Abstract
Heme oxygenase (HO, EC 1.14.99.3) catalyzes the oxidative conversion of heme to biliverdin IXα with the concomitant release of carbon monoxide and iron. Recently, HO has been involved in the protection against oxidative stress in plants. The fact that nitric oxide (NO), an endogenous signaling molecule in animals and plants mediates responses to abiotic and biotic stresses, prompted us to study whether this molecule could modulate HO-1 gene transcription. To fulfill this objective leaves of soybean (Glycine max L.) plants were stimulated with Cd, employing an acute intoxication model. Cadmium caused dehydration, chlorophyll loss and ion leakage. Semi-quantitative RT-PCR analysis showed no augmentation of HO-1 transcript levels with respect to controls. Pretreatment with 100 μM sodium nitroprussiate (SNP), a well-known NO donor, prevented the effects caused by Cd. When the HO-1 mRNA levels were analyzed, a significant augmentation (54%) was observed with respect to Cd-treated plants. On the other hand, 50 or 300 μM SNP did not fully prevent the effects elicited by Cd. When HO-1 transcript levels were analyzed, no significant enhancement or a down-regulation was observed. The potassium salt of 2-(4-carboxylphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), a specific NO scavenger, arrested NO-mediated protective effects against to Cd-induced oxidative damage. These data provide an understanding of one of the possible roles that NO can play against an oxidative insult. NO is cytoprotective depending on its concentration, and it was further demonstrated that this protection could be, at least in part, mediated by an enhancement of HO-1 mRNA, as it happens with genes associated with the antioxidant defense system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen species (ROS) are generated in small amounts in the normal metabolism of the cells and in increased amounts under many conditions of altered cell physiology; they are responsible for many kinds of cell injuries (Sies 1993) and have been shown to induce a significant reprogramming of gene expression (Colburn 1992) Heme oxygenases (HO, EC 1.14.99.3) catalyze the oxidative conversion of heme to biliverdin IXα (BV), with the concomitant release of carbon monoxide and free iron. HO activity has been detected in different organisms including bacteria, animals and plants (Tenhunen et al. 1968) and it has been demonstrated that it plays an important role in modulating cellular sensitivity to oxidant’s insults through the generation of BV, which is rapidly reduced to the potent antioxidant bilirubin (Stocker 1990; Llesuy and Tomaro 1994; Reiter and Tyrrell 2000; Clark et al. 2000b; Tomaro and Batlle 2002; Noriega et al. 2003). One of the three known mammalian isoforms, heme oxygenase-1 (HO-1), is induced in animal tissues by many factors including its own substrate heme, heavy metals, UV-A radiation among others (Tomaro and Batlle 2002). While earlier studies pointed to plant HO as a source of phytochrome chromophore (Terry et al. 2002), a more recent work showed that HO synthesis increases in plants subjected to oxidative stress conferring resistance to a subsequent insult (Noriega et al. 2004; Balestrasse et al. 2005).
In plants, nitric oxide (NO) is used for a number of intercellular and intracellular signaling functions such as resistance to pathogens, stomatal closure, germination, growth, flowering and apoptosis, among others (Beligni and Lamattina 2001; Wendehenne et al. 2004). Plants can produce NO through either two main enzymatic systems, namely NO synthase and nitrate reductase, or by several nonenzymatic reactions such as liberation of NO from nitrite under different conditions (Crawford 2006). Nevertheless, the existence of an animal-like NO synthase in plants is still questioned (Zemojtel et al. 2006). The effect of cytoprotective or cytotoxic action of NO on plant metabolism depends to a large extent on the local concentration of the molecule and is affected by the rate of synthesis, displacement and efficiency of removal of this reactive nitrogen species.
Previous reports demonstrated that as a consequence of 200 μM Cd treatment for 48 h, HO-1 activity and protein synthesis were increased in soybean plants as a signal of cell protection against oxidative stress (Noriega et al. 2004). Moreover, when experiments were carried out in the presence of Zn protoporphyrin IX, a strong irreversible inhibitor of HO activity, the studied oxidative stress parameters were far altered with respect to controls, indicating the involvement of this enzyme in the cell protection against the oxidative damage. On the other hand, recent studies in pea demonstrated that as a consequence of Cd treatment a reduction in NO content occurred (Barroso et al. 2006; Rodriguez-Serrano et al. 2006), but pretreatment of soybean plants with low NO concentrations leads to the up-regulation of several enzymes involved in the antioxidant defense (Parani et al. 2004; Shi et al. 2005). In this context, we want to evaluate the response of HO-1 gene to NO pretreatment. Using Cd as the stimulus, our data let us demonstrate that the overexpression of HO-1 gene is a key component of the stress response in soybean leaves, that NO can offer cytoprotection against an oxidative insult in a dose-depending manner, and we demonstrated for the first time that this behavior is positive correlated with HO-1 gene expression. Experiments were carried out with plants pretreated with or without different concentrations of sodium nitroprussiate (SNP), a well-known NO donor, prior to Cd administration. In addition, oxidative stress markers were determined.
Materials and methods
Plant material and growing conditions
Seeds of soybean (Glycine max L.) were surface sterilized with 5% v/v sodium hypochlorite for 10 min and then washed with distilled water four times. The seeds were planted in vermiculite for 5 days. After germination, plants were removed from pots; roots were gently washed and transferred to separate containers for hydroponics. Plants were germinated and grown in a controlled climate room at 24 ± 2°C and 50% relative humidity, with a photoperiod of 16 h and a light intensity of 175 μmol m−2 s−1. The hydroponics medium was Hoagland nutrient solution (Hoagland and Arnon 1950). The medium was continuously aerated, protected from light and replaced every 3 days. After 4 weeks’ growth, plants were pretreated for 12 h with different SNP concentrations (50, 100 or 300 μM) or nutrient solution (control). Afterward, Cl2Cd solution was added up to 200 μM. After 48 h of treatment leaves were isolated and used for determinations. When the potassium salt of 2-(4-carboxyphenil)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) was used as a NO scavenger, it was added together with SNP.
Ion leakage assay
Leaves were harvested and cut into 30-mm pieces. They were washed in deionized water to remove surface-added electrolytes and placed in Petri dishes with 15 ml of deionized water at 25°C for 3 h. Electrical conductivity in the bathing solution was determined (C1). Then, the samples were heated at 80°C for 2 h and the conductivity was read again in the bathing solution (C2). Relative ion leakage was expressed as a percentage of the total conductivity after heating at 80°C (relative ion leakage % = C1/C2 × 100) (Zhao et al. 2004).
Chlorophyll content determination
Leaves (0.5 g of fresh weight) were homogenized with 96% ethanol (1:30 w/v). Extracts were heated in a boiling bath until complete bleaching. After centrifugation, the absorbance was measured in the supernatant at 665, 649 and 654 nm as described by Wintermans and de Mots (1965).
Relative water content
Relative water content (RWC), expressed as a percentage, was determined in soybean leaves according to the formula: RWC (%) = (FW − DW) × 100/DW; where FW and DW means fresh weight and dry weight, respectively. FW was measured just after the leaves were collected at the end of the experiment and DW was measured after drying the leaves at 80°C for 48 h.
H2O2 localization in situ
Leaves from control and treated plants were excised and immersed in a 1% solution of 3,3′-diamino benzidine (DAB) in Tris–HCl buffer (pH 6.5), vacuum-infiltrated for 5 min and then incubated at room temperature for 16 h in the absence of light. Leaves were illuminated until the appearance of brown spots, characteristic of the reaction of DAB with H2O2. Leaves were bleached by immersing in boiling ethanol to visualize the brown spots. H2O2 deposits were determined by scanning the spots from leaf pictures and the numbers of pixels were quantified using the public domain NIH Image program (developed at the US National Institutes of Health). The results were expressed as percentage of spot area versus total leaf area [(spot area/total leaf area) × 100] in order to compensate the differences in leaves size.
\( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) localization in situ
Leaves from control and treated plants were excised and immersed in a 0.1% solution of nitroblue tetrazolium in K-phosphate buffer (pH 6.4), containing 10 mM Na-azide, and were vacuum-infiltrated for 5 min and illuminated until the appearance of dark spots, characteristic of blue formazan precipitate. Leaves were bleached by immersing in boiling ethanol to visualize the dark spots. Superoxide deposits were quantified by scanning spots from leaf pictures as mentioned above.
H2O2 determination in leaf extracts
The H2O2 concentration of crude extracts from soybean leaves was determined by spectrofluorometry method as described by Creissen et al. (1999). Leaves (0.5 g) were homogenized in 1.2 ml of 25 mM HCl, the crude extracts were filtered through two nylon layers, and the pigments were removed by mixing with 15 mg of charcoal. The pigment-containing charcoal was separated by centrifugation at 5,000×g for 5 min, and the supernatants were clarified by filtration through a 0.20-μm filter unit. The pH of leaf disk extracts was adjusted to 7.0 with NaOH and these extracts were used to measure the H2O2 concentration. The reaction mixtures (3 ml) contained 50 mM HEPES buffer, pH 7.6, 5 mM homovanillic acid and 100 μl of sample. The reaction was started by adding 40 μM horseradish peroxidase and the fluorescence produced was measured in a spectrofluorophotometer Shimadzu RF-540 (Kyoto, Japan), at excitation and emission wavelengths of 315 and 425 nm, respectively. The H2O2 concentration was determined from a calibration curve of H2O2 in the range 0.1–20 μM.
\( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) determination in leaf extracts
The \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \)concentration of crude extracts from soybean leaves was determined by spectrometry method as described by Boveris (1984). Leaves (0.5 g) were homogenized in 1.2 ml of 25 mM HCl, and the crude extracts were filtered through two nylon layers, and the pigments were removed by mixing with 15 mg of charcoal. The pigment-containing charcoal was separated by centrifugation at 5,000×g for 5 min, and the supernatants were clarified by filtration through 0.20-μm filter unit. The pH of leaf disk extracts was adjusted to 7.0 with NaOH and these extracts were used to measure the \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) concentration. The reaction medium consisted of 40 mM potassium phosphate buffer (pH 7.4), 120 mM KCl, 1 mM EDTA and 1 mM epinephrine. The rate of production of \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) was determined as the superoxide dismutase-sensitive rate of adrenochrome formation, measured at 485–575 nm (ε = 2.97 mM−1 cm−1) in a Perkin-Elmer dual-wavelength spectrophotometer.
Heme oxygenase preparation and assay
Leaves (0.3 g) were homogenized in a Potter-Elvehjem homogenizer using 1.2 ml of ice-cold 0.25 M sucrose solution containing 1 mM phenylmethyl sulfonyl fluoride, 0.2 mM EDTA and 50 mM potassium phosphate buffer (pH 7.4). Homogenates were centrifuged at 20,000×g for 20 min and chloroplasts were used for activity determination. HO activity was assayed as previously described with minor modifications (Muramoto et al. 2002). The assays (1-ml final volume) contained 250 μl of extract (0.5 mg protein), 10 μM hemin, 0.15 mg ml−1 bovine serum albumin, 50 μg ml−1 (4.2 μM) spinach (Spinacia oleracea) ferredoxin (Sigma Chemical Co.), 0.025 U ml−1 spinach ferredoxin-NADP+ reductase (Sigma Chemical Co.). The reaction was started by adding NADPH to a final concentration of 100 μM, samples were incubated at 37°C during 30 min and BV formation was calculated using the absorbance change at 650 nm. The concentration of BV was estimated using a molar absorption coefficient at 650 nm of 6.25 mM−1 cm−1 in 0.1 M HEPES–NaOH buffer (pH 7.2).
Isolation of RNA and RT-PCR analysis
Total RNA was isolated using Trizol reagent (Gibco BRL), treated with RNase-free DNase I (Promega), and reverse transcribed into cDNA using random hexamers and M-MLV Superscript II RT (Gibco BRL). PCR reactions were carried out using G. max HO-1 and 18S specific primers, as previously described (Yannarelli et al. 2006). Each primer set was amplified using an optimized number of PCR cycles to ensure the linearity requirement for semi-quantitative RT-PCR analysis. Ethidium bromide stained gels were scanned (Photodyne Incorporated, WI, USA) and analyzed using Gel-Pro Analyzer 3.1 software (Media Cybernetics, MD, USA). The ratio of HO-1 mRNA to 18S mRNA was quantified.
Protein determination
Protein concentration was evaluated by the method of Bradford (1976), using bovine serum albumin as a standard.
Statistics
Continuous variables are expressed as mean ± SE. Differences among treatments were analyzed by one-way ANOVA, taking P < 0.05 as significant according to Tukey’s multiple range test.
Results
Effect of NO on relative water content in leaves of Cd-treated plants
Relative water content was tested to evaluate whether NO could counteract tissue dehydration provoked by Cd. Table 1 shows that RWC decreased considerably under Cd treatment (75% respect to controls). Pretreatment with 50 and 100 μM SNP protected against this effect, whereas 300 μM SNP did not show any protective effect (73% diminution respect to controls). When experiments were performed in the presence of 100 μM cPTIO, a specific NO scavenger, RWC loss was similar to that found in Cd-treated plants. cPTIO alone did not have any effect on RWC compared with controls (data not shown).
Nitric oxide prevents chlorophyll loss in Cd-treated plants
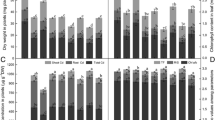
Chlorophyll can be bleached under oxidative stress. Figure 1 shows that chlorophyll content decreased under Cd treatment (47% respect to controls). Experiments carried out in the presence of 50 or 100 μM SNP revealed that these concentrations partially protected or prevented chlorophyll loss against Cd insult (31.4 and 12.4% decrease compared to control, respectively). On the other hand, 300 μM SNP were not able to prevent chlorophyll loss (49% respect to controls). As shown in Fig. 1 (inset) the protective effect of 100 μM SNP could be reversed by the addition of 100 μM cPTIO. Treatment with cPTIO alone did not have any effect on this parameter compared with controls (data not shown).
Effect of NO on total chlorophyll content in soybean leaves treated with Cd. Plants were pretreated with different SNP concentrations ranging from 0 to 300 μM prior to 200 μM Cd exposure for 48 h. Experiments were performed as described in Sect. “Materials and methods”. Control was considered as 100% of chlorophyll content. Data in the inset show that 100 μM cPTIO negated the protection exerted by 100 μM SNP on chlorophyll loss in Cd-treated plants. Values are the mean of three independent experiments and bars indicate SE. *P < 0.01, #P < 0.001 compared to control according to Tukey’s multiple range test
Nitric oxide protects soybean leaves from Cd-induced ion leakage
Ion leakage reflects the membrane injury as a result of oxidative damage. Figure 2 shows that ion leakage increased by 510% when plants were subjected to Cd treatment. To examine whether NO could alleviate this damage, plants were pretreated with different SNP concentrations. Pretreatment with 50 or 300 μM SNP had no effect on this parameter with respect to plants treated with Cd alone (491 and 534% enhancement respect to controls). Nevertheless, 100 μM SNP revealed only a 146% increase respect to the same controls; hence membrane injury was partially protected. To further clarify the role of NO in preventing Cd-induced oxidative damage cPTIO was used. As shown in Fig. 2 (inset), the protective effect of 100 μM SNP on Cd-induced ion leakage could be reversed by the addition of 100 μM cPTIO. Treatment with cPTIO alone did not have any effect on ion leakage compared with controls (data not shown).
Effect of NO on ion leakage in soybean leaves treated with Cd. Plants were pretreated with different SNP concentrations ranging from 0 to 300 μM prior to 200 μM Cd exposure for 48 h. Experiments were performed as described in Sect. “Materials and methods”. Control was considered as 100% of ion leakage. Data in the inset show that 100 μM cPTIO negated the protection exerted by 100 μM SNP on ion leakage in Cd-treated plants. Data are mean values of three independent experiments and bars indicate SE. *P < 0.001 compared to control according to Tukey’s multiple range test
H2O2 and \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) contents in soybean leaves
To check the possible role of NO in Cd-induced oxidative stress, H2O2 and \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) contents were determined. Table 2 shows that H2O2 and \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) levels were increased by 6.7- and 9.3-folds respectively, after treatment with 200 μM Cd. In the presence of 50 μM SNP, no differences were observed with respect to Cd-treated plants, whereas pretreatment with 100 μM SNP partially protected against H2O2 and \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) formation. No effective protection was observed in experiments carried out in the presence of 300 μM SNP.
H2O2 and \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) localization in situ
Accumulation of H2O2 and \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) were also evaluated in situ by histochemical methods as shown in Fig. 3. Cd produced 41% H2O2 spots area versus total leaf area, while pretreatment with 100 μM SNP prevented this effect and spot area was similar to controls (Fig. 3b). Pretreatment with 50 μM SNP avoided partially H2O2 accumulation, while 300 μM SNP did not avoid H2O2 formation with respect to Cd-treated plants. Data in Fig. 3c showed that leaves treated with 200 M Cd produced 16% \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) spots area versus total leaf area. Pretreatments with 100 μM SNP completely prevented the \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) production induced by Cd, while 50 and 300 μM SNP could only partially counteract this effect.
Histochemical detection of H2O2 and \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) in soybean leaves treated with Cd. Plants were pretreated with different SNP concentrations ranging from 0 to 300 μM prior to 200 μM Cd exposure for 48 h. The second pair of fully expanded leaves above the cotyledons was used for the assays as described in Sect. “Materials and methods”. a Leaves from control and Cd-treated plants stained for H2O2 (DAB) and \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) (NBT) content. The figure is representative of three different experiments with duplicate measurements for each treatment. Hydrogen peroxide (b) and superoxide anion deposits (c) were quantified by measuring the number of pixels of spots using the NIH Image program (National Institutes of Health, USA). Results are expressed as percentage of spot area versus total leaf area. *P < 0.05, #P < 0.01, †P < 0.001 and ‡P < 0.01, §P < 0.001 compared to control and Cd alone, respectively, according to Tukey’s multiple range test
Effect of NO on HO-1 gene expression and enzyme activity
We analyzed the expression of HO-1 mRNA in soybean leaves in response to different pretreatments with SNP (0, 50, 100 or 300 μM) before Cd administration. Semi-quantitative RT-PCR revealed that 100 μM SNP brought about the highest induction of HO-1 gene, and the level of 18S was unaffected throughout all the experiments (Fig. 4a). Densitometric analysis showed that HO-1 mRNA level was not affected in Cd-treated plants with respect to control values, while a 54% enhancement was observed when plants were pretreated with 50 μM SNP and a strong diminution (63%) occurred in plants pretreated with 300 μM SNP (Fig. 4b). These results demonstrated that 50 and 100 μM SNP treated plants overexpressed the HO-1 gene transcript. As shown in Table 3, these data were positively paralleled with enzyme activities. The highest enzyme activity was obtained in plants pretreated with 100 μM SNP and a strong diminution was observed in the presence of 300 μM SNP. The addition of cPTIO totally prevented the enhancement of HO activity produced by 100 μM SNP. To further clarify the effect of SNP on HO-1 mRNA expression, experiments were performed in the presence of 100 μM SNP without Cd. Figure 5 shows an increase of gene transcription (57%), whereas when plants were afterward exposed to Cd this response was still more pronounced (83%). Once again the presence of cPTIO prevented HO-1 up-regulation.
Effect of NO on HO-1 gene expression in soybean leaves treated with Cd. Plants were pretreated with different SNP concentrations ranging from 0 to 300 μM prior to 200 μM Cd exposure for 48 h. a HO-1 mRNA expression was analyzed by semi-quantitative RT-PCR as described in Sect. “Materials and methods”. The 18S amplification band is shown to confirm equal loading of RNA and RT efficiency. b Relative HO-1 transcript expression taking control as 1 U. Data are mean values of three independent experiments and bars indicate SE. *P < 0.05, #P < 0.01 and ‡P < 0.05 compared to control and Cd alone, respectively, according to Tukey’s multiple range test
Effect of 100 μM SNP on HO-1 gene expression in soybean leaves. Experiments were performed adding 100 μM SNP to the nutrient solution. The following cases were analyzed: effect of SNP in the absence of 200 μM Cd (−Cd), the effect of SNP pretreatment on 200 μM Cd exposure (+Cd), the combined effect of SNP with 100 μM cPTIO before Cd treatment (cPTIO). a HO-1 mRNA expression was analyzed by semi-quantitative RT-PCR as described in Sect. “Materials and methods.” The 18S amplification band is shown to confirm equal loading of RNA and RT efficiency. b Relative HO-1 transcript expression taking control as 1 U. Data are mean values of three independent experiments and bars indicate SE. *P < 0.05, #P < 0.01 compared to control according to Tukey’s multiple range test
Discussion
The present study first provided evidence that 100 μM SNP, a NO donor, can scavenge ROS and up-regulates HO-1 gene in an attempt to protect leaf tissues against the oxidative damage caused by a chemical stressor, such as Cd. It has been already demonstrated that an enhancement of HO-1 activity leads to increased levels of BV, an efficient scavenger of ROS (Stocker 1990). We have chosen a high Cd concentration in order to perform an acute intoxication model, based on previous reports that demonstrated that after 48 h, 200 μM Cd caused oxidative stress (Wagner 1993; Balestrasse et al. 2001).
Cadmium brought about an increase in ion leakage and a loss in relative water and chlorophyll content. Measurements of HO-1 mRNA expression did not reveal any augmentation under this treatment. Previous experiments carried out under the same conditions showed an enhancement of HO-1 protein levels and a higher activity in soybean plants (Noriega et al. 2004; Balestrasse et al. 2005). As expected, in our model, mRNA increase occurred at an earlier time (24 h) in an attempt to prevent the effects caused by Cd (data not shown). Nevertheless, this enhancement was not high enough to mitigate the oxidative damage caused by this metal. In this regard, an augmentation of HO-1 mRNA may be attributed to ROS, as it has been already demonstrated in soybean leaves treated with UV-B radiation (Yannarelli et al. 2006).
It is interesting to note that ion leakage parameters were more affected than chlorophyll content. This fact indicates that membranes are highly prone to be affected by ROS, rendering this conductimetric parameter as a very reliable index for this oxidative insult. It is well known that H2O2 and \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) can be an index of oxidative stress. Plants pre-treated with 100 μM SNP showed a loss of H2O2 and \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) in the leaves with respect to plants treated only with 200 μM Cd. Employing 300 μM SNP higher amounts of \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) as well as H2O2 were observed with respect to the other SNP concentrations. The reaction of NO with \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) generates peroxinitrite, which has been shown to mediate the tyrosine nitration of proteins, diminishing in this way the availability of ROS (Zaninotto et al. 2006). Furthermore, it is noteworthy that the effect of NO depends on its concentration (Shi et al. 2005) and consequently, at 300 μM SNP, instead of a stimulation, an inhibition of the antioxidant defense system may be observed (Clark et al. 2000a). In this way, although \( {\text{O}}^{{{\hbox{${\scriptscriptstyle \bullet}$}} - }}_{{\text{2}}} \) is captured by NO, its degradation is diminished, explaining the higher amounts observed at 300 μM SNP. Similarly, higher amounts of H2O2 under this condition are also explained by the diminution of its breakdown. In order to get hints of HO-1 regulation, gene expression was analyzed in plants treated with different SNP concentrations prior to Cd addition. Results here presented clearly demonstrated that 100 μM SNP can protect leaf tissues against oxidative stress elicited by Cd, up-regulates HO-1 transcript levels and enhances HO-1 enzyme activity. As the protective effect of SNP was abolished by the NO scavenger cPTIO, this fact could be taken as an evidence for the antioxidative role of this NO concentration in this experimental system. Data from experiments carried out in the presence of 100 μM SNP with or without Cd indicated that this gene could be regulated not only by ROS but also by NO. It has been demonstrated in a murine macrophage cell line that iron is more critical than NO in SNP induction of HO-1 (Kim et al. 2006). Nevertheless, results obtained employing cPTIO strongly suggest that in our system exogenous NO and not iron is involved in this response. It is interesting to note that ferritins play an essential role in iron homeostasis by sequestering iron in a bioavailable and non-toxic form. In plants, ferritin mRNAs are highly and quickly accumulated in response to iron overload. Moreover, it has been also reported that SNP induces accumulation of ferritin transcripts in Arabidopsis (Murgia et al. 2004). Such accumulation leads to a subsequent ferritin protein synthesis and iron storage (Arnaud et al. 2006).
It has been reported that low concentrations of NO could act as an efficient scavenger breaking the oxidative chain, but high NO concentrations could provoke a major oxidative insult, since this molecule is itself a nitrogen reactive species (Shi et al. 2005). Our data demonstrated that 50 μM SNP could not totally prevent the changes observed in the presence of 200 μM Cd. Nevertheless, a low mRNA HO up-regulation was observed as well as an enhancement of its activity, not being high enough to avoid this damage. Employing 300 μM SNP,resulted in a down-regulation. These results agree with the data that showed a lack of antioxidant defense and an important diminution of HO-1 activity in this tissue under this condition. This down-regulation could occur, on one hand because NO is itself a reactive nitrogen species and could have a toxic effect and on the other hand, it is well known that HO-1 is a regulatory enzyme in heme catabolism (Kikuchi et al. 2005) and with heme involved in NO detoxification a diminution of its pool could lead to a down-regulation of this gene. In this context, hemoglobins (Hbs) are recognized for their ability to act either as O2 carriers or stores to facilitate O2 delivery, even though they are also well-known regulators of NO homeostasis. Thus, the primordial function of Hbs, present not only in erythrocytes but also in plants may well be to protect nitrosoactive stress and modulate the signaling function of NO (Gow et al. 1999; Perazzolli et al. 2004). HO-1 has been widely reported as playing a major part in the antioxidant cell machinery of animal and plant cells (Stocker 1990; Llesuy and Tomaro 1994; Noriega et al. 2004; Balestrasse et al. 2005), and gene induction was observed upon UV-B radiation (Yannarelli et al. 2006). Application of the NO donor NOR-3 to Arabidopsis cell suspension culture resulted in the induction of several genes (Huang et al. 2002). Microarray analysis showed that NO can modulate the expression of a substantial number of genes at the transcriptional level, among them those of glutathione peroxidase, glutathione S-transferase, glutaredoxins and other antioxidant genes (Parani et al. 2004). Until now no information is available about the effect of NO on HO behavior in plants. The data reported here let us conclude that HO-1 is not only induced by ROS but also by NO and they can be extrapolated to other situations where NO is produced as a response to oxidative stress.
In this study it was demonstrated that depending on its concentration, NO could protect against the oxidative insult caused by an abiotic stress increasing the levels of HO-1 expression, as it occurs with other genes involved in the antioxidant defense system.
Abbreviations
- BV:
-
Biliverdin IXα
- cPTIO:
-
2-(4-Carboxyphenil)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- DAB:
-
3,3′-Diamino benzidine
- HO:
-
Heme oxygenase
- NBT:
-
Nitroblue tetrazolium
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthetase
- SNP:
-
Sodium nitroprussiate
References
Arnaud N, Murgia I, Boucherez J, Briat JF, Cellier F, Gaymard F (2006) An iron-induced nitric oxide burst precedes ubiquitin-dependent protein degradation for Arabidopsis AtFer1 ferritin gene expression. J Biol Chem 281:23579–23588
Balestrasse KB, Gardey L, Gallego SM, Tomaro ML (2001) Response of antioxidative defense system in soybean nodules and roots subjected to cadmium stress. Aust J Plant Physiol 28:497–504
Balestrasse KB, Noriega GO, Batlle A, Tomaro ML (2005) Involvement of heme oxygenase as antioxidant defense in soybean nodules. Free Radic Res 39:145–151
Barroso JB, Corpas FJ, Carreras A, Rodriguez-Serrano M, Esteban FJ, Fernandez-Ocana A, Chaki M, Romero-Puertas MC, Valderrama R, Sandalio LM, del Rio LA (2006) Localization of S-nitrosoglutathione and expression of S-nitrosoglutathione reductase in pea plants under cadmium stress. J Exp Bot 57:1785–1793
Beligni NV, Lamattina L (2001) Nitric oxide: a non-traditional regulator of plant growth. Trends Plant Sci 6:508–509
Boveris A (1984) Oxygen radicals in biological systems. Methods Enzymol 105:429–435
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Clark D, Durner J, Navarre DA, Klessig DF (2000a) Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol Plant Microbe Interact 13:1380–1384
Clark JE, Foresti R, Green CJ, Motterlini R (2000b) Dynamics of haem-oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem J 348:615–619
Colburn NH (1992) Gene regulation by active oxygen and other stress inducers. In: Spatz L, Bloom AD (eds) Biological consequences of oxidative stress. Oxford University Press, New York, pp 121–137
Crawford NM (2006) Mechanisms for nitric oxide synthesis in plants. J Exp Bot 57:471–478
Creissen G, Firmin J, Fryer M (1999) Elevated glutathione biosynthesis capacity in the chloroplast of transgenic tobacco plants paradoxically causes oxidative stress. Plant Cell 11:1277–1291
Gow AJ, Luchsinger BP, Pawloski JR, Singel DJ, Stamler JS (1999) The oxyhemoglobin reaction of nitric oxide. Proc Natl Acad Sci USA 96:9027–9032
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Calif Agric Exp Stn Circ 347:1–32
Huang X, von Rad U, Durner J (2002) Nitric oxide induces the nitric oxide tolerant alternative oxidase in Arabidopsis suspension cells. Planta 215:914–923
Kikuchi G, Yoshida T, Noguchi M (2005) Heme oxygenase and heme degradation. Biochem Biophys Res Commun 338:558–567
Kim HJ, Tsoy I, Park MK, Lee YS, Lee JH, Seo HG, Chang KC (2006) Iron released by sodium nitroprusside contributes to heme oxygenase-1 induction via the cAMP-protein kinase A-mitogen activated protein kinase pathway in RAW 264.7 cells. Mol Pharmacol 69:1633–1640
Llesuy SF, Tomaro ML (1994) Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim Biophys Acta 1223:9–14
Muramoto T, Tsurui N, Terry MJ, Yokota A, Kohchi T (2002) Expression and biochemical properties of a ferredoxin-dependent heme oxygenase required for phytochrome chromophore synthesis. Plant Physiol 130:1958–1966
Murgia I, De Pinto MC, Delledonne M, Soave C, De Gara L (2004) Comparative effects of various nitric oxide donors on ferritin regulation, programmed cell death, and cell redox state in plant cells. J Plant Physiol 161:777–783
Noriega GO, Tomaro ML, Batlle AMC (2003) Bilirubin is highly effective in preventing in vivo d-aminolevulinic acid-induced oxidative cell damage. Biochim Biophys Acta 1638:173–178
Noriega GO, Balestrasse KB, Batlle A, Tomaro ML (2004) Heme oxygenase exerts a protective role against oxidative stress in soybean leaves. Biochem Biophys Res Commun 323:1003–1008
Parani MR, Myers R, Wireich H, Smith B, Leaman DW, Goldman SL (2004) Microarray analysis of nitric oxide responsive transcripts in Arabidopsis. Plant Biotechnol J 2:359–366
Perazzolli M, Dominici P, Romero-Puertas MC, Zago E, Zeier J, Sonoda M, Lamb C, Delledonne M (2004) Arabidopsis non symbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell 16:2785–2794
Reiter SW, Tyrrell RM (2000) The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 28:289–309
Rodriguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gomez M, Del Rio LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544
Shi S, Wang G, Wang Y, Zhang L, Zhang L (2005) Protective effect of nitric oxide against oxidative stress under ultraviolet-B radiation. Nitric Oxide 13:1–9
Sies H (1993) Strategies of antioxidant defense. Eur J Biochem 215:213–219
Stocker R (1990) Induction of haem oxygenase as a defence against oxidative stress. Free Radic Res Commun 9:101–112
Tenhunen R, Marver HS, Schmid R (1968) The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA 61:748–755
Terry MJ, Linley PJ, Kochi T (2002) Making light of it: the role of plants heme oxygenases in phytochrome chromophore synthesis. Biochem Soc Trans 30:604–609
Tomaro ML, Batlle A (2002) Bilirubin: its role in cytoprotection against oxidative stress. Int J Biochem Cell Biol 34:216–220
Wagner GJ (1993) Accumulation of cadmium in crop plants and its consequences to human health. Adv Agron 51:173–212
Wendehenne D, Durner J, Klessig DF (2004) Nitric oxide: a new player in plant signaling and defence responses. Curr Opin Plant Biol 7:449–455
Wintermans J, de Mots A (1965) Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta 109:448–453
Yannarelli GG, Noriega GO, Batlle A, Tomaro ML (2006) Heme oxygenase up-regulation in ultraviolet-B irradiated soybean plants involves reactive oxygen species. Planta 224:1154–1162
Zaninotto F, La Camera S, Polverari A, Delledonne M (2006) Cross talk between reactive nitrogen and oxygen species during the hypersensitive disease resistance response. Plant Physiol 141:379–383
Zemojtel T, Frohlich A, Palmieri MC, Kolanczyk M, Mikula I, Wyrwicz LS, Wanker EE, Mundlos S, Vingron M, Martasek P, Durner J (2006) Plant nitric oxide synthase: a never-ending story? Trends Plant Sci 11:524–525
Zhao L, Zhang F, Guo J, Yang Y, Li B, Zhang L (2004) Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed (Phragmites communis Trin.). Plant Physiol 134:849–857
Acknowledgments
This work was supported by grants from the Universidad de Buenos Aires (Argentina) and from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (Argentina). K.B.B., A.B. and M.L.T. are Career Investigators from CONICET.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noriega, G.O., Yannarelli, G.G., Balestrasse, K.B. et al. The effect of nitric oxide on heme oxygenase gene expression in soybean leaves. Planta 226, 1155–1163 (2007). https://doi.org/10.1007/s00425-007-0561-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0561-8