Abstract
Recent results discovered the protective roles of methane (CH4) against oxidative stress in animals. However, the possible physiological roles of CH4 in plants are still unknown. By using physiological, histochemical and molecular approaches, the beneficial role of CH4 in germinating alfalfa seeds upon copper (Cu) stress was evaluated. Endogenous production of CH4 was significantly increased in Cu-stressed alfalfa seeds, which was mimicked by 0.39 mM CH4. The pretreatment with CH4 significantly alleviated the inhibition of seed germination and seedling growth induced by Cu stress. Cu accumulation was obviously blocked as well. Meanwhile, α/β amylase activities and sugar contents were increased, all of which were consistent with the alleviation of seed germination inhibition triggered by CH4. The Cu-triggered oxidative stress was also mitigated, which was confirmed by the decrease of lipid peroxidation and reduction of Cu-induced loss of plasma membrane integrity in CH4-pretreated alfalfa seedlings. The results of antioxidant enzymes, including ascorbate peroxidase (APX), superoxide dismutase (SOD), catalase (CAT), and guaiacol peroxidase (POD) total or isozymatic activities, and corresponding transcripts (APX1/2, Cu/Zn SOD and Mn-SOD), indicated that CH4 reestablished cellular redox homeostasis. Further, Cu-induced proline accumulation was partly impaired by CH4, which was supported by the alternation of proline metabolism. Together, these results indicated that CH4 performs an advantageous effect on the alleviation of seed germination inhibition caused by Cu stress, and reestablishment of redox homeostasis mainly via increasing antioxidant defence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper (Cu) is one of the essential micronutrient for plant development and growth (Yruela 2005). It was well known that Cu plays a vital role in oxidative phosphorylation, protein trafficking machinery, photosynthesis, oxidative stress responses, cell wall remodeling, and catalytic activities of various enzymes (Yruela 2005; Burkhead et al. 2009). Despite of its key roles in many physiological processes, Cu at a higher concentration is also considered as a stress factor. Normally, the excess Cu exhibits a detrimental effect on plant growth and causes severe plant growth inhibition, even leading to plant death (Janas et al. 2010). Besides the inhibition of seed germination, for example, another primary and distinct symptom of Cu toxicity is the inhibition of root growth due to its accumulation in the root tissue with little translocation to the shoots (Sheldon and Menzies 2005; Burkhead et al. 2009). Additionally, the reduced growth rate and severe browning in Cu-stressed Prunus cerasifera and wheat, were observed (Lombardi and Sebastiani 2005; Zhang et al. 2008).
As a redox active transition metal, superoptimal concentration of Cu can induce the overproduction of reactive oxygen species (ROS), such as superoxide anion radicals (O ·−2 ), hydrogen peroxide (H2O2), and even hydroxyl radicals (OH·). As a consequence, ROS overproduction leads to oxidative damage to several macromolecules, such as DNA, proteins, and lipids, thus disturbing membrane integrity and resulting in the inhibition of plant root and shoot growth (Yruela 2005; Wang et al. 2011). To cope with heavy metals, plants have evolved various homeostatic mechanisms to alleviate its toxicity. For instance, the overproduction of ROS is scavenged by enzymatic and non-enzymatic mechanism, and the enzymatic system includes superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and guaiacol peroxidase (POD), etc. (Foyer and Shigeoka 2011). In response to heavy metal exposure, one of the most common is the reestablishment of redox homeostasis in survived plants. Previous study showed that overexpressing a gene encoding γ-glutamylcysteine synthetase-glutathione synthetase (GCS-GS), which synthesizes glutathione, an important antioxidant in plants, enhanced the tolerance of sugar beet to Cu stress (Liu et al. 2016a). Meanwhile, some reports indicated that activities of some antioxidant enzymes were increased in Cu-stressed plants, which is usually accompanied by enhanced lipid peroxidation (Wang et al. 2011; Yonar et al. 2015; Jiang et al. 2016). Ample evidence also showed that enhanced antioxidant defence might be beneficial for seed germination and seedling growth, particularly, in plants subjected to abiotic stress (Patui et al. 2014; Zhang et al. 2014; Chen et al. 2016a, b).
A growing amount of evidence has emerged that proline accumulation regulated at transcriptional level of both synthesis and degradation, could function as important adaptive response to a wide range of abiotic stress, since ROS might be efficiently scavenged by proline (Verbruggen and Hermans 2008). It was well-known that the first committing step in proline synthesis is catalyzed by ∆1-pyrroline-5-carboxylate synthetase (P5CS). Meanwhile, proline dehydrogenase (PDH) is involved in the first step of the conversion of proline to glutamic acid. On the other hand, some results suggested that hyperaccumulation of proline in salt-sensitive barley did not alleviate the sensitivity to salinity, but instead, appeared to be a symptom of injury (Chen et al. 2007).
Methane (CH4) is a highly inflammable gas with a characteristic of colorless, odorless, nontoxic, lighter than air and slightly soluble in water. In addition, high concentrations of CH4 can cause headaches even asphyxia in a very short period of time (Barker et al. 1986). Meanwhile, CH4 is the second most important potent greenhouse gas after carbon dioxide (Wang et al. 2013). Since the last three decades, some species of plant under the normal growth conditions and environmental stimuli (UV radiation, etc.) were found to generate CH4 (Seiler et al. 1983; Bruhn et al. 2012, 2014). This suggests that it may be an endogenous chemical and suitable to be functioning as a newly identified signalling molecule. Besides plants, ample evidence discovered the emission of CH4 from a wide range of microbial and non-microbial sources, such as archaea and animals, etc. (Conrad 2005, 2009; Wang et al. 2013). Previously, by using methane-rich saline or methane gas, it was proposed that CH4 might be a fundamental molecule playing a significant role against oxidative and nitrosative stresses in mammals (Boros et al. 2012; Chen et al. 2016a, b; He et al. 2016).
Previous results showed that CH4 could trigger cucumber adventitious root formation (Cui et al. 2015) and ameliorate salinity toxicity in alfalfa plants (Zhu et al. 2016). However, whether or how CH4 enhances tolerance against Cu toxicity is not clear. In the present study, the beneficial role of CH4 in enhancing plant tolerance against Cu stress was evaluated during Medicago sativa (alfalfa) seed germination. Firstly, we observed that endogenous production of CH4 was significantly increased during seed germination in response to Cu stress, which was mimicked by exogenously applied methane-rich water containing 0.39 mM CH4. Moreover, we studied the ameliorative effects elicited by CH4 on the inhibition of seed germination and the partial reversal of Cu accumulation. Most importantly, besides the alteration of proline metabolism, the alleviation of oxidative stress elicited by CH4 was discovered, which was confirmed by the decrease of lipid peroxidation, and the modulation of antioxidant enzymes as well as their transcripts.
Materials and methods
Preparation of CH4
Purified CH4 gas (99.9%, v/v) in a compressed gas cylinder (8 L) was purchased from Nanjing Special Gases Factory Co., Ltd. The purified CH4 gas was bubbled into 500 ml distilled water at a rate of 160 ml min−1 for 30 min at 25 °C (the concentration of CH4 has no longer increased; we defined this as saturation stock solution, 100% of saturation). Afterwards, this corresponding saturation stock solution was immediately diluted to the required concentrations (10, 30, 50, and 100% of saturation). By using gas chromatography (GC), the contents of CH4 in fresh saturation solution (10, 30, 50, and 100% of saturation) were 0.13, 0.39, 0.65, and 1.3 mM, respectively, and maintained at relatively constant level for at least 12 h.
Plant materials, growth conditions, and experimental designs
Commercially available alfalfa seeds (Medicago sativa L. cv. Biaogan) were surface-sterilized with 5% NaClO for 10 min, washed extensively in distilled water, and then dried. To examine the toxic effect of Cu stress on the seed germination, alfalfa seeds with approximately equal size were selected and allocated randomly in 96- × 16-mm round petri dishes (50 seeds per dish) containing 5 ml treatment solution in the following experiments. After pretreatment with distilled water for 12 h, seeds were treated with different concentrations of CuCl2 solution for the designated time points (related schematic representation of seeds culture was shown in Fig. S1). Thus, above experiments were carried out in an aerobic condition. The sample without chemicals was the control (Con). All samples were kept at 25 °C in a growth chamber in darkness. Germination tests were carried out on at least 3 replicates of 150 seeds each. The germination rate was calculated as a standard that the emerging root length reaches approximately the length of the seeds. Additionally, the growth parameters, including root length and fresh weight, were determined.
To examine the possible protective effect of CH4 on the Cu-induced inhibition of alfalfa seed germination, alfalfa seeds were presoaked with the indicated concentrations of CH4 as described in the corresponding figure legends for 12 h, and subsequently exposed to Cu stress for the indicated time points. The CuCl2-treating solution was substituted by a fresh batch every 24 h, maintaining the unchanged stressed conditions. Similarly, above experiments were carried out in an aerobic condition. After the indicated time points, the seedlings were sampled, and growth parameters were determined. Meanwhile, samples were used immediately, or frozen in liquid nitrogen at −80 °C, and stored until further analysis.
Estimation of endogenous CH4 content
CH4 concentration was determined with a gas chromatograph (GC, Agilent 7820, USA) equipped with a flame ionization detector (FID) and Poropak column (1/8 inch, 8 foot), which was previously used to determine endogenous carbon monoxide (CO) and hydrogen gas (H2) contents in animals and plants (Renwick et al. 1964; Bernardi et al. 2008; Jin et al. 2013; Bruhn et al. 2014) with minor variations. After washed several times by sterile water, about 0.3 g of fresh samples was homogenized with sterile water (7 ml) and transferred to a vial. Pure nitrogen gas was purged into the vial to displace the air, followed by the addition of 100 μl sulfuric acid and placed them for overnight to digest plant material. A headspace aliquot was sampled from the vials. Quantification of CH4 was performed by direct comparison of peak area with that obtained with a reference standard mixture containing 2 ppm CH4 in N2, as the carrier gas, and air pressure was 0.5 MPa. All samples were prepared in triplicate.
Cu content determination
Fresh samples were oven-dried at 60 °C for 3 days. Afterwards, the dried samples (0.1 g) were ground in mortars, and then digested with 2 ml HNO3 using a Microwave Digestion System (Milestone/Ethos T) for 30 min. After digestion, the residual volume was adjusted to 10 ml with deionized H2O, and Cu contents were measured by an Inductively Coupled Plasma Optical Emission Spectrometer (Perkin Elmer Optima 2100DV). All data are from three independent experiments with at least three replicates for each, and Cu content was expressed as mg g−1 dry weight (DW).
Determination of thiobarbituric acid reactive substances (TBARS)
Lipid peroxidation was estimated by evaluating the amount of TBARS, as an indicator of oxidative stress (Han et al. 2008). About 250 mg of fresh tissues was ground with 0.25% 2-thiobarbituric acid (TBA) in 10% trichloroacetic acid (TCA) using a mortar and pestle. The mixture was heating at 95 °C for 30 min, then quickly cooled in an ice bath and centrifuged at 12,000g for 10 min. The absorbance of the supernatant was read at 532 nm and corrected for unspecific turbidity by subtracting the absorbance at 600 nm. The blank was 0.25% TBA in 10% TCA. The concentration of lipid peroxides, together with oxidatively modified proteins of plants, was thus quantified in terms of TBARS amount using an extinction coefficient of 155 mM−1 cm−1 and expressed as nmol g−1 fresh weight (FW).
Histochemical analyses
Histochemical detection of lipid peroxidation in root tissues was studied using the method described by Yamamoto et al. (2001), which involves the staining with Schiff’s reagent, and histochemical detection of loss of plasma membrane integrity in root apexes was performed with Evans blue. Afterwards, the roots stained with Schiff’s reagent were rinsed with a solution containing 0.5% (w/v) K2S2O5 (prepared in 0.05 M HCl) to retain the staining color. Meanwhile, the roots stained with Evans blue were washed three times with distilled water, after which the dye was no longer eluted from the roots. Finally, above roots were immediately examined under a light microscope (Model Stemi 2000-C; Carl Zeiss, Germany), and photographed on color film (Powershot A620, Canon Photo Film, Japan).
Enzyme extraction and assays
Fresh samples (approximately 0.1 g) were ground in a mortar and pestle in 2 ml of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA and 1% polyvinylpyrrolidone (PVP) for determining activities of catalase (CAT, EC 1.11.1.6), guaiacol peroxidase (POD, EC 1.11.1.7), and superoxide dismutase (SOD, EC 1.15.1.1), or with the addition of 1 mM ascorbic acid (ASC) in the case of ascorbate peroxidase (APX, EC 1.11.1.11) assay. The homogenates were centrifuged at 15,000g for 20 min at 4 °C, and the supernatants were used for assays of enzymatic activities.
Total SOD activity was measured on the basis of its capacity of inhibiting the reduction of nitroblue tetrazolium (NBT) by the superoxide anion radicals generated by the riboflavin system under illumination. One unit of SOD (U) was defined as the amount of crude enzyme extract required to inhibit the reduction rate of NBT by 50% (Beauchamp and Fridovich 1971; Wang et al. 2012). POD activity was determined by measuring the oxidation of guaiacol (extinction coefficient 26.6 mM−1 cm−1) at 470 nm (Han et al. 2008). APX activity was determined by monitoring the decrease in absorbance at 290 nm as ASC was oxidized (extinction coefficient 2.8 mM−1 cm−1) for at least 1 min in 3 ml reaction mixture (Xie et al. 2008). Activities of CAT were determined spectrophotometrically by monitoring the consumption of H2O2 (extinction coefficient 39.4 mM−1 cm−1) at 240 nm for at least 3 min (Aebi 1984; Huang et al. 2006).
∆1-pyrroline-5-carboxylate synthetase (P5CS) activity was performed spectrophotometrically according to the method described by Zhang et al. (1995). The reaction mixture contained 100 mM Tris–HCl buffer (pH 7.2), 25 mM MgCl2, 75 mM Na-glutamate, 5 mM ATP, 0.4 mM NADPH, and the enzyme extract. P5CS activity was measured as the rate of consumption of NADPH (extinction coefficient 6.22 mM−1 cm−1) monitored by the decrease in absorbance at 340 nm for at least 2 min. Total protein concentration was determined using bovine serum albumin (BSA) as a standard reported by Bradford (1976).
Assay of amylase activity and total soluble sugar contents
The content of total soluble sugar and the activities of α/β-amylase were determined based on the methods described previously by Xu et al. (2013). The content of total soluble sugar was expressed as mg g−1 dry weight (DW). Also, one unit (U) of the activity was calculated by taking the quantity of the enzyme to reach 50% of the original color intensity.
qPCR analysis
The isolation of total RNA from 100 mg fresh samples was homogenized with mortar and pestle in liquid nitrogen until a fine powder appeared, and isolated by using Trizol reagent (Invitrogen, Gaithersburg, MD, USA) according to the manufacturer’s instructions. The isolated RNA samples were treated with RNase-free DNase (TaKaRa Bio Inc., Dalian, China) for preventing DNA contamination, and RNA concentration and quality were checked using the NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA). cDNA was synthesized from 2 μg of total RNA using an oligo (dT) primer and M-MLV reverse transcriptase (BioTeke, Beijing, China).
Real-time quantitative RT-PCR (qPCR) reactions were performed using a Mastercycler® ep realplex real-time PCR system (Eppendorf, Hamburg, Germany) with SYBR® Premix Ex Taq™ (TaKaRa Bio Inc., China) according to the manufacturer’s instructions. The cDNA was amplified using the following primers: for APX1 (accession number DQ122791), forward TCCTCTTATGCTCCGTTTG and reverse GTTCCACCCAGTAATCCCA; for APX2 (accession number AY054988), forward GGAACCATCAAGCACCAAGC and reverse ACAGCAACAACACCAGCCAAC; for Cu/Zn-SOD (accession number AF056621), forward TAATTGCTGATGCCAACG and reverse ACCACAGGCTAATCTTCCAC; for Mn-SOD (accession number AY145894.1), forward TGTCATCAGCGGCG TAATCAT and reverse GGGCTTCCTTTGGTGGTTCA; for proline dehydrogenase (PDH; accession number AY556386), forward TGGAATGTCTGAGGCACTA and reverse CAGCCAACACTCCTCTATT; for MSC 27 (Medicago sativa cDNA 27; accession number X63872), forward AGAATGGAATGTTGTGGGAGG and reverse GTCATCAACACCCTCATCTTCTC. Gene-specific primers were designed using Primer Express software (Applied Biosystems, Foster City, CA, USA). Relative expression levels were presented as values relative to that of the corresponding control samples at the indicated time points, after normalization to MSC 27 transcript levels. All reactions were set up in triplicate.
Determination of proline content
Proline content was determined by ninhydrin assay and high performance liquid chromatography (HPLC). For ninhydrin assay, according to the method described by Bates et al. (1973), about 0.1 g fresh samples was homogenized with 2 ml 3% sulfosalicylic acid. Afterwards, homogenate was mixed and centrifuged at 12,000g for 20 min. One milliliter of the supernatant was then mixed with equal volume of glacial acetic acid and acid ninhydrin reagent, incubated for 1 h at 100 °C. Afterwards, the reaction mixture was quickly cooled with water. The reaction product was extracted with 4 ml toluene by shaking, and the absorbance of upper toluene phase was read at 520 nm. Proline content was expressed as µmole g−1 FW. For HPLC analysis, proline was extracted from 0.1 g fresh samples by digesting in 0.01 M HCl for 24 h, and then performed HPLC (Agilent Technologies, 1200 series Quaternary, Foster City, CA, USA) analysis according to the method described by Inoue et al. (1996).
Statistical analysis
Where indicated, results are expressed as mean ± standard error (SE) of three independent experiments with at least three replicates for each. Statistical analysis was performed using SPSS 18.0 software. For statistical analysis, Duncan’s multiple comparison (P < 0.05) was selected.
Results
Cu-triggered inhibition of seed germination, Cu accumulation, and endogenous CH4 production
To investigate the toxic effects of Cu stress on growth performance, the germinating alfalfa seeds were exposed to different concentrations of CuCl2 (Schematic representation of seed culture was shown in Fig. S1). In comparison with the control sample, the germination rate, root length, and fresh weight of seedlings declined progressively with the increasing concentrations of Cu from 0.75 to 6.0 mM (Table 1; Fig. 1). For example, upon 1.5 mM CuCl2 stress, the germination rate was inhibited by 54.9%; root length and fresh weight were reduced by 82.0% and 62.7%, respectively. Meanwhile, the germination rate and corresponding root length upon 6.0 mM Cu were totally inhibited. Based on these results, 1.5 mM Cu, a high but not lethal concentration, was selected for the further experiments.
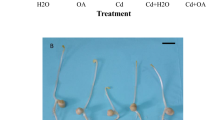
CH4 alleviates the inhibition of alfalfa seed germination and seedling growth caused by Cu stress. Seeds were presoaked with mediums containing different concentrations of CH4 for 12 h, and then shifted to 1.5 mM CuCl2 solution (Cu) for the designated time. The sample without chemicals was the control (Con). Afterwards, germination rate (a; 24 h), fresh weight of 50 seedlings (b; 72 h), root length per 20 seedlings (c; 72 h), were determined, and representative photographs of seeds with or without 0.39 mM CH4 pretreatment (d; 24 h) were also taken. Values are mean ± SE of three independent experiments with at least three replicates for each. Bars with different letters are significantly different at P < 0.05 according to Duncan’s multiple comparison
Further, the accumulation of Cu in alfalfa seedlings was measured. As shown in Fig. 2, Cu stress resulted in Cu accumulation respect to the Cu-free control. A subsequent work was performed to evaluate whether CH4 was involved in Cu response in plants. By contrast, compared to the control plants, the production of CH4 was noticeably increased upon Cu stress for 72 h (Fig. 3; related time course model of Cu treatment was shown in Fig. S2). These results indicated that endogenous CH4 may be involved in the response of Cu stress.
CH4 decreases the accumulation of Cu. Alfalfa seeds were presoaked with mediums containing 0.39 mM CH4 for 12 h, and then shifted to 1.5 mM CuCl2 solution for another 72 h. The sample without chemicals was the control (Con). Values are mean ± SE of three independent experiments with at least three replicates for each. Within individual stress time points, bars with different letters denote significant differences at P < 0.05 according to Duncan’s multiple comparison
Endogenous production of CH4. Alfalfa seed were presoaked with mediums containing 0.39 mM CH4 for 12 h, and then shifted to 1.5 mM CuCl2 solution for another 72 h. The sample without chemicals was the control (Con). Values are the mean ± SE of three independent experiments with three replicates for each. Within the similar time points, bars with different letters are significantly different at P < 0.05 according to Duncan’s multiple comparison
CH4 counteracts the inhibition of alfalfa seed germination and seedling growth, and Cu accumulation
To investigate whether CH4 exhibits beneficial roles in the alleviation of alfalfa seed germination and seedling growth, seeds were pretreated with methane-rich water containing CH4 at the concentrations of 0.13, 0.39, 0.65, and 1.30 mM, and then subsequently subjected to 1.5 mM CuCl2. Interestingly, the pretreatment with all the tested concentrations of CH4, was differentially effective in ameliorating the adverse effects of Cu stress, compared with the control, especially in germination rate (Fig. 1). For example, pretreatments with CH4 at 0.39, 0.65, and 1.30 mM differentially brought about the improvement of seed germination rate by 60.0, 44.6, and 18.5%, by compared with the Cu-stressed alone (Fig. 1a). A similar but less conspicuous alleviating performance against the Cu-induced inhibition of seedling growth was also observed (Fig. 1b, c). We also noticed that CH4 treatment alone differentially increased the root length of alfalfa seedling. By contrast, it has no significant effect on germination rate and fresh weight (except the decrease in fresh weight by 0.13 and 1.3 mM CH4 alone). Comparatively, the maximal inducible response was observed in 0.39 mM CH4-treated samples. Therefore, 0.39 mM CH4 was applied in the following experiment.
In addition, we measured Cu contents and CH4 production in alfalfa plants in the absence and presence of exogenously applied CH4. As shown in Figs. 2 and 3, CH4 pretreatment not only significantly suppressed Cu accumulation in alfalfa seedlings, but also mimicked the endogenous production of CH4 triggered by Cu stress alone. Above results revealed that CH4 may enhance plant tolerance against Cu stress during seed germination.
Increased amylase activities and total sugar contents by CH4
For better understanding and exploring the beneficial alleviating effect of CH4 on Cu-stressed alfalfa seed germination, α/β-amylase activities and total sugar contents in alfalfa seedlings were investigated. Enzyme assay showed that upon Cu stress, the activities of α-amylase (in particular) and β-amylase were reduced (Fig. 4a). Meanwhile, total sugar content was dramatically decreased, as a result of Cu toxicity (Fig. 4b). By contrast, CH4 pretreatments resulted in significant increases in α/β-amylase activities compared with Cu stress alone, both of which were consistent with the changes in total sugar contents. Compared to the stress conditions, CH4 pretreatment alone displayed slightly higher β-amylase activity and total sugar content, as compared with that of the control. Meanwhile, almost all seeds germinated in the control and all CH4-pretreated alone samples.
Regulation of α/β-amylase activity (a) and contents of total sugar (b) by CH4. Alfalfa seed were presoaked with mediums containing 0.39 mM CH4 for 12 h, and then shifted to 1.5 mM CuCl2 solution for another 24 h. The sample without chemicals was the control (Con). Values are mean ± SE of three independent experiments with at least three replicates for each. Within each parameter, bars with different letters denote significant differences at P < 0.05 according to Duncan’s multiple comparison
Cu-induced redox imbalance were blocked by CH4
To assess whether the beneficial effect of CH4 on Cu stress was related to oxidative stress and further the redox imbalance, the TBARS formation, which is a reliable maker of lipid peroxidation and free radical generation, was measured. As expected, the exposure of plants to Cu stress for 72 h caused a significant increase in TBARS level, compared with chemical-free control (Fig. 5a). This result suggested that redox imbalance occurred in stressed plants. However, CH4 produced a pronounced reduction of TBARS content upon Cu stress.
CH4 alleviates lipid peroxidation and loss of plasma membrane integrity in the root tissues of alfalfa seedlings upon Cu stress. Seed were presoaked with mediums containing 0.39 mM CH4 for 12 h, and then shifted to 1.5 mM CuCl2 solution for another 72 h. The sample without chemicals was the control (Con). Subsequently, the content of TBARS was determined (a). Meanwhile, the seedling roots were stained with Evans blue (b) or Schiff’s reagent (c), and immediately photographed under a light microscope. Bars: 1 mm. Values are mean ± SE of three independent experiments with at least three replicates for each. Bars with different letters denote significant differences at P < 0.05 according to Duncan’s multiple comparison
The estimation of the loss of plasma membrane integrity and lipid peroxidation in alfalfa seedling roots were further carried out by histochemical staining with Evans blue and Schiff’s reagent. Consistent with the changes in TBARS, roots of alfalfa seedling treated with Cu alone were stained extensively, while those pretreated with CH4 exhibited light staining (Fig. 5b, c). Interestingly, CH4 treated alone also showed slightly staining, in comparison with control. Hence, these results manifested that CH4 could protect alfalfa seedlings from oxidative stress caused by Cu stress to some extent.
Modulation of antioxidant enzyme activities and their transcripts
In order to get an insight into the in vivo function of CH4, the activities of representative antioxidant enzymes were investigated. The results showed that, upon Cu stress exposure, the total activities of APX, SOD, and CAT in alfalfa seedlings were significantly decreased to 47.8, 71.3, and 8.7% of the control samples, whereas the activity of POD was increased by 40.4% (Fig. 6). By contrast, the pretreatment with CH4 significantly blocked Cu-decreased APX, SOD, and CAT activities. Meanwhile, the increased activity of POD triggered by Cu was strengthened by CH4. Meanwhile, the decreases in the abundance of APX1, APX2, Cu/Zn-SOD, and Mn-SOD transcripts caused by Cu stress were differentially weakened by CH4 (Fig. 7). These results suggested that CH4 modulated gene expression of antioxidant enzymes at transcriptional and enzymatic levels.
Changes in antioxidant enzymatic activities. Alfalfa seeds were presoaked with mediums containing 0.39 mM CH4 for 12 h, and then shifted to 1.5 mM CuCl2 solution for another 24 h. The sample without chemicals was the control (Con). Afterwards, total activities of APX (a), SOD (b), CAT (c), and POD (d), were determined. Values are mean ± SE of three independent experiments with at least three replicates for each. Bars with different letters denote significant differences at P < 0.05 according to Duncan’s multiple comparison
Changes in the transcripts of APX1/2 (a), Cu/Zn-SOD and Mn-SOD (b). Alfalfa seeds were presoaked with mediums containing 0.39 mM CH4 for 12 h, and then shifted to 1.5 mM CuCl2 solution for another 24 h. The sample without chemicals was the control (Con). Afterwards, corresponding transcriptional profiles were analyzed by qPCR, normalized against the internal reference gene. Values are mean ± SE of three independent experiments with at least three replicates for each. Within each set of experiments, bars with different letters denote significant differences at P < 0.05 according to Duncan’s multiple comparison
Effect of CH4 on proline metabolism under Cu stress
We next determined the proline accumulation in alfalfa seeds under Cu and CH4 treatments. Since the ninhydrin-based assay is not that specific for proline, we also used HPLC method for further verification. As expected, a similar tendency was observed by using two methods, showing that Cu stress alone significantly increased the content of proline (Fig. 8a). By contrast, the increase of proline content was obviously blocked by CH4.
Effects of CH4 pretreatments on the proline content (a), P5CS enzyme activity (b) and gene expression of PDH (c) in alfalfa germinating seeds upon Cu exposure. Seeds were presoaked with mediums containing 0.39 mM CH4 for 12 h, and then shifted to 1.5 mM CuCl2 solution for another 24 h. The sample without chemicals was the control (Con). Proline contents were determined by ninhydrin-based and HPLC assay. Values are mean ± SE of three independent experiments with at least three replicates for each. Within each set of experiments, bars with different letters denote significant differences at P < 0.05 according to Duncan’s multiple comparison
To obtain a deep understanding of above results, the enzyme involved in the proline metabolic pathway was analyzed. Similar to the results of proline, the activity of P5CS, the key enzyme in proline synthesis, was up-regulated by 86.6% upon Cu stress alone (Fig. 8b), which was weaken by the pretreatment with CH4. Meanwhile, molecular evidence showed that Cu-down regulated PDH transcript was reversed by CH4 (Fig. 8c). These results clearly suggested that CH4 could reduce proline accumulation under Cu stress, at least partially, by restraining proline synthesis and promoting proline degradation.
Discussion
Ample evidence showed that Cu stress exhibits a detrimental effect on plant growth and development (Alaoui-Sossé et al. 2004). Besides seed germination and growth inhibition, a series of physiological and biochemical processes were triggered by Cu stress. Among these, Cu accumulation and redox imbalance occupy main parts (Zhang et al. 2008, Burkhead et al. 2009). CH4 is the second most important greenhouse gas and could emit from the terrestrial plants, soil, peat, and fungi etc. A comprehensive understanding of CH4 in plants is that the living plants and fungi can not only emit CH4 to the atmosphere (Lenhart et al. 2012; a major discovery in CH4 plant biology), but also produce CH4 in vivo (Jia et al. 2001). Similar discovery occurred in nitric oxide (Rockel et al. 2002; Planchet et al. 2005) and carbon monoxide (Tarr et al. 1995; Guenther et al. 2000), as well as in hydrogen gas (H2; Das and Veziroglu 2001). In this work, we aim to figure out whether CH4 could alleviate Cu toxicity in alfalfa plants, and how it works.
Similar to the response in salt stress (Zhu et al. 2016), the increased production of endogenous CH4 in germinating alfalfa seeds upon Cu stress, was firstly observed (Fig. 3). The endogenous CH4 production was determined by gas chromatography (GC) according to the method of CO and H2 production (Renwick et al. 1964; Anderson and Wu 2005; Bernardi et al. 2008). The CH4 gas in samples would emit only after sulfuric acid was added. The purpose of sulfuric acid is to digest plant materials, which was previously adopted in the determination of CO and H2 production (Renwick et al. 1964; Anderson and Wu 2005). In fact, the determination of CH4 related to emission often skips this step (Lenhart et al. 2012). Thus, the measurement of CH4 only reflects the in vivo production rather than emission to atmosphere. In this case, we focused on the production of endogenous CH4 rather than the emission from seedlings to atmosphere. Together, our study suggested that CH4 production can be induced by copper stress in Medicago sativa.
Subsequent results suggested the beneficial role of CH4 against Cu toxicity. It was confirmed by its alleviating growth and development inhibition caused by Cu stress, including seed germination (in particular) and seedlings growth (Fig. 1). Besides the alteration of proline metabolism, the reestablishment of redox homeostasis and lowing Cu accumulation may be associated with the above beneficial role of CH4. In this report, Therefore, consistent with the beneficial role of CH4 in animals (Boros et al. 2012; Chen et al. 2016a, b; He et al. 2016), we suggested that the CH4, at least in our experimental condition, might counteract Cu toxicity during seed germination. Several explanations may account for this conclusion and are addressed in this study.
First, our results revealed that the pretreatment with different concentrations of CH4 (from 0.13 to 1.3 mM) could differentially alleviate the inhibition of seed germination and seedling growth caused by Cu stress, with the most effective response at 0.39 mM CH4 (Fig. 1). Besides, the beneficial effects of CH4 on amylase activities, total sugar and TBARS contents exhibited the similar tendencies, also showing dose-dependent responses (Fig. S3, S4). Meanwhile, Cu stress induced an increase in the CH4 production in alfalfa plants, which was mimicked by exogenously applied 0.39 mM CH4 (Fig. 3). Similar results have been suggested by several previous reports under various environmental stress conditions, including UV radiation, high temperature, and water stress (Keppler et al. 2009; Messenger et al. 2009; Wishkerman et al. 2011; Bruhn et al. 2012, 2014; Wang et al. 2013). Although we have not characterized the possible enzymatic or non-enzymatic resource(s) of CH4 synthesis and degradation in this study, our data clearly indicated that endogenous production of CH4 occurred in germinating alfalfa seeds under Cu stress.
Notably, the accumulation of Cu was also partly blocked by CH4 (Fig. 2). Similarly, previous reports showed that exogenously applied with various gaseous molecules [i.e. carbon monoxide (CO), hydrogen sulfide (H2S), hydrogen gas (H2), etc.], has beneficial effects on plant tolerance against cadmium and copper stress by lowing their accumulation (Han et al. 2008; Zhang et al. 2008; Cui et al. 2013).
In accordance with the previous observations (El-Tayeb et al. 2006; Zhang et al. 2008), our results showed that Cu stress decreased the activities of amylase and sugar content during seed germination (Fig. 4a, b). By contrast, the pretreatment with CH4 significantly increased the activities of α-amylase (in particular) and β-amylase and the contents of total sugar. It might be contributed to increase germination rates (Fig. 1a), since the mobilization of starch endosperm triggered by amylase was essential for seed germination. It was reported that nitric oxide (NO)-triggered increase of amylase might be beneficial for elevated germination of wheat seeds under Cu stress (Hu et al. 2007). Similarly, exogenous application of H2S is able to enhance activity of amylase and further alleviate Cu stress (Zhang et al. 2008). Consistent with the advantageous roles of NO and H2S, our results further confirmed a potential role for CH4 in the tolerance of alfalfa against Cu stress.
It was well-known that cellular redox perturbation caused by overproduction of ROS is one of the main features caused by heavy metal stress (Sharma and Dietz 2009). Previous study showed that transgenic Arabidopsis plant overexpressing a barely APX gene exhibited zinc and cadmium tolerance (Xu et al. 2008). Our results indicated that CH4 counteracts the redox imbalance caused by Cu stress. This conclusion could be confirmed by the increased activities of representative antioxidant enzymes, including APX, SOD, CAT, and POD (Fig. 6), or up-regulation of corresponding transcripts (Fig. 7) elicited by CH4 pretreatment, all of which were positively correlated with alfalfa Cu tolerance (Fig. 1). These results were consistent with the observations in animals. For example, CH4 treatment by using methane-rice saline increased antioxidant enzyme activities (SOD, CAT, etc.) in the retinas with ischemia/reperfusion injury, and further suppressed oxidative damage (Liu et al. 2016b). Similar result was also presented, showing that CH4 protected liver against concanavalin A-induced injury through activating antioxidant mechanism (He et al. 2016). Therefore, it’s easily deduced that increased antioxidant enzyme activities could partially alleviate oxidative damage and further membrane injury. This deduction was supported by the fact, showing that CH4 significantly decreased TBARS overproduction (Fig. 5a). Such alleviating effects were further supported by the results of histochemical staining for detection of lipid peroxidation (Fig. 5b) and loss of plasma membrane integrity (Fig. 5c). Similar results were shown in a dose–response pattern in CH4 alleviated TBARS overproduction (Fig. S4). Together, above results clearly suggested that CH4-triggered alleviation of Cu toxicity might be, at least partially, related to the attenuation of oxidative damage.
Proline accumulation has been considered as a common physiological response to a various biotic and abiotic stress in many plants (Verbruggen and Hermans 2008). Generally, proline functions as a compatible osmolyte and a repository for carbon and nitrogen (Hare and Cress 1997). Proline accumulation during stresses mainly depends on the activation of synthesis and suppression of degradation. Similar to the previous results (Bassi and Sharma 1993; Chen et al. 2004; Ku et al. 2012; Tripathi et al. 2013), Cu stress increased proline content, which was accompanied by enhancing the activity of P5CS, and down-regulation of PDH transcripts (Fig. 8). Contrastingly, pretreatment with CH4 blocked the accumulation of proline by suppressing its synthesis and increasing degradation. Interestingly, the blocking effect in proline accumulation by CH4, was in agreement with those reports by the application of salicylic acid (SA) and NO under various stresses. For example, the repression of proline accumulation is reported in barley under cadmium stress by application of SA (Metwally et al. 2003). Likewise, exogenous application of sodium nitroprusside (SNP), a NO donor, decreased the proline accumulation and oxidative damage in Chinese cabbage under salt stress (Lopez-Carrion et al. 2008). Our results indicated that proline accumulation might be not responsible for the Cu stress tolerance, given that increase in proline level didn’t bring out superior tolerance. Proline toxicity was also observed in many plants by exogenous application of proline (Hellmann et al. 2000; Mani et al. 2002; Ayliffe et al. 2005). Genetic evidence conferred an inverse relationship between proline level and yeast cell growth (Maggio et al. 2002). In fact, higher proline levels did not always mean better stress tolerance (Verbruggen and Hermans 2008). On the contrary, the degradation of proline may beneficial for stress adaption, due to the fact that the conversion of proline to glutamate offers reducing equivalents which accelerates mitochondrial oxidative phosphorylation (Hare and Cress 1997). Combined with these results, we deduced that the alteration in proline metabolism caused by CH4 might function in an indirect way, not just as a compatible solute or molecular chaperone to maintain cell homeostasis in our study. Certainly, further investigation needed to be carried out in near future.
In conclusion, our results present a new insight in CH4-triggered alleviation of Cu toxicity during alfalfa seeds germination. The beneficial roles of CH4 are partly mediated by reducing Cu accumulation, increasing amylase activities, and reestablishing redox homeostasis. Thus, our results will provide an effective strategy for plant tolerance against heavy metals.
Abbreviations
- APX:
-
Ascorbate peroxidase
- ASC:
-
Ascorbic acid
- CAT:
-
Catalase
- CH4 :
-
Methane
- FID:
-
Flame ionization detector
- GC:
-
Gas chromatograph
- H2O2 :
-
Hydrogen peroxide
- NBT:
-
Nitroblue tetrazolium
- O2 − :
-
Superoxide anion radicals
- P5C:
-
∆1-pyrroline-5-carboxylate
- P5CS:
-
∆1-pyrroline-5-carboxylate synthetase
- PDH:
-
Proline dehydrogenase
- POD:
-
Guaiacol peroxidase
- PVP:
-
Polyvinylpyrrolidone
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TBA:
-
Thiobarbituric acid
- TBARS:
-
Thiobarbituric acid reactive substances
- TCA:
-
Trichloroacetic acid
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
Alaoui-Sossé B, Genet P, Vinit-Dunand F et al (2004) Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci 166:1213–1218. doi:10.1016/j.plantsci.2003.12.032
Anderson CR, Wu WH (2005) Analysis of carbon monoxide in commercially treated tuna (Thunnus spp) and mahi-mahi (Coryphaena hippurus) by gas chromatography/mass spectrometry. J Agric Food Chem 53:7019–7023. doi:10.1021/jf0514266
Ayliffe MA, Mitchell HJ, Deuschle K et al (2005) Comparative analysis in cereals of a key proline catabolism gene. Mol Genet Genomics 274:494–505. doi:10.1007/s00438-005-0048-x
Barker J, Curtis S, Hogsett O et al (1986) Safety in swine production systems. Pork Industry Handbook 104
Bassi R, Sharma SS (1993) Proline accumulation in wheat seedlings exposed to zinc and copper. Phytochem 33:1339–1342. doi:10.1016/0031-9422(93)85086-7
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207. doi:10.1007/BF00018060
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. doi:10.1016/0003-2697(71)90370-8
Bernardi C, Chiesa LM, Soncin S et al (2008) Determination of carbon monoxide in tuna by gas chromatography with micro-thermal conductivity detector. J Chromatogr Sci 46:392–394. doi:10.1093/chromsci/46.5.392
Boros M, Ghyczy M, Érces D et al (2012) The anti-inflammatory effects of methane. Crit Care Med 40:1269–1278. doi:10.1097/CCM.0b013e31823dae05
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Bruhn D, Møller IM, Mikkelsen TN et al (2012) Terrestrial plant methane production and emission. Physiol Plant 144:201–209. doi:10.1111/j.1399-3054.2011.01551.x
Bruhn D, Mikkelsen TN, Rolsted MMM et al (2014) Leaf surface wax is a source of plant methane formation under UV radiation and in the presence of oxygen. Plant Biol 16:512–516. doi:10.1111/plb.12137
Burkhead JL, Gogolin Reynolds KA, Abdel-Ghany SE et al (2009) Copper homeostasis. New Phytol 182:799–816. doi:10.1111/j.1469-8137.2009.02846.x
Chen CT, Chen TH, Lo KF et al (2004) Effects of proline on copper transport in rice seedlings under excess copper stress. Plant Sci 166:103–111. doi:10.1016/j.plantsci.2003.08.015
Chen Z, Cuin TA, Zhou M et al (2007) Compatible solute accumulation and stress-mitigating effects in barely genotypes contrasting in their salt tolerance. J Exp Bot 58:4245–4255. doi:10.1093/jxb/erm284
Chen O, Ye Z, Cao Z et al (2016a) Methane attenuates myocardial ischemia injury in rats through anti-oxidative, anti-apoptotic and anti-inflammatory actions. Free Radic Biol Med 90:1–11. doi:10.1016/j.freeradbiomed.2015.11.017
Chen Z, Yu L, Wang X et al (2016b) Changes of phenolic profiles and antioxidant activity in canaryseed (Phalaris canariensis L.) during germination. Food Chem 194:608–618. doi:10.1016/j.foodchem.2015.08.060
Conrad R (2005) Quantification of methanogenic pathways using stable carbon isotopic signatures: a review and a proposal. Org Geochem 36:739–752. doi:10.1016/j.orggeochem.2004.09.006
Conrad R (2009) The global methane cycle: recent advances in understanding the microbial processes involved. Environ Microbiol Rep 1:285–292. doi:10.1111/j.1758-2229.2009.00038.x
Cui W, Gao C, Fang P et al (2013) Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J Hazard Mater 260:715–724. doi:10.1016/j.jhazmat.2013.06.032
Cui W, Qi F, Zhang Y et al (2015) Methane-rich water induces cucumber adventitious rooting through heme oxygenase1/carbon monoxide and Ca2+ pathways. Plant Cell Rep 34:435–445. doi:10.1007/s00299-014-1723-3
Das D, Veziroǧlu TN (2001) Hydrogen production by biological processes: a survey of literature. Int J Hydrogen Energ 26:13–28. doi:10.1016/S0360-3199(00)00058-6
El-Tayeb MA, El-Enany AE, Ahmed NL (2006) Salicylic acid-induced adaptive response to copper stress in sunflower (Helianthus annuus L). Plant Growth Regul 50:191–199. doi:10.1007/s10725-006-9118-2
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155:93–100. doi:10.1104/pp.110.166181
Guenther A, Geron C, Pierce T et al (2000) Natural emissions of non-methane volatile organic compounds, carbon monoxide, and oxides of nitrogen from North America. Atmos Environ 34:2205–2230. doi:10.1016/S1352-2310(99)00465-3
Han Y, Zhang J, Chen X, Gao Z, Xuan W, Xu S, Ding X, Shen WB (2008) Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol 177:155–166. doi:10.1111/j.1469-8137.2007.02251.x
Hare PD, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21:79–102. doi:10.1023/A:1005703923347
He R, Wang L, Zhu J et al (2016) Methane-rich saline protects against concanavalin A-induced autoimmune hepatitis in mice through anti-inflammatory and anti-oxidative pathways. Biochem Biophys Res Commun 470:22–28. doi:10.1016/j.bbrc.2015.12.080
Hellmann H, Funck D, Rentsch D et al (2000) Hypersensitivity of an Arabidopsis sugar signaling mutant toward exogenous proline application. Plant Physiol 122:357–368. doi:10.1104/pp.122.2.357
Hu KD, Hu LY, Li YH et al (2007) Protective roles of nitric oxide on germination and antioxidant metabolism in wheat seeds under copper stress. Plant Growth Regul 53:173–183. doi:10.1007/s10725-007-9216-9
Huang BK, Xu S, Xuan W et al (2006) Carbon monoxide alleviates salt-induced oxidative damage in wheat seedling leaves. J Integr Plant Biol 48:249–254. doi:10.1111/j.1744-7909.2006.00220.x
Inoue H, Date Y, Kohashi K et al (1996) Determination of total hydroxyproline and proline in human serum and urine by HPLC with fluorescence detection. Biol Pharm Bull 19:163–166. doi:10.1248/bpb.19.163
Janas KM, Zielińska-Tomaszewska J, Rybaczek D et al (2010) The impact of copper ions on growth, lipid peroxidation, and phenolic compound accumulation and localization in lentil (Lens culinaris Medic.) seedlings. J Plant Physiol 167:270–276. doi:10.1016/j.jplph.2009.09.016
Jia Z, Cai Z, Xu H et al (2001) Effect of rice plants on CH4 production, transport, oxidation and emission in rice paddy soil. Plant Soil 230:211–221. doi:10.1023/A:1010366631538
Jiang WD, Qu B, Feng L et al (2016) Histidine prevents Cu-induced oxidative stress and the associated decreases in mRNA from encoding tight junction proteins in the intestine of Grass Carp (Ctenopharyngodon idella). PLoS ONE 11:e0157001. doi:10.1371/journal.pone.0157001
Jin Q, Zhu K, Cui W et al (2013) Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell Environ 36:956–969. doi:10.1111/pce.12029
Keppler F, Boros M, Frankenberg C et al (2009) Methane formation in aerobic environments. Environ Chem 6:459–465. doi:10.1071/EN09137
Ku HM, Tan CW, Su YS et al (2012) The effect of water deficit and excess copper on proline metabolism in Nicotiana benthamiana. Biol Plant 56:337–343. doi:10.1007/s10535-012-0095-1
Lenhart K, Bunge M, Ratering S et al (2012) Evidence for methane production by saprotrophic fungi. Nat Commun 3:1046. doi:10.1038/ncomms2049
Liu DL, An ZG, Mao ZJ et al (2016a) Enhanced heavy metal tolerance and accumulation by transgenic sugar beets expressing Streptococcus thermophilus StGCS-GS in the presence of Cd, Zn and Cu alone or in combination. PLoS ONE 10:e0128824. doi:10.1371/journal.pone.0128824
Liu L, Sun Q, Wang R et al (2016b) Methane attenuates retinal ischemia/reperfusion injury via anti-oxidative and anti-apoptotic pathways. Brain Res 1646:327–333. doi:10.1016/j.brainres.2016.05.037
Lombardi L, Sebastiani L (2005) Copper toxicity in Prunus cerasifera: growth and antioxidant enzymes responses of in vitro grown plants. Plant Sci 168:797–802. doi:10.1016/j.plantsci.2004.10.012
Lopez-Carrion AI, Castellano R, Rosales MA et al (2008) Role of nitric oxide under saline stress: implications on proline metabolism. Biol Plant 52:587–591. doi:10.1007/s10535-008-0117-1
Maggio A, Miyazaki S, Veronese P et al (2002) Does proline accumulation play an active role in stress-induced growth reduction? Plant J 31:699–712. doi:10.1046/j.1365-313X.2002.01389.x
Mani S, Van De Cotte B, Van Montagu M et al (2002) Altered levels of proline dehydrogenase cause hypersensitivity to proline and its analogs in Arabidopsis. Plant Physiol 128:73–83. doi:10.1104/pp.010572
Messenger DJ, McLeod AR, Fry SC (2009) Reactive oxygen species in aerobic methane formation from vegetation. Plant Signal Behav 4:629–630. doi:10.1111/j.1365-3040.2008.01892.x
Metwally A, Finkemeier I, Georgi M et al (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281. doi:10.1104/pp.102.018457
Patui S, Clincon L, Peresson C et al (2014) Lipase activity and antioxidant capacity in coffee (Coffea arabica L.) seeds during germination. Plant Sci 219–220:19–25. doi:10.1016/j.plantsci.2013.12.014
Planchet E, Jagadis Gupta K, Sonoda M et al (2005) Nitric oxide emission from tobacco leaves and cell suspensions: rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J 41:732–743. doi:10.1111/j.1365-313X.2005.02335.x
Renwick GM, Giumarro C, Siegel SM (1964) Hydrogen metabolism in higher plants. Plant Physiol 39:303–306. doi:10.1104/pp.39.3.303
Rockel P, Strube F, Rockel A et al (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53:103–110. doi:10.1093/jexbot/53.366.103
Seiler W, Holzapfel-Pschorn A, Conrad R et al (1983) Methane emission from rice paddies. J Atmos Chem 1:241–268. doi:10.1007/BF00058731
Sharma SS, Dietz K (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14:43–50. doi:10.1016/j.tplants.2008.10.007
Sheldon AR, Menzies NW (2005) The effect of copper toxicity on the growth and root morphology of Rhodes grass (Chloris gayana Knuth.) in resin buffered solution culture. Plant Soil 278:341–349. doi:10.1007/s11104-005-8815-3
Tarr MA, Miller WL, Zepp RG (1995) Direct carbon monoxide photoproduction from plant matter. J Geophys Res 100:11403–11413. doi:10.1029/94JD03324
Tripathi BN, Singh V, Ezaki B et al (2013) Mechanism of Cu- and Cd-induced proline hyperaccumulation in triticum aestivum (wheat). Plant Growth Regul 32:799–808. doi:10.1007/s00344-013-9343-7
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759. doi:10.1007/s00726-008-0061-6
Wang SH, Zhang H, Zhang Q et al (2011) Copper-induced oxidative stress and responses of the antioxidant system in roots of Medicago sativa. J Agron Crop Sci 197:418–429. doi:10.1111/j.1439-037X.2011.00476.x
Wang Y, Li L, Cui W et al (2012) Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil 351:107–119. doi:10.1007/s11104-011-0936-2
Wang ZP, Chang SX, Chen H et al (2013) Widespread non-microbial methane production by organic compounds and the impact of environmental stresses. Earth-Sci Rev 127:193–202. doi:10.1016/j.earscirev.2013.10.001
Wishkerman A, Greiner S, Ghyczy M et al (2011) Enhanced formation of methane in plant cell cultures by inhibition of cytochrome c oxidase. Plant Cell Environ 34:457–464. doi:10.1111/j.1365-3040.2010.02255.x
Xie Y, Ling T, Han Y et al (2008) Carbon monoxide enhances salt tolerance by nitric oxide-mediated maintenance of ion homeostasis and up-regulation of antioxidant defence in wheat seedling roots. Plant Cell Environ 31:1864–1881. doi:10.1111/j.1365-3040.2008.01888.x
Xu WF, Shi WM, Liu F et al (2008) Enhanced zinc and cadmium tolerance and accumulation in transgenic Arabidopsis plants constitutively overexpressing a barley gene (HvAPX1) that encodes a peroxisomal ascorbate peroxidase. Botany 86:567–575. doi:10.1139/B08-025
Xu S, Zhu S, Jiang Y et al (2013) Hydrogen-rich water alleviates salt stress in rice during seed germination. Plant Soil 370:47–57. doi:10.1007/s11104-013-1614-3
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208. doi:10.1104/pp.125.1.199
Yonar ME, Ispir U, Mise Yonar S et al (2015) Effect of copper sulphate on the antioxidant parameters in the rainbow trout fry, Oncorhynchu mykiss. Cell Mol Biol (Noisy-le-grand) 62:55–58
Yruela I (2005) Copper in plants. Braz J Plant Physiol 17:145–156. doi:10.1590/S1677-04202005000100012
Zhang CS, Lu Q, Verma DPS (1995) Removal of feedback inhibition of ∆1-pyrroline-5-carboxylate synthetase, a bifunctional enzyme catalyzing the first two steps of proline biosynthesis in plants. J Biol Chem 270:20491–20496. doi:10.1074/jbc.270.35.20491
Zhang H, Hu LY, Hu KD et al (2008) Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol 50:1518–1529. doi:10.1111/j.1744-7909.2008.00769.x
Zhang HJ, Zhang N, Yang RC et al (2014) Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J Pineal Res 57:269–279. doi:10.1111/jpi.12167
Zhu K, Cui W, Dai C et al (2016) Methane-rich water alleviates NaCl toxicity during alfalfa seed germination. Environ Exper Bot 129:37–47. doi:10.1016/j.envexpbot.2015.11.013
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (J1210056 and J1310015) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Muhammad Kaleem Samma and Heng Zhou have contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Samma, M.K., Zhou, H., Cui, W. et al. Methane alleviates copper-induced seed germination inhibition and oxidative stress in Medicago sativa . Biometals 30, 97–111 (2017). https://doi.org/10.1007/s10534-017-9989-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-017-9989-x