2008). These results suggest that NO functions downstream of CO in physiological processes in the plants. This conclusion is also true under HM stress conditions; the difference is that NO is not induced but is inhibited by the HO/CO system. In detail, HY1 null mutant of Arabidopsis was able to induce NO overproduction and Cd accumulation. Overexpression of HY1 enhanced Cd exclusion via modulation of the expression levels of FRO2, IRT1 and heavy metal ATPase 2/4 (HMA2/4), suggesting that HY1 is beneficial to plants under Cd stress by decreasing NO production and improving Fe homeostasis in the root tissues thus contributing to a decrease in Cd accumulation and phytotoxicity (Han et al. 2014). However, in an attempt to protect soybean leaves against oxidative damage caused by Cd, Noriega et al. (2007) demonstrated that supplementation with 100 µM SNP, which is an NO donor, can upregulate the expression of the HO-1 gene, whereas cPTIO, a specific NO scavenger, blocked the NO-induced upregulation of HO-1 and NO-mediated protective effects. This result suggests that in response to Cd stress, NO acts upstream of CO. Currently, the mechanism of NO-CO interaction in plants is unclear; however, it is known that NO binds to Fe2+ of the heme group of human heme oxygenase HO-1 in vitro. Exploration of the putative feedback mechanism that controls the biological activity of NO and CO in plants is an interesting subject for future studies.
3.2 Crosstalk between nitric oxide and hydrogen sulfide
Since both NO and H2S are involved in plant responses to HM and share the same mechanisms of action, including antioxidant system maintenance, inhibition of HM accumulation, and interplay with Ca2+, it is intuitive to ask whether and how NO and H2S interact to coordinate their functions in these processes. H2S treatment reduced the negative charge in the root cell wall by decreasing the activity of PMEs, blocked Al-binding sites in the root cell wall, increased the secretion of citrate to reduce Al biding, enhanced antioxidant enzyme activities, and compartmentalized Al into the vacuole (Zhu et al. 2018). Zhu et al. (2018) found that applications of H2S and the NO donor SNP reduced and increased Al content, respectively, in the roots of Al-treated rice plants. The authors also observed a decrease in the NO content in rice roots after H2S treatment thereby implying that a decrease in the endogenous NO levels is involved in the H2S-induced alleviation of Al toxicity. In alfalfa seedlings, NaHS induced Cd tolerance phenotypes, including mitigated lipid peroxidation, arrested growth inhibition, and reduced Cd accumulation, and these effects were reversed by NO scavenger cPTIO (Li et al. 2012). In the roots of Cd-stressed wheat plants, NaHS and SNP application induced endogenous production of H2S and NO. Interestingly, the effect of NaHS was reversed by H2S scavenger hypotaurine (HT) and partly inhibited by cPTIO, whereas the effect of SNP was only sensitive to cPTIO (Kaya et al. 2020). These results suggest that NO acts as a downstream component of the H2S signaling pathway in Cd stress responses. Nevertheless, in Cd-stressed bermudagrass, induction of H2S by SNP was reversed by cPTIO and H2S scavenger/inhibitors, while NaHS-induced H2S generation was specifically blocked by HT indicating that NO plays a role upstream of H2S in Cd responses (Shi et al. 2014). Collectively, these experiments suggest a complex interplay of the two GTs that may depend on the plant species, tissue type and HM stress condition. Extensive future studies are thus needed to clarify this process.
4 Conclusions and future perspectives
Studies on GT regulation of important traits and environmental responses have become a research hotspot in the plant science. In the specific area of HM-related stress biology, accumulating evidence has proven the induction or inhibition of endogenous GTs in plants by different HM stimuli (Fig. 1; Table 2).The vast majority of the published work indicate that GTs enhance plant tolerance to HM stress, except for certain studies on NO that described negative roles, such as an increase in HM uptake and accumulation and promotion of HM-induced oxidative stress (Besson-Bard et al. 2009; Xu et al. 2010b; Yu et al. 2012; Terron-Camero et al. 2020). Identified mechanisms of GT regulation of HM stress include suppression of HM accumulation, regulation of antioxidant enzymes, restoration of redox and GSH homeostasis, maintenance of ion homeostasis, and promotion of photosynthesis (Fig. 2). From an agricultural perspective, a better understanding of how GTs regulate HM translocation in various parts of a plant is of particular interest because it may provide a foundation for breeding safer crops (low HM accumulation in edible parts) without compromising the ability of bioremediation (high HM accumulation in nonedible parts). In reality, GTs do not independently but coordinately work with others to modulate HM response (Fig. 3). The understanding of the biological interplay between GTs and other molecules is improving. For example, H2S was found to be necessary for abscisic acid (ABA)-stimulated NO accumulation and stomatal closure (Scuffi et al. 2014). The stimulatory effect of SNP on Ca2+ accumulation and calmodulin 1 (CaM1) expression was abolished by inhibiting H2S synthesis suggesting a “mediator” role of H2S between the NO and Ca2+ signaling pathways. However, currently published studies are far from creating a comprehensive picture of how these GTs modulate the protective and signaling pathways by interacting with phytohormones, second messengers and each other. More efforts are required to identify the global network mechanisms of the effects of GT on plant responses to HM.
To achieve more in-depth mechanistic understandings of GTs, some very interesting questions are worth to be explored in the near future: What are the direct target proteins of these GTs in the HM response? What types of chemical reactions/modifications occur during these processes? Is the information about GT crosstalk gained in other growth conditions/treatment scenarios transferable to the HM stress conditions? Although a large number of studies showed that GTs may be either induced or inhibited by HM stimulus, future studies on the metabolic pathways of these GTs should focus on the molecular details of their production pathways under HM stress. The intricate mechanisms associated with their responses to HM stimuli remain a subject of great interest. So far, proteomic studies have revealed at least three types of posttranslational modifications of the target proteins by NO: metal nitration, tyrosine nitration, and S-nitrosation (Astier and Lindermayr 2012). H2S may directly modify protein thiol groups via S-sulfhydration (Qiao et al. 2015). However, direct protein targets of these two GTs under HM stress are largely unknown. Answers to these questions will provide a clearer picture.
Abbreviations
- AACT :
-
Al-activated citrate transporter
- ABC :
-
ATP-binding cassette
- ALMT1 :
-
Al-activated malate transporter 1
- APX :
-
Ascorbate peroxidase
- AR :
-
Adventitious root
- AsA:
-
Ascorbic acid
- CA :
-
Carbonic anhydrase
- CAS :
-
Cyanoalanine synthase
- CAT :
-
Catalase
- CDPKs :
-
Calcium-dependent protein kinases
- cGMP :
-
Cyclic guanosine monophosphate
- CH4 :
-
Methane
- CO2 :
-
Carbon dioxide
- CO :
-
Carbon monoxide
- cPTIO :
-
2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxy-3-oxide
- CS :
-
Cysteine synthase
- D-DES/DCD:
-
D-cysteine desulfhydrase
- DHAR :
-
Dehydroascorbate reductase
- FRO2 :
-
Ferric reduction oxidase
- GA :
-
Gibberellin
- GPX :
-
Glutathione peroxidase
- GR :
-
Glutathione reductase
- GSH :
-
Glutathione
- GSNO :
-
S-nitrosoglutathione
- GSNOR :
-
S-nitrosoglutathione reductase
- GST :
-
Glutathione-S-transferase
- GT :
-
Gasotransmitter
- H2S:
-
Hydrogen sulfide
- HT :
-
Hypotaurine
- HMA2/4:
-
Heavy metal ATPase 2/4
- HM :
-
Heavy metal
- HO :
-
Heme oxygenase
- H2O2 :
-
Hydrogen peroxide
- HY1:
-
Heme oxygenase1, HO-1
- IRT1/2:
-
Iron-regulated transporter 1/2
- L-DES/LCD:
-
L-cysteine desulfhydrase
- L-NAME:
-
L-NG-nitro arginine methyl ester
- LR :
-
Lateral root
- MDA :
-
Malondialdehyde
- MDH1/2 :
-
Malate dehydrogenase 1/2
- MDHAR :
-
Monodehydroascorbate reductase
- MPK6 :
-
Mitogen-activated protein kinase 6
- MT :
-
Metallothionein
- NADPH :
-
Nicotinamide-adenine dinucleotide phosphate
- NaHS :
-
Sodium hydrosulfide hydrate
- NO :
-
Nitric oxide
- NOS :
-
Nitric oxide synthesis
- NRAMP :
-
Natural resistance-associated macrophage protein
- NR :
-
Nitrate reductase
- OAS :
-
O-acetyl-L-serine
- PCD :
-
Programmed cell death
- PC :
-
Phytochelatin
- PME :
-
Pectin methyl esterase
- POD :
-
Peroxidase
- RNS :
-
Reactive nitrogen species
- ROS :
-
Reactive oxygen species
- SAT1 :
-
Serine acetyltransferase 1
- SiR :
-
Sulfite reductase
- SNP :
-
Sodium nitroprusside
- SOD :
-
Superoxide dismutase
- TBARS :
-
Thiobarbituric acid reactive substances
- UV :
-
Ultraviolet radiation
- ZIP :
-
Zinc-regulated transporter
References
Abdel-Kader DEZ (2007) Role of nitric oxide, glutathione and sulfhydryl groups in zinc homeostasis in plants. Am J Plant Physiol 2:59–75. https://doi.org/10.3923/ajpp.2007.59.75
Abdulmajeed AM, Derby SR, Strickland SK, Qaderi MM (2017) Interactive effects of temperature and UVB radiation on methane emissions from different organs of pea plants grown in hydroponic system. J Photochem Photobiol B 166:193–201. https://doi.org/10.1016/j.jphotobiol.2016.11.019
Abe K, Kimura H (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16:1066–1071. https://doi.org/10.1523/jneurosci.16-03-01066.1996
Akinyemi AJ, Faboya OL, Olayide I, Faboya OA, Ayodeji O, Ijabadeniyi T (2017) Effect of cadmium stress on non-enzymatic antioxidant and nitric oxide levels in two varieties of maize (Zea mays). Bull Environ Contam Toxicol 98:845–849. https://doi.org/10.1007/s00128-017-2069-7
Akopyan TN, Braunstein AE, Goryachenkova EV (1975) Beta-cyanoalanine synthase: purification and characterization. Proc Natl Acad Sci 72:1617–1621. https://doi.org/10.1073/pnas.72.4.1617
Ali S, Farooq MA, Hussain S, Yasmeen T, Abbasi GH, Zhang GP (2013) Alleviation of chromium toxicity by hydrogen sulfide in barley. Environ Toxicol Chem 32:2234–2239. https://doi.org/10.1002/etc.2309
Ali B, Gill RA, Yang S, Gill MB, Ali S, Rafiq MT, Zhou WJ (2014) Hydrogen sulfide alleviates cadmium-induced morpho-physiological and ultrastructural changes in Brassica napus. Ecotoxicol Environ Saf 110:197–207. https://doi.org/10.1016/j.ecoenv.2014.08.027
Althoff F, Benzing K, Comba P, McRoberts C, Boyd DR, Greiner S, Keppler F (2014) Abiotic methanogenesis from organosulphur compounds under ambient conditions. Nat Commun 5:4205. https://doi.org/10.1038/ncomms5205
Álvarez C, Calo L, Romero LC, García I, Gotor C (2010) An O-acetylserine (thiol) lyase homolog with L-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiol 152:656–669. https://doi.org/10.1104/pp.109.147975
Amooaghaie R, Tabatabaei F, Ahadi AM (2015) Role of hematin and sodium nitroprusside in regulating Brassica nigra seed germination under nanosilver and silver nitrate stresses. Ecotoxicol Environ Saf 113:259–270. https://doi.org/10.1134/S000368381201005X
Arao T, Ishikawa S, Murakami M, Abe K, Maejima Y, Makino T (2010) Heavy metal contamination of agricultural soil and countermeasures in Japan. Paddy Water Environ 8:247–257. https://doi.org/10.1007/s10333-010-0205-7
Aroca A, Serna A, Gotor C, Romero LC (2015) S-sulfhydration: a cysteine posttranslational modification in plant systems. Plant Physiol 168:334–342. https://doi.org/10.1104/pp.15.00009
Aroca A, Gotor C, Romero LC (2018) Hydrogen sulfide signaling in plants: emerging roles of protein persulfidation. Front Plant Sci 9:1369. https://doi.org/10.3389/fpls.2018.01369
Astier J, Lindermayr C (2012) Nitric oxide-dependent posttranslational modification in plants: an update. Int J Mol Sci 13:15193–15208. https://doi.org/10.3390/ijms131115193
Barroso JB, Corpas FJ, Carreras A, Rodríguez-Serrano M, Esteban FJ, Fernandez-Ocana A, Chaki M, Romero-Puertas MC, Valderrama R, Sandalio LM, del Rio LA (2006) Localization of S-nitrosoglutathione and expression of S-nitrosoglutathione reductase in pea plants under cadmium stress. J Exp Bot 57:1785–1793. https://doi.org/10.1093/jxb/erj175
Bartha B, Kolbert Z, Erdei L (2005) Nitric oxide production induced by heavy metals in Brassica juncea L. Czern. and Pisum sativum L. Acta Biol Szeged 49:9–12. https://doi.org/10.2307/2443681
Bauer M, Huse K, Settmacher U, Claus RA (2008) The heme oxygenase-carbon monoxide system: regulation and role in stress response and organ failure. Intensive Care Med 34:640–648. https://doi.org/10.1007/s00134-008-1010-2
Besson-Bard A, Astier J, Rasul S, Wawer I, Dubreuil-Maurizi C, Jeandroz S, Wendehenne D (2009) Current view of nitric oxide-responsive genes in plants. Plant Sci 177:302–309. https://doi.org/10.1016/j.plantsci.2009.06.006
Bruggemann N, Meier R, Steigner D, Zimmer I, Louis S, Schnitzler J (2009) Nonmicrobial aerobic methane emission from poplar shoot cultures under low-light conditions. New Phytol 182:912–918. https://doi.org/10.1111/j.1469-8137.2009.02797.x
Cao ZY, Huang BK, Wang QY, Xuan W, Ling TF, Zhang B, Chen X, Nie L, Shen WB (2007) Involvement of carbon monoxide produced by heme oxygenase in ABA-induced stomatal closure in Vicia faba and its proposed signal transduction pathway. Chin Sci Bull 52:2365–2373. https://doi.org/10.1007/s11434-007-0358-y
Cerana R, Malerba M (2015) Role of nitric oxide in heavy metal stress. In: Khan M, Mobin M, Mohammad F, Corpas F (eds) Nitric oxide action in abiotic stress responses in plants. Springer, Cham, pp 181–192
Chen J, Wu FH, Wang WH, Zheng CJ, Lin GH, Dong XJ, He JX, Pei ZM, Zheng HL (2011) Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J Exp Bot 62:4481–4493. https://doi.org/10.1093/jxb/err145
Chen J, Wang WH, Wu FH, You CY, Liu TW, Dong XJ, He JX, Zheng HL (2013) Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362:301–318. https://doi.org/10.1007/s11104-012-1275-7
Chen Q, Zhang XY, Liu YY, Wei JY, Shen WB (2017a) Hemin-mediated alleviation of zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; suppressed metal uptake and oxidative stress in rice seedlings. Plant Growth Regul 81:253–264. https://doi.org/10.1007/s10725-016-0202-y
Chen Z, Chen M, Jiang M (2017b) Hydrogen sulfide alleviates mercury toxicity by sequestering it in roots or regulating reactive oxygen species productions in rice seedlings. Plant Physiol Biochem 111:179–192. https://doi.org/10.1016/j.plaphy.2016.11.027
Chen Q, Gong CY, Ju X, Zhu ZB, Shen WB, Shen ZG, Cui J (2018) Hemin through the heme oxygenase 1/ferrous iron, carbon monoxide system involved in zinc tolerance in Oryza Sativa L. Plant Growth Regul 37:947–957. https://doi.org/10.1007/s00344-018-9793-z
Chungopast S, Duangkhet M, Tajima S, Ma JF, Nomura M (2017) Iron-induced nitric oxide leads to an increase in the expression of ferritin during the senescence of Lotus japonicus nodules. J Plant Physiol 208:40–46. https://doi.org/10.1016/j.jplph.2016.11.004
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719. https://doi.org/10.1016/j.biochi.2006.07.003
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182. https://doi.org/10.1146/annurev.arplant.53.100301.135154
Conrad R (2009) The global methane cycle: recent advances in understanding the microbial processes involved. Environ Microbiol Rep 1:285–292. https://doi.org/10.1111/j.1758-2229.2009.00038.x
Cooney RV, Harwood PJ, Custer LJ, Franke AA (1994) Light-mediated conversion of nitrogen dioxide to nitric oxide by carotenoids. Environ Health Perspect 102:460–462. https://doi.org/10.1289/ehp.94102460
Corpas FJ, Barroso JB, Carreras A et al (2007) Nitrosative stress in plants: a new approach to understand the role of NO in abiotic stress. In: Lamattina L, Polacco JC et al (eds) Nitric oxide in plant growth. Springer, Berlin, pp 187–205
Corpas FJ, Palma JM, Sandalio LM, Valderrama R, Barroso JB, Del Rio LA (2008) Peroxisomal xanthine oxidoreductase:characterization of the enzyme from pea (Pisum sativum L.) leaves. J Plant Physiol 165:1319–1330. https://doi.org/10.1016/j.jplph.2008.04.004
Corpas FJ, Palma JM, Del Rio LA, Barroso JB (2009) Evidence supporting the existence of L-arginine-dependent nitric oxide synthase activity in plants. New Phytol 184:9–14. https://doi.org/10.1111/j.1469-8137.2009.02989.x
Cui W, Fu G, Wu H, Shen W (2011) Cadmium-induced heme oxygenase-1 gene expression is associated with the depletion of glutathione in the roots of Medicago sativa. Biometals 24:93–103. https://doi.org/10.1007/s10534-010-9377-2
Cui W, Li L, Gao Z, Wu H, Xie Y, Shen W (2012) Haem oxygenase-1 is involved in salicylic acid- induced alleviation of oxidative stress due to cadmium stress in Medicago sativa. J Exp Bot 63:5521–5534. https://doi.org/10.1093/jxb/ers201
Cui W, Zhang J, Xuan W, Xie Y (2013) Up-regulation of heme oxygenase-1 contributes to the amelioration of aluminum-induced oxidative stress in Medicago sativa. J Plant Physiol 170:1328–1336. https://doi.org/10.1016/j.jplph.2013.05.014
Cui WT, Chen HP, Zhu KK, Jin QJ, Xie YJ, Cui J, Xia Y, Zhang J, Shen WB (2014) Cadmium-induced hydrogen sulfide synthesis is involved in cadmium tolerance in Medicago sativa by reestablishment of reduced (homo) glutathione and reactive oxygen species homeostases. PLoS ONE 9:e109669. https://doi.org/10.1371/journal.pone.0109669
Cui W, Qi F, Zhang Y, Cao H, Zhang J, Wang R, Shen W (2015) Methane-rich water induces cucumber adventitious rooting through heme oxygenase1/carbon monoxide and Ca2+ pathways. Plant Cell Rep 34:435–445. https://doi.org/10.1007/s00299-014-1723-3
Cui W, Cao H, Yao P, Pan J, Gu Q, Xu S, Wang R, Ouyang Z, Wang Q, Shen W (2017) Methane enhances aluminum resistance in alfalfa seedlings by reducing aluminum accumulation and reestablishing redox homeostasis. Biometals 30:719–732. https://doi.org/10.1007/s10534-017-0040-z
Dalcorso G, Farinati S, Furini A (2010) Regulatory networks of cadmium stress in plants. Plant Signal Behav 5:663–667. https://doi.org/10.4161/psb.5.6.11425
Da-Silva CJ, Modolo LV (2018) Hydrogen sulfide: a new endogenous player in an old mechanism of plant tolerance to high salinity. Acta Bot Bras 32:150–160. https://doi.org/10.1590/0102-33062017abb0229
De Michele R, Vurro E, Rigo C, Costa A, Elviri L, Di Valentin M, Careri M, Zottini M, Sanita di Toppi L, Lo Schiavo F (2009) Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol 150:217–228. https://doi.org/10.1104/pp.108.133397
Du X, Jin Z, Zhang L, Liu X, Yang G, Pei Y (2018) H2S is involved in ABA-mediated stomatal movement through MPK4 to alleviate drought stress in Arabidopsis thaliana. Plant Soil 435:295–307. https://doi.org/10.1007/s11104-018-3894-0
Dulak J, Jozkowicz A (2003) Carbon monoxide: a “new” gaseous modulator of gene expression. Acta Biochim Pol 50:31–47. https://doi.org/10.18388/abp.2003_3712
Emborg TJ, Walker JM, Noh B, Vierstra RD (2006) Multiple heme oxygenase family members contribute to the biosynthesis of the phytochrome chromophore in Arabidopsis. Plant Physiol 140:856–868. https://doi.org/10.1104/pp.105.074211
Fang T, Cao Z, Li J, Shen W, Huang L (2014) Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol Biochem 76:44–51. https://doi.org/10.1016/j.plaphy.2013.12.024
Fang HH, Liu ZQ, Jin ZP, Zhang LP, Liu DM, Pei YX (2016) An emphasis of hydrogen sulfide-cysteine cycle on enhancing the tolerance to chromium stress in Arabidopsis. Environ Pollut 213:870–877. https://doi.org/10.1016/j.envpol.2016.03.035
Fang LC, Ju WL, Yang CL, Duan CJ, Cui YX, Han F, Shen GT, Zhang C (2019) Application of signaling molecules in reducing metal accumulation in alfalfa and alleviating metal-induced phytotoxicity in Pb/Cdcontaminated soil. Ecotoxicol Environ Saf 182:109459. https://doi.org/10.1016/j.ecoenv.2019.109459
Farnese FS, Menezes-Silva PE, Gusman GS, Oliveira JA (2016) When bad guys become good ones: the key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front Plant Sci 7:471. https://doi.org/10.3389/fpls.2016.00471
Filippou P, Antoniou C, Fotopoulos V (2013) The nitric oxide donor sodium nitroprusside regulates polyamine and proline metabolism in leaves of Medicago truncatula plants. Free Radical Biol Med 56:172–183. https://doi.org/10.1016/j.freeradbiomed.2012.09.037
Freschi L (2013) Nitric oxide and phytohormone interactions: current status and perspectives. Front Plant Sci 4:398. https://doi.org/10.3389/fpls.2013.00398
Fu GQ, Zhang LF, Cui WT, Wang YQ, Shen WB, Ren Y, Zheng TQ (2011) Induction of heme oxygenase-1 with β-CD-hemin complex mitigates cadmium-induced oxidative damage in the roots of Medicago sativa. Plant Soil 345:271–285. https://doi.org/10.1007/s11104-011-0779-x
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetyl-choline. Nature 288:373–376. https://doi.org/10.1038/288373a0
Garcia-Mata C, Lamattina L (2010) Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol 188:977–984. https://doi.org/10.1111/j.1469-8137.2010.03465.x
García-Mata C, Lamattina L (2013) Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci 201–202:66–73 https://doi.org/10.1016/j.plantsci.2012.11.007
Garthwaite J, Charles SL, Chess-Williams R (1988) Endothelium-derived relaxing factor release on activation of NMDA receptors suggests a role as intracellular messenger in the brain. Nature 336:385–388. https://doi.org/10.1038/336385a0
Gilvanova IR, Enikeev AR, Stepanov SY, Rakhmankulova ZF (2012) Involvement of salicylic acid and nitric oxide in protective reactions of wheat under the influence of heavy metals. Appl Biochem Micro 48:90–94
Gill SS, Hasanuzzaman M, Nahar K, Macovei A, Tuteja N (2013) Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol Biochem 63:254e261. https://doi.org/10.1016/j.plaphy.2012.12.001
Gong B, Nie W, Yan Y, Gao Z, Shi Q (2017) Unravelling cadmium toxicity and nitric oxide induced tolerance in Cucumis sativus: insight into regulatory mechanisms using proteomics. J Hazard Mater 336:202–213. https://doi.org/10.1016/j.jhazmat.2017.04.058
Gotor C, Alvarez C, Bermúdez MA, Moreno I, García I, Romero LC (2010) Low abundance does not mean less importance in cysteine metabolism. Plant Signal Behav 5:1028–1030. https://doi.org/10.4161/psb.5.8.12296
Gotor C, García I, Aroca A, Laureano A, Arenas-Alfonseca L, Jurado-Flores A, Moreno I, Romero (2019) Signaling by hydrogen sulfide and cyanide through posttranslational modification. J Exp Bot 70:4251–4265. https://doi.org/10.1093/jxb/erz225
Gu Q, Chen Z, Cui W, Zhang Y, Hu H, Yu X, Wang Q, Shen W (2018) Methane alleviates alfalfa cadmium toxicity via decreasing cadmium accumulation and reestablishing glutathione homeostasis. Ecotoxicol Environ Saf 147:861–871. https://doi.org/10.1016/j.ecoenv.2017.09.054
Guala SD, Vega FA, Covelo EF (2010) The dynamics of heavy metals in plant–soil interactions. Ecol Model 221:1148–1152. https://doi.org/10.1016/j.ecolmodel.2010.01.003
Guo K, Xia K, Yang ZM (2008) Regulation of tomato lateral root development by carbon monoxide and involvement in auxin and nitric oxide. J Exp Bot 59:3443–3452. https://doi.org/10.1093/jxb/ern194
Gupta KJ, Fernie AR, Kaiser WM, Van Dongen JT (2011) On the origins of nitric oxide. Trends Plant Sci 16:160e168. https://doi.org/10.1016/j.tplants.2010.11.007
Han Y, Xuan W, Yu T, Fang W, Lou T, Gao Y, Chen X, Xiao X, Shen W (2007) Exogenous hematin alleviates mercury-induced oxidative damage in the roots of Medicago sativa. J Integr Plant Biol 49:1703–1713. https://doi.org/10.1111/j.1744-7909.2007.00592.x
Han Y, Zhang J, Chen X, Gao Z, Xuan W, Xu S, Ding X, Shen W (2008) Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol 177:155–166. https://doi.org/10.1111/j.1469-8137.2007.02251.x
Han B, Yang Z, Xie Y, Nie L, Cui J, Shen W (2014) Arabidopsis HY1 confers cadmium tolerance by decreasing nitric oxide production and improving iron homeostasis. Mol Plant 7:388–403. https://doi.org/10.1093/mp/sst122
Han B, Duan X, Wang Y, Zhu K, Zhang J, Wang R, Hu H, Qi F, Pan J, Yan Y, Shen W (2017) Methane protects against polyethylene glycol-induced osmotic stress in maize by improving sugar and ascorbic acid metabolism. Sci Rep UK 7:46185. https://doi.org/10.1038/srep46185
Hancock JT (2018) Hydrogen sulfide and environmental stresses. Environ Exp Bot 161:50–56. https://doi.org/10.1016/j.envexpbot.2018.08.034
Hasan MK, Liu CC, Wang FN, Ahammed GJ, Zhou J, Xu MX, Yu JQ, Xia XJ (2016) Glutathione-mediated regulation of nitric oxide, S-nitrosothiol and redox homeostasis confers cadmium tolerance by inducing transcription factors and stress response genes in tomato. Chemosphere 161:536–545. https://doi.org/10.1016/j.chemosphere.2016.07.053
He HY, He LF (2014) Heme oxygenase 1 and abiotic stresses in plants. Acta Physiol Plant 36:581–588. https://doi.org/10.1007/s11738-013-1444-1
Hu YF, You J, Liang XL (2015) Nitrate reductase-mediated nitric oxide production is involved in copper tolerance in shoots of hulless barley. Plant cell Rep 34:367–379. https://doi.org/10.1007/s00299-014-1715-3
Hu LB, Li H, Huang SJ, Wang C, Sun WJ, Mo HZ, Shi ZQ, Chen J (2018) Eugenol confers cadmium tolerance via intensifying endogenous hydrogen sulfide signaling in Brassica rapa. J Agric Food Chem 66:9914–9922. https://doi.org/10.1021/acs.jafc.8b03098
Hyvelin JM, Maurel B, Uzbekov R, Motterlini R, Lermusiaux P (2010) Hemin prevents in-stent stenosis in rat and rabbit models by inducing heme-oxygenase-1. J Vasc Surg 51:417–428. https://doi.org/10.1016/j.jvs.2009.09.004
Jia HL, Wang XF, Dou YH, Liu D, Si WT, Fang H, Zhao C, Chen SL, Xi JJ, Li JS (2016) Hydrogen sulfide-cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots. Sci Rep 6:39702. https://doi.org/10.1038/srep39702
Jiang J, Chan A, Ali S et al (2016) Hydrogen sulfide-mechanisms of toxicity and development of an antidote. Sci Rep 6:20831. https://doi.org/10.1038/srep20831
Jin Z, Xue S, Luo Y, Tian B, Fang H, Li H, Pei Y (2013) Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol Biochem 62:41–46. https://doi.org/10.1016/j.plaphy.2012.10.017
Jin Z, Wang Z, Ma Q et al (2017) Hydrogen sulfide mediates ion fluxes inducing stomatal closure in response to drought stress in Arabidopsis thaliana. Plant Soil 419:141–152. https://doi.org/10.1007/s11104-017-3335-5
Jin XX, LiY, Lu RF, Cheng PF, Zhang YH, Li LN, Wang R, Cui J, Shen WB (2020) Methane-induced lateral root formation requires the participation of nitric oxide signaling. Plant Physiol Biochem 147:262–271. https://doi.org/10.1016/j.plaphy.2019.12.029
Kabala K, Zboińska M, Dorota G, Reda M, Jakubowska D, Janicka M (2019) Interaction between the signaling molecules hydrogen sulfide and hydrogen peroxide and their role in vacuolar H+-ATPase regulation in cadmium: tressed cucumber roots. Physiol Plantarum 166:688–704. https://doi.org/10.1111/ppl.12819
Kaur G, Singh HP, Batish DR, Mahajan P, Kohli RK, Rishi V (2015) Exogenous nitric oxide (NO) interferes with lead (Pb)-induced toxicity by detoxifying reactive oxygen species in hydroponically grown wheat (Triticum aestivum) roots. PLoS ONE 10:e0138713. https://doi.org/10.1371/journal.pone.0138713
Kaya C (2020) Salicylic acid-induced hydrogen sulphide improves lead stress tolerance in pepper plants by upraising the ascorbate-glutathione cycle. Physiol Plantarum. https://doi.org/10.1111/ppl.13159
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020) Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol Plantarum 168:345–360. https://doi.org/10.1111/ppl.13012
Keppler F, Hamilton JTG, Braß M, Röckmann T (2006) Methane emissions from terrestrial plants under aerobic conditions. Nature 439:187–191. https://doi.org/10.1038/nature04420
Keppler F, Boros M, Frankenberg C, Lelieveld J, McLeod A, Pirttilä AM, Röckmann T, Schnitzler JP (2009) Methane formation in aerobic environments. Environ Chem 6:459–465. https://doi.org/10.1071/EN09137
Kikuchi G, Yoshida T, Noguchi M (2005) Heme oxygenase and heme degradation. Biochem Biophys Res Commun. 338:558-567. https://doi.org/10.1016/j.bbrc.2005.08.020
Kirschke S, Bousquet P, Ciais P, Saunois M, Zeng G (2013) Three decades of global methane sources and sinks. Nat Geosci 6:813–823. https://doi.org/10.1038/NGEO1955
Kou NH, Xiang ZX, Cui WT, Li LN, Shen WB (2018) Hydrogen sulfide acts downstream of methane to induce cucumber adventitious root development. J Plant Physiol 228:113–120. https://doi.org/10.1016/j.jplph.2018.05.010
Krishnan N, Fu CX, Pappin DJ, Tonks NK (2011) H2S-induced sulfhydration of the phosphatase ptp1b and its role in the endoplasmic reticulum stress response. Sci Signal 4:ra86. https://doi.org/10.1126/scisignal.2002329
Lamattina L, García-Mata C (2016) Gasotransmitters in plants: the rise of a new paradigm in cell signaling. Springer, Switzerland
Lenhart K, Bunge M, Ratering S, Neu TR, Schuettmann I, Greule M, Kammann C, Schnell S, Mueller C, Zorn H, Keppler F (2012) Evidence for methane production by saprotrophic fungi. Nat Commun 3:1046. https://doi.org/10.1038/ncomms2049
Leterrier M, Airaki M, Palma JM, Chaki M, Barroso JB, Corpas FJ (2012) Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ Pollut 166:136–143. https://doi.org/10.1016/j.envpol.2012.03.012
Li MY, Cao ZY, Shen WB, Cui J (2011) Molecular cloning and expression of a cucumber (Cucumis sativus L.) heme oxygenase-1 gene, CsHO1, which is involved in adventitious root formation. Gene 486:47–55. https://doi.org/10.1016/j.gene.2011.07.008
Li L, Wang YQ, Shen WB (2012) Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. Biometals 25:617–631. https://doi.org/10.1007/s10534-012-9551-9
Li L, Wei S, Shen WB (2019) The role of methane in plant physiology: a review. Plant cell Rep 39:171–179. https://doi.org/10.1007/s00299-019-02478-y
Lin YT, Li MY, Cui WT, Lu W, Shen WB (2012) Haem oxygenase-1 is involved in hydrogen sulfide-induced cucumber adventitious root formation. Plant growth Regul 31:519–528. https://doi.org/10.1007/s00344-012-9262-z
Liu YH, Xu S, Ling TF, Xu LL, Shen WB (2010) Heme oxygenase/carbon monoxide system participates in regulating wheat seed germination under osmotic stress involving the nitric oxide pathway. J Plant Physiol 167:1371–1379. https://doi.org/10.1016/j.jplph.2010.05.021
Liu X, Chen J, Wang GH, Wang WH, Shen ZJ, Luo MR, Gao GF, Simon M, Ghoto K, Zheng HL (2016) Hydrogen sulfide alleviates zinc toxicity by reducing zinc uptake and regulating genes expression of antioxidative enzymes and metallothioneins in roots of the cadmium/zinc hyperaccumulator Solanum nigrum L. Plant Soil 400:177–192. https://doi.org/10.1007/s11104-015-2719-7
Liu SL, Yang RJ, Tripathi DK, Li X, Jiang MY, Lv BY, Ma MD, Chen QB (2018) Signalling cross-talk between nitric oxide and active oxygen in Trifolium repens L. plants responses to cadmium stress. Environ Pollut 239:53–68. https://doi.org/10.1016/j.envpol.2018.03.106
Ma D, Ding H, Wang C, Qin H, Han Q, Hou J, Lu H, Xie Y, Guo T (2016) Alleviation of drought stress by hydrogen sulfide is partially related to the abscisic acid signaling pathway in wheat. PLoS ONE 11:e0163082. https://doi.org/10.1371/journal.pone.0163082
Mahar A, Wang P, Ali A, Awasthi MK, Lahori AH, Wang Q, Li R, Zhang Z (2016) Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotoxicol Environ Saf 126:111–121. https://doi.org/10.1016/j.ecoenv.2015.12.023
Maines MD (1988) Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J 2:2557–2568. https://doi.org/10.1096/fasebj.2.10.3290025
Martel AB, Qaderi MM (2017) Light quality and quantity regulate aerobic methane emissions from plants. Physiol Plant 159:313–328. https://doi.org/10.1111/ppl.12514
McLeod AR, Fry SC, Loake GJ, Messenger DJ, Reay DS, Smith KA, Yun BW (2008) Ultraviolet radiation drives methane emissions from terrestrial plant pectins. New Phytol 180:124–132. https://doi.org/10.1111/j.1469-8137.2008.02571.x
Mei YD, Zhao YY, Jin XX, Wang R, Xu N, Hu JW, Huang LQ, Guan RZ, Shen WB (2019) L-Cysteine desulfhydrase-dependent hydrogen sulfide is required for methane-induced lateral root formation. Plant Mol Biol 99:283–298. https://doi.org/10.1007/s11103-018-00817-3
Meng DK, Chen J, Yang Z (2011) Enhancement of tolerance of Indian mustard (Brassica juncea) to mercury by carbon monoxide. J Hazard Mater 186:1823–1829. https://doi.org/10.1016/j.jhazmat.2010.12.062
Messenger DJ, Mcleod AR, Fry SC (2010) The role of ultraviolet radiation, photosensitizers, reactive oxygen species and ester groups in mechanisms of methane formation from pectin. Plant Cell Environ 32:1–9. https://doi.org/10.1111/j.1365-3040.2008.01892.x
Mostofa MG, Rahman A, Ansary MMU, Watanabe A, Fujita M, Tran LP (2015) Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci Rep 5:14078. https://doi.org/10.1038/srep14078
Mustafa AK, Gadalla MM, Sen N (2009) H2S signals through protein s-sulfhydration. Sci Signal 2:ra72. https://doi.org/10.1126/scisignal.2000464
Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I (2008) Nitric oxide, stomatal closure and abiotic stress. J Exp Bot 59:165–176. https://doi.org/10.1093/jxb/erm293
Noriega GO, Yannarelli GG, Balestrasse KB, Batlle A, Tomaro ML (2007) The effect of nitric oxide on heme oxygenase gene expression in soybean leaves. Planta 226:1155–1163. https://doi.org/10.1007/s00425-007-0561-8
Notni J, Schenk S, Protoschill-Krebs G, Kesselmeier J, Anders E (2007) The missing link in COS metabolism: a model study on the reactivation of carbonic anhydrase from its hydrosulfide analogue. Chembiochem 8:530–536. https://doi.org/10.1002/cbic.200600436
Papanatsiou M, Scuffi D, Blatt MR, Garcia-Mata C (2015) Hydrogen sulfide regulates inward-rectifying K+ channels in conjunction with stomatal closure. Plant Physiol 168:29–35. https://doi.org/10.1104/pp.114.256057
Perez-Chaca MV, Rodríguez-Serrano M, Molina SA, Pedranzani HE, Zirulnik F, Sandalio LM, Romero-Puertas MC (2014) Cadmium induces two waves of reactive oxygen species in Glycine max (L.) roots. Plant Cell Environ 37:1672–1687. https://doi.org/10.1111/pce.12280
Qaderi MM, Reid DM (2009) Methane emissions from six crop species exposed to three components of global climate change: temperature, ultraviolet-B radiation and water stress. Physiol Plant 137:139–147. https://doi.org/10.1111/j.1399-3054.2009.01268.x
Qi F, Xiang Z, Kou N, Cui W, Xu D, Wang R, Zhu D, Shen W (2017) Nitric oxide is involved in methane-induced adventitious root formation in cucumber. Physiol Plant 159:366–377. https://doi.org/10.1111/ppl.12531
Qiao ZJ, Jing T, Liu ZQ, Zhang LP, Jin ZP, Liu DM (2015) H2S acting as a downstream signaling molecule of SA regulates Cd tolerance in Arabidopsis. Plant Soil 393:137–146. https://doi.org/10.1007/s11104-015-2643-x
Rascio N, Izzo FN (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180:169–181. https://doi.org/10.1016/j.plantsci.2010.08.016
Riemenschneider A, Wegele R, Schmidt A, Papenbrock J (2005) Isolation and characterization of a d-cysteine desulfhydrase protein from Arabidopsis thaliana. J FEBS 272:1291–1304. https://doi.org/10.1111/j.1742-4658.2005.04567.x
Rizwan M, Mostofa MG, Ahmad MZ, Zhou YY, Adeel M, Mehmood S, Ahmad MA, Javed R, Imtiaz M. Aziz O, Ikram M, Tu S, Liu YX (2019) Hydrogen sulfide enhances rice tolerance to nickel through the prevention of chloroplast damage and the improvement of nitrogen metabolism under excessive nickel. Plant Physiol Biochem 138:100–111. https://doi.org/10.1016/j.plaphy.2019.02.023
Rockel P (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53:103–110. https://doi.org/10.1093/jexbot/53.366.103
Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gomez M, Del Rio LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544. https://doi.org/10.1111/j.1365-3040.2006.01531.x
Rodríguez-Serrano M, Romero-Puertas MC, Pazmin DM, Testillano PS, Risueo MC, Del Rio LA, Sandalio LM (2009) Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150:229–243. https://doi.org/10.1111/ppl.13012
Romero LC, García I, Gotor C (2013) L-cysteine desulfhydrase 1 modulates the generation of the signaling molecule sulfide in plant cytosol. Plant Signal Behav 8:4621–4634. https://doi.org/10.4161/psb.24007
Rudenko NN, Ignatova LK, Fedorchuk TP, Ivanov BN (2015) Carbonic anhydrases in photosynthetic cells of higher plants. Biochemistry 80:674–687. https://doi.org/10.1134/S0006297915060048
Rumer S, Gupta KJ, Kaiser WM (2009) Plant cell soxidize hydroxylamines to NO. J Exp Bot 60:2065–2072. https://doi.org/10.1093/jxb/erp077
Ryter SW, Alam J, Choi AMK (2006) Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86:583–650. https://doi.org/10.1152/physrev.00011.2005
Samma MK, Zhou H, Cui W, Zhu K, Zhang J, Shen W (2017) Methane alleviates copper-induced seed germination inhibition and oxidative stress in Medicago sativa. Biometals 30:97–111. https://doi.org/10.1007/s10534-017-9989-x
Saxena I, Shekhawat GS (2013) Nitric oxide (NO) in alleviation of heavy metal induced phytotoxicity and its role in protein nitration. Nitric Oxide 32:13–20. https://doi.org/10.1016/j.niox.2013.03.004
Schmidt HH, Walter U (1994) NO at work. Cell 78:919–925. https://doi.org/10.1016/0092-8674(94)90267-4
Scuffi D, Álvarez C, Laspina N, Gotor C, Lamattina L, García-Mata C (2014) Hydrogen sulfide generated by L-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol 166:2065–2076. https://doi.org/10.1104/pp.114.245373
Shen Q, Jiang M, Li H, Che L, Yang Z (2011) Expression of a Brassica napus heme oxygenase confers plant tolerance to mercury toxicity. Plant Cell Environ 34:752–763. https://doi.org/10.1111/j.1469-8137.2007.02251.x
Shi H, Wang Y, Chen Z, Ye T, Chan Z (2012) Analysis of natural variation in bermudagrass (Cynodon dactylon) reveals physiological responses underlying drought tolerance. PLoS ONE 7:e53422. https://doi.org/10.1371/journal.pone.0053422
Shi HT, Ye TT, Chan ZL (2013) Exogenous application of hydrogen sulfide donor sodium hydrosulfide enhanced multiple abiotic stress tolerance in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol Biochem 71:226–234. https://doi.org/10.1016/j.plaphy.2013.07.021
Shi HT, Ye TT, Chan ZL (2014) Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol Biochem 74:99e107. https://doi.org/10.1016/j.plaphy.2013.11.001
Shivaraj SM, Vats S, Bhat JA, Dhakte P, Goyal V, Khatri P, Kumawat S, Singh A, Prasad M (2020) Nitric oxide and hydrogen sulfide crosstalk during heavy metal stress in plants. Physiol Plantarum 168:437–455. https://doi.org/10.1111/ppl.13028
Sukmansky OI, Reutov VP (2016) Gasotransmitters: physiological role and involvement in the pathogenesis of the diseases. Usp Fiziol Nauk 47:30–58. https://doi.org/10.3389/fpls.2016.00277
Sun J, Wang R, Zhang X, Yu YC, Zhao R, Li ZY, Chen SL (2013) Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes in Populus euphratica cells. Plant physiol Biochem 65:67–74. https://doi.org/10.1016/j.plaphy.2013.01.003
Sun CL, Liu LJ, Yu Y, Liu WJ, Lu LL, Jin CW, Lin XY (2015) Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. J Integr Plant Biol 57:550–561. https://doi.org/10.1111/jipb.12298
Terron-Camero LC, del Val C, Sandalio LM, Romero-Puertas MC (2020) Low endogenous NO levels in roots and antioxidant systems are determinants for the resistance of Arabidopsis seedlings grown in Cd. Environ Pollut 256:113411. https://doi.org/10.1016/j.envpol.2019.113411
Tian QY, Sun DH, Zhao MG, Zhang WH (2007) Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos. New Phytol 174:322–331. https://doi.org/10.1111/j.1469-8137.2007.02005.x
Tian B, Qiao Z, Zhang L, Li H, Pei Y (2016) Hydrogen sulfide and proline cooperate to alleviate cadmium stress in foxtail millet seedlings. Plant Physiol Biochem 109:293–299. https://doi.org/10.1016/j.plaphy.2016.10.006
Toth G, Hermann T, Silva MRD, Montanarella L (2016) Heavy metals in agricultural soils of European Union with implications for food safety. Environ Int 88:299–309. https://doi.org/10.1016/j.envint.2015.12.017
Tripathi DK, Singh S, Singh S et al (2017) Nitric oxide alleviates silver nanoparticles (AgNps)- induced phytotoxicity in Pisum sativum seedlings. Plant Physiol Biochem 110:167–177. https://doi.org/10.1016/j.plaphy.2016.06.015
Tun NN, Santa-Catarina C, Begum T, Silveira V, Handro W, Floh EIS, Scherer GFE (2006) Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol 47:346–354. https://doi.org/10.1093/pcp/pci252
Vandivder MS, Paul BD, Xu RS et al (2013) Sulfhydration mediates neuroprotective actions of parkin. Nat Commun 4:1626. https://doi.org/10.1038/ncomms2623
Verma K, Mehta SK, Shekhawat GS (2013) Nitric oxide (NO) counteracts cadmium induced cytotoxic processes mediated by reactive oxygen species (ROS) in Brassica juncea: cross-talk between ROS, NO and antioxidant responses. Biometals 26:255–269. https://doi.org/10.1007/s10534-013-9608-4
Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat J, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14:1223–1233. https://doi.org/10.1105/tpc.001388
Vigano I, Van Weelden H, Holzinger R, Keppler F, Röckmann T (2008) Effect of UV radiation and temperature on the emission of methane from plant biomass and structural components. Biogeosciences 5:243–270. https://doi.org/10.5194/bg-5-937-2008
Wang R (2002) Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16:1792–1798. https://doi.org/10.1096/fj.02-0211hyp
Wang R (2014) Gasotransmitters: growing pains and joys. Trends Biochem Sci 39:227–232. https://doi.org/10.1016/j.tibs.2014.03.003
Wang YS, Yang ZM (2005) Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant Cell Physiol 46:1915–1923. https://doi.org/10.1093/pcp/pci202
Wang HH, Huang JJ, Bi YR (2010a) Nitrate reductase-dependent nitric oxide production is involved in aluminum tolerance in red kidney bean roots. Plant Sci 179:281–288. https://doi.org/10.1016/j.plantsci.2010.05.014
Wang LN, Yang LM, Yang FJ, Li XG, Song YP, Wang XF, Hu XY (2010b) Involvements of H2O2 and metallothionein in NO-mediated tomato tolerance to copper toxicity. J Plant Physiol 167:1298–1306. https://doi.org/10.1016/j.jplph.2010.04.007
Wang Z, Keppler F, Greule M, Hamilton JTG (2011) Non-microbial methane emissions from fresh leaves: effects of physical wounding and anoxia. Atmos Environ 45:4915–4921. https://doi.org/10.1016/j.atmosenv.2011.06.001
Wang L, Wan R, Shi Y, Xue S (2016) Hydrogen sulfide activates S-type anion channel via OST1 and Ca2+ modules. Mol Plant 9:489–491. https://doi.org/10.1016/j.molp.2015.11.010
Wei YY, Zheng Q, Liu ZP, Yang ZM (2011) Regulation of tolerance of chlamydomonas reinhardtii to heavy metal toxicity by heme oxygenase-1 and carbon monoxide. Plant Cell Physiol 52:1665–1675. https://doi.org/10.1093/pcp/pcr102
Wilks SS (1959) Carbon monoxide in green plants. Science 129:964–966. https://doi.org/10.1126/science.129.3354.964
Wirtz M, Hell R (2006) Functional analysis of the cysteine synthase protein complex from plants: structural, biochemical and regulatory properties. J Plant Physiol 163:273–286. https://doi.org/10.1016/j.jplph.2005.11.013
Wojtaszek P (2000) Nitric oxide in plants: to NO or not to NO. Phytochemistry 54:1–4. https://doi.org/10.1002/chin.200033282
Xie YJ, Ling TF, Han Y, Liu KL, Zheng QS, Huang LQ, Yuan XX, Ziyi HE, Bing HU, Fang L (2008) Carbon monoxide enhances salt tolerance by nitric oxide-mediated maintenance of ion homeostasis and up-regulation of antioxidant defence in wheat seedling roots. Plant Cell Environ 31:1864–1881. https://doi.org/10.1111/j.1365-3040.2008.01888.x
Xie YJ, Zhang C, Lai DW, Sun Y, Samma MK, Zhang J, Shen WB (2014) Hydrogen sulfide delays GA-triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-1 expression. J Plant Physiol 171:53–62. https://doi.org/10.1016/j.jplph.2013.09.018
Xiong J, An L, Lu H, Zhu C (2009) Exogenous nitric oxide enhances cadmium tolerance by increasing pectin and hemicellulose contents in root cell wall. Planta 230:755–765. https://doi.org/10.1007/s00425-009-0984-5
Xu J, Wang WY, Yin HX, Liu XJ, Sun H, Min Q (2010a) Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil 326:321–330. https://doi.org/10.1007/s11104-009-0011-4
Xu J, Yin H, Li Y, Liu X (2010b) Nitric oxide is associated with long-term zinc tolerance in Solanum nigrum. Plant Physiol 154:1319–1334. https://doi.org/10.1104/pp.110.162982
Xu J, Wang W, Sun J, Zhang Y, Ge Q, Du L, Yin H, Liu X (2011) Involvement of auxin and nitric oxide in plant Cd-stress response. Plant Soil 346:107–119. https://doi.org/10.1007/s11104-011-0800-4
Xuan W, Xu S, Yuan X. Shen W (2008a) Carbon monoxide: a novel and pivotal signal molecule in plants? Plant Signal Behav 3:381–382. https://doi.org/10.4161/psb.3.6.5374
Xuan W, Zhu FY, Xu S et al (2008b) The heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious rooting process. Plant Physiol 148(2):881–893. https://doi.org/10.1104/pp.108.125567
Xuan W, Xu S, Li M, Han B, Zhang B, Zhang J, Lin Y, Huang J, Shen W, Cui J (2012) Nitric oxide is involved in hemin-induced cucumber adventitious rooting process. J Plant Physiol 169:1032–1039. https://doi.org/10.1016/j.jplph.2012.02.021
Xuan LJ, Li J, Wang XY, Wang CY (2020) Crosstalk between hydrogen sulfide and other signal molecules regulates plant growth and development. Int J Mol Sci 21:4593. https://doi.org/10.3390/ijms21134593
Yang GD, Zhao KX, Ju YJ, Mani S, Cao QH, Puukila S, Khaper N, Wu LY, Wang R (2013) Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and Activation of Nrf2. Antioxid Redox Sign 18:1906–1919. A. https://doi.org/10.1089/ars.2012.4645
Yang L, Ji J, Harris-Shultz KR, Wang H, Wang H, Abd-Allah EF, Luo Y, Hu X (2016) The dynamic changes of the plasma membrane proteins and the protective roles of nitric oxide in rice subjected to heavy metal cadmium stress. Front Plant Sci 7:190. https://doi.org/10.3389/fpls.2016.00190
Yang LP, Zeng J, Wang P, Zhu J (2018) Sodium hydrosulfide alleviates cadmium toxicity by changing cadmium chemical forms and increasing the activities of antioxidant enzymes in salix. Environ Exp Bot 156:161–169. https://doi.org/10.1016/j.envexpbot.2018.08.026
Ye Y, Li Z, Xing D (2013) Nitric oxide promotes MPK6-mediated caspase-3-like activation in cadmium-induced Arabidopsis thaliana programmed cell death. Plant Cell Environ 36:1–15. https://doi.org/10.4161/psb.21893
Yu Q, Sun L, Jin H, Chen Q, Chen Z, Xu M (2012) Lead-induced nitric oxide generation plays a critical role in lead uptake by Pogonatherum crinitum root cells. Plant Cell Physiol 53:1728–1736. https://doi.org/10.1093/pcp/pcs116
Yu Y, Zhou XY, Zhu ZH, Zhou KJ (2019) Sodium hydrosulfide mitigates cadmium toxicity by promoting cadmium retention and inhibiting its translocation from roots to shoots in brassica napus. J Agric Food Chem 67:433–440. https://doi.org/10.1021/acs.jafc.8b04622
Yuan HM, Huang X (2016) Inhibition of root meristem growth by cadmium involves nitric oxide-mediated repression of auxin accumulation and signaling in Arabidopsis. Plant Cell Environ 39:120–135. https://doi.org/10.1111/pce.12597
Zhang H, Hu LY, Hu KD, He YD, Wang SH, Luo JP (2008) Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol 50:1518–1529. https://doi.org/10.1111/j.1744-7909.2008.00769.x
Zhang H, Hu LY, Li P, Hu KD, Jiang CX, Luo JP (2010a) Hydrogen sulfide alleviated chromium toxicity in wheat. Biol Plantarum 54:743–747. https://doi.org/10.1007/s10535-010-0133-9
Zhang H, Tan ZQ, Hu LY, Wang SH, Luo JP, Jones RL (2010b) Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. J Integr Plant Biol 52:556–567. https://doi.org/10.1111/j.1744-7909.2010.00946.x
Zhang H, Hu SL, Zhang ZJ, Hu LY, Jiang CX, Wei ZJ, Liu J, Wang HL, Jiang ST (2011) Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol Technol 60:251–257. https://doi.org/10.1016/j.postharvbio.2011.01.006
Zhang LP, Pei YX, Wang HJ, Jin ZP, Liu ZQ, Qiao ZJ, Fang HH, Zhang YJ (2015) Hydrogen sulfide alleviates cadmium-induced cell death through restraining ROS accumulation in roots of brassica rapa L. ssp. pekinensis. Antioxid Redox Sign 2015:1–11. https://doi.org/10.1155/2015/804603
Zhang Y, Su J, Cheng D, Wang R, Mei Y, Hu H, Shen W, Zhang Y (2018) Nitric oxide contributes to methane-induced osmotic stress tolerance in mung bean. BMC Plant Biol 18:207. https://doi.org/10.1186/s12870-018-1426-y
Zhao YY, Zhang YH, Liu FJ, Wang R, Huang LQ, Shen WB (2019) Hydrogen peroxide is involved in methane-induced tomato lateral root formation. Plant Cell Rep 38:377–389. https://doi.org/10.1007/s00299-019-02372-7
Zhou ZH, Wang Y, Ye XY, Li ZG (2018) Signaling molecule hydrogen sulfide improves seed germination and seedling growth of maize (Zea mays L.) under high temperature by inducing antioxidant system and osmolyte biosynthesis. Front Plant Sci 9:1288. https://doi.org/10.3389/fpls.2018.01288
Zhu XF, Jiang T, Wang ZW, Lei GJ, Shi YZ, Li GX, Zhang SJ (2012) Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J Hazard Mater 239–240:302–307. https://doi.org/10.1016/j.jhazmat.2012.08.077
Zhu K, Cui W, Dai C, Wu M, Zhang J, Zhang Y, Xie Y, Shen W (2016) Methane-rich water alleviates NaCl toxicity during alfalfa seed germination. Environ Exp Bot 129:37–47. https://doi.org/10.1016/j.envexpbot.2015.11.013
Zhu CQ, Zhang JH, Sun LM, Zhu LF, Abliz B, Hu WJ, Zhong C, Bai ZG, Sajid H, Cao XC (2018) Hydrogen sulfide alleviates aluminum toxicity via decreasing apoplast and symplast Al contents in rice. Front Plant Sci 9:294. https://doi.org/10.3389/fpls.2018.00294
Zhu ZB, Huang YF, Wu X, Liu ZL, Zou JW, Chen YH, Su NN (2019) Increased antioxidative capacity and decreased cadmium uptake contribute to hemin-induced alleviation of cadmium toxicity in Chinese cabbage seedlings. Ecotoxicol Environ Saf 177:47–57. https://doi.org/10.1016/j.ecoenv.2019.03.113
Funding
This work was supported by the Key Research Program of Zhejiang Province (2021C02041) and the start-up funds for high-end talents from China Jiliang University (Grant No. 19072501).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fang, P., Sun, T., Wang, Y. et al. Plant gasotransmitters: light molecules interplaying with heavy metals. Rev Environ Sci Biotechnol 20, 31–53 (2021). https://doi.org/10.1007/s11157-020-09562-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-020-09562-w