Abstract
Background and aims
Hydrogen sulfide (H2S) acting as the third gasotransmitter following NO and CO has important physiological functions in both animals and plants. In plants, H2S plays a critical role in alleviating toxicity of Cd stresses. It is well known that Calcium-Dependent Protein Kinases (CDPKs) can regulate cell recognition and signal transduction through reversible protein phosphorylation, but how CDPKs regulate H2S signal remains unclear.
Methods
The genetic and pharmacological method together with spectrophotometry and LC-MS/MS were used in this study.
Results
Our results indicated that Arabidopsis pretreated with H2S exhibited enhanced tolerance to Cd. After treatment by trifluoroperazine (TFP), the toxicity of Cd was exacerbated. Meanwhile, the activity of L-cysteine desulfhydrase (LCD) was reduced and the content of endogenous H2S decreased. In vitro experiments demonstrated that CDPK3 could raise LCD activity. Interestingly, expressions of Cd associated genes could not normally respond to Cd stress in cdpk3 whereas increased when this mutant was pretreated with H2S. S-sulfhydration results revealed that the content of glutathione persulfide (GSSH) was significantly lower in lcd and cdpk3 mutants. The results indicated that the decrease in GSSH content was mainly due to the reduction in H2S, which further caused the increased sensitivity to Cd.
Conclusions
CDPKs can enhance the tolerance to Cd in Arabidopsis through the way of intensifying H2S signal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen sulfide (H2S), a colorless gas with the odor of rotten eggs, has long been considered toxic to living organisms (Chen et al. 2011; Dooley et al. 2013; Garcia-Mata and Lamattina 2013; Jin et al. 2013; Li et al. 2011; Lisjak et al. 2013). However, recent studies have indicated that H2S, identified as the third signaling gasotransmitter following NO and CO, has important physiological functions in animals and more recently in plants as well (Wang 2012). In plants, two enzymes known as L-cysteine desulfhydrase (LCD) and D-cysteine desulfhydrase (DCD) have been discovered and regarded to be capable of decomposing L-cysteine and D-cysteine into H2S, pyruvate, and NH3 respectively (Riemenschneider et al. 2005). LCD has a higher catalytic activity as compared to DCD, and the function of LCD (At3g62130) has been studied by many research groups (Jin et al. 2011, 2013). Shi found that the overexpression LCD plants could exhibit enhanced tolerance to abiotic stresses (Shi et al. 2014b). Alvarez and Papenbrock later on revealed that DES1 protein encoded by DES1 (At5g28030), a member of the OASTL gene family, also has LCD activity (Alvarez et al. 2010; Papenbrock et al. 2007). Studies with exogenous H2S treatment have demonstrated that H2S has multiple effects on plants, which includes promotion of seed germination and organogenesis (Zhang et al. 2009a), enhanced tolerance to osmotic stress (Shi et al. 2013; Zhang et al. 2009b), salt stress and extreme temperatures (Li et al. 2013a, b), and increased resistance to oxidative stress and heavy metal stress (Dawood et al. 2012; Li et al. 2012a; Qiao et al. 2015; Shi et al. 2014a; Zhang et al. 2008, 2010).
It is proposed that H2S could modify cysteine residues on proteins through S-sulfhydration and subsequently alter the functions of target proteins. For example, in animals, a total of 39 proteins modified by S-sulfhydration were first discovered through biotin switch method and all of them have critical functions, including ion channel flux (Munaron et al. 2013), suppression of apoptosis and cellular senescence (Sen et al. 2012; Yang et al. 2013), regulation of ER stress response (Krishnan et al. 2011) and enhancement of various enzymatic activities (Vandiver et al. 2013). However, the study of protein S-sulfhydration in plants has just begun. Ángeles Aroca in early 2015 published the first report of S-sulfhydration as a posttranslational modification in plants, which opens a door to further study of this area in plant world (Aroca et al. 2015; Romeroa et al. 2013).
GSSH acts as a link between H2S and a target protein during protein S-sulfhydration. At first, GSSH is formed through the transfer of sulfhydryl group from H2S to the GSSG. Then the GSSH further attacks cysteine residues on the target protein for the completion of S-sulfhydration. In plants, the metabolism of GSSH involves two enzymes, ETHE1 and STR1. ETHE1 highly specific for GSSH catalyzes its conversion to thiosulfate, which results in the reduction in S-sulfhydration (Krussel et al. 2014; Lu et al. 2013).
In mammals, the modified biotin switch method is a commonly used way to detect specific protein sulfhydration sites, while in plants; this method is not widely used. In order to better investigate protein S-sulfhydration in plants, MBB solution together with LC-MS/MS technique is used to quantify the content of GSSH, which is an indicator of the level of S-sulfhydration (Lu et al. 2013).
Reversible protein phosphorylation plays a crucial role in regulating a wide spectrum of cellular processes, ranging from cell fate control to cell signal transduction. In particular, protein phosphorylation is regulated by protein kinases. Currently, many protein kinases have been identified and implicated to function as regulators involved in multiple signaling pathways induced by phytohormones or extracellular signals in plants. Among a large number of PKs is a type of protein kinases known as calcium-dependent protein kinases (CDPKs). They are widely distributed and can be detected across the entire plants, such as in roots, stems, leaves, fruits and seeds. Moreover, they are widespread in most of the plant organelles, including plasma membranes, vacuolar membranes and cytoplasm. More importantly, they are Ca2+-dependent instead of CaM dependent (Tuteja and Mahajan 2007; Zhu et al. 2007).
CDPKs are ubiquitous in plants. Some studies proposed that Ca signaling could alleviate Cr stress to some extent (Fang et al. 2014). However, whether CDPKs are involved in H2S mediated signaling pathway and their potential functions in defending against Cd in H2S dependent pathway remain to be discovered.
H2S as a signaling molecule plays a crucial role in Cd stress. Protein phosphorylation mediated by CDPKs is an important regulatory mechanism during cell signal transduction. However, the relationship between CDPKs and H2S remains unclear. We hypothesize that under Cd stress, CDPKs might raise LCD activity to generate more endogenous H2S and this intensifying signal could probably promote protein S-sulfhydration, regulate the expression of stress-associated genes and enhance antioxidative capacity, thereby potentially elevating Cd tolerance in plants. In this study, we used wild type Arabidopsis, LCD-knockout mutants (lcd) and CDPK3-knockout plants (cdpk3) as experimental materials, to investigate this issue and further explore the molecular mechanism of plants’ response against heavy metal stresses.
Materials and methods
Plant materials
Seeds of Arabidopsis thaliana (Columbia-0), CDPKs mutants (cdpk3, cdpk4, cdpk6 and cdpk32) and lcd mutants (SAIL_793_C08/CS835466) obtained from ABRC were surface sterilized in 70 % ethanol for 30 s and then incubated in 5 % (w/v) sodium hypochlorite for 10 min. After three washes with distilled water, seeds (50–60 per bottle) were planted in 20 ml 1/2 Murashige-Skoog (1/2 MS) medium supplemented with 0.7 % (w/v) agar, and 1 % (w/v) sucrose, pH 5.8. Unless otherwise stated, the trays were placed in growth chambers with the conditions of 23 ± 2°C, 16 h photoperiod, 200 μE · m−2 · s−1 of light and 70–80 % relative humidity. In our study, NaHS fumigation method was used to provide H2S. Basically, a small container of NaHS solution was placed in a petri dish containing 14 day-old seedlings. Since NaHS can release H2S slowly, seedlings can be fumigated in this way. In order to make a 50 μM H2S in the petri dish, we added 3.5 μL of 1 M NaHS to the small container placed in the petri dish. HT and TFP were directly added to the 1/2 MS medium. The working concentrations of HT and TFP are 100 μM and 10 μM respectively. They are all 1000× pre-made. Fourteen days post germination; the seedlings were treated with 50 μM H2S or 10 μM TFP (inhibitor of CDPKs) for 6 h. Subsequently, treated seedlings were transferred to 1/2 MS medium containing 100 μM CdCl2 for 120 h.
Detection of ROS and MDA content assay in A. thaliana leaves
The leaves of 14 day-old plants treated with various chemicals (Control; 100 μM CdCl2; 50 μM H2S + 100 μM CdCl2) were collected and then incubated in 0.1 mg/ml NBT (Sigma, MO, USA) solution (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and 2 mM NaH2PO4, pH 7.5) for 1 h at 30°C in the dark place. After three washes with distilled water, the leaves were soaked in boiled 95 % ethanol for 10 min. ROS in the form of blue coloration was visualized. MDA content was determined according to previously described methods (Halliwell and Chirico 1993).
Extraction of total RNA and RT-PCR
All molecular manipulations were carried out according to standard methods (Sambrook and Manistis 1987). The primers are as follows:
-
LCD: 5′-ATGGCAATGGAGGCGGGAGAGCGGCGCAATG-3′ 5′-CTACAATGCAGGAAGGTTTTGACAAG-3′;
-
CDPK3: 5′-ATGGGCCACAGACACAGC-3′
-
5′-GTTCATCGCCCGGAATTG-3′;
-
MYB107: 5′-ATGGGGAGATCACCGTGTTGC-3′
-
5′-CTATTCACGAAATGGCCAAGGC-3′
-
CAX3: 5′-ATGGGAAGTATCGTGGAGCCA-3′
-
5′-TTAAGCTGAGAAACTTCTCCCA-3′
-
POX1: 5′-ATGTTGAGTG AGAAACAAGCA-3′
-
5′-TCAGATA TTGACAAATC TACAA-3′
-
MT3: 5′-ATGTCAAGC AACTGCGGAAG-3′
-
5′-TTAGTTGGGGCAGCAAGTGCA-3′
-
PCS1: 5′-ATGGCTATGGCGAGTTTATATCG-3′
-
5′-CTAATAGGCAGGAGCAGCGAGA-3′
-
ETHE1: 5′-CGTGGTTGTGGGAGGACTGACT-3′
-
5′-CCTTTGGCAATGTAAATATCTGTGA-3′
-
STR1: 5′-GACCGCTTCTTATTCCCT-3′
-
5′-AAGTTCCAGCCACATCTA-3′
-
ACTIN: 5′-CTCAGCACCTTCCAACAGATGTGGA-3′
-
5′-CCAAAAAAATGAACCAAGGACCAAA-3′
Enzyme preparations and assays
Whole plants in three groups were harvested. SOD (EC 1.15.1.1) activity was measured by using a nitroblue tetrazolium (NBT) reduction method (Becana et al. 1986), GR (EC 1.6.4.2) activity was measured by following the decrease in absorbance at 340 nm due to NADPH oxidation (Schaedle 1977) and Catalase (CAT, EC1.11.1.6) activity was determined by measuring the decrease in absorption at 240 nm, in a reaction solution containing 50 mM potassium phosphate buffer (pH 7.2) and 2 mM H2O2 (Chance et al. 1979). The data presented are the mean ± SE from three independent repetitions.
Determination of H2S production rate
The total activity of cysteine desulfhydrases was measured by detecting the rate of H2S production from L-cysteine. At first, the whole plant material was ground in a 20 mM phosphate buffer (pH 8.0) by using a mortar and pestle. After centrifugation at 10,000 g for 5 min at 4 °C, the resulting supernatant (plant soluble extract) was used for LCD activity measurements. The assay was performed in a flask with a center vial containing a filter paper (1 cm × 2 cm) and 500 μL trapping solution (1 % zinc acetate; 2 % sodium EDTA, pH 12.7). The reaction solution contains 100 mM Tris/HCl pH 9.0, 10 % plant extracts, 2.5 mM DTT and 0.8 mM L-cysteine, for a total volume of 1 ml. When the reaction began, the flask was sealed with a rubber stopper. After incubation at 37°C for 15 min, 100 μL of 30 mM FeCl3 dissolved in 1.2 M HCl together with 100 μl 20 mM N,N-dimethyl-p-phenylenediamine dihydrochloride dissolved in 7.2 M HCl were added to the trapping vial. The vial was vortexed gently and placed in dark for 20 min. The formation of methylene blue was detected at 670 nm using a spectrophotometer. D-A standard curve was made by using solutions with different concentrations of NaHS (Jin et al. 2011).
Measurement of endogenous H2S content
In order to understand the effect of SA treatment on endogenous H2S content, H2S produced in coleoptiles and roots of seedlings was measured by monitoring the formation of methylene blue from 20 mM N,N-dimethyl-p-phenylenediamine dihydrochloride dissolved in 7.2 M HCl according to previously described methods (Chen et al. 2011; Li et al. 2013b; Shi et al. 2014a).
The measurement of GSSH content
GSSH content was determined according to the previously described methods (Lu et al. 2013). Freshly harvested tissues were added to a 2 mL Eppendorf tube containing 1 mL of CH3OH:H2O (80 : 20) with deoxygenated 2 mM monobrombimane (MBB) and 0.1 mM EDTA. The tube was incubated at 4°C for 2 h, followed by additional 2 h incubation at room temperature. The tissue disruption was then carried out by using stainless steel beads in a TissueLyser (Qiagen). After 20 min incubation at 4°C and additional 30 min incubation at room temperature, homogenized solution was centrifuged at 13,000 g for 10 min at 4°C. The supernatant was analyzed by LC-MS/MS. MBB and its derivatives are light sensitive; so all manipulations must be in the dark. GSSH is unstable at pH < 5.5, so solutions should be freshly prepared and kept pH between 7.0-8.0.
The interaction of CDPK3 and LCD
The coding sequences were amplified using primer pairs:
-
LCD-R, 5′-ATGGCAATGGAGGCGGGAGAGCGGCGCAATG-3′,
-
LCD-F, 5′-CTACAATGCAGGAAGGTTTTGACAAG-3′.
-
CDPK3-R, 5′-ATGGGCCACAGACACAGC-3′,
-
CDPK3-F, 5′-GTTCATCGCCCGGAATTG-3′.
After verifying its sequence, the PCR fragment was ligated into the pET-28a expression vector, and the resulting pET28a-LCD plasmid was delivered into E. coli strain BL21 (DE3). Bacterial cultures were grown to an OD600 of 0.6 in LB medium containing 50 mg L−1 kanamycin with agitation at 37°C, and production of the recombinant protein was induced over the course of 6 h by the addition of 1 mM IPTG. The protein was purified with a His-Tag Protein Purification Kit as described. The prokaryotic expression of CDPK3 and LCD were first carried out. Then 1 μg of CDPK3 and 30 μg of LCD were mixed gently on ice. The mixture was transferred to the 100 μL reaction system (20 mM Tris–HCl (pH 7.5), l0 mM MgCl2, 1 mM CaCl2 and 100 μM ATP) for 30 min reaction. Subsequently, LCD activity was measured in 100 μL reaction solution. 30 μL of LCD was used as a control (Cheng et al. 2002).
Statistical analysis
All experiments were repeated at least three times. All treatments were analyzed by one-way ANOVA, and the differences between WT and other treatments analyzed using Tukey test were statistically significant. Error bars represent standard error and each data in the figure represents the mean ± SE of three experiments, and asterisk and double asterisks indicate significant difference (P < 0.05) and very significant difference (P < 0.01) respectively. All data analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Both H2S and CDPKs enhanced the tolerance to Cd in A. thaliana
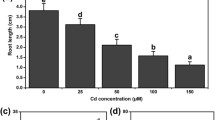
Fourteen day-old seedlings pretreated with 50 μM H2S for 6 h were transferred to 1/2 MS medium with 100 μM CdCl2 to grow for 120 h. Length of the hook was measured by using root tip bending method. Our results indicated that there was no obvious phenotypic change between H2S treatment alone and the WT control whereas root growth of plants treated by Cd only was strongly inhibited and diminished as compared to the control (P < 0.05, Fig. 1b). When the plants were pretreated with H2S and subsequently treated with Cd, the growth of the roots was almost back to normal (no significant difference to the control), suggesting that treatment with physiological concentration of H2S could enhance Cd tolerance in A. thaliana (Fig. 1).
The influences of Cd stress on Arabidopsis thaliana. (a) Phenotypes of Arabidopsis seedlings with various treatments. (b) The length of the hook in cm under different treatments. TFP: an inhibitor of CDPKs; HT: a H2S scavenger. Data are mean ± SE of three independent repeats. * indicates P < 0.05 compared to WT and ** indicates P < 0.01 compared to WT
CDPKs play a critical role in cell signal transduction. In order to explore whether CDPKs could affect Cd stress, TFP, an inhibitor of CDPKs was applied to study the Cd tolerance in Arabidopsis seedlings.
Fourteen day-old seedlings were moved to 1/2 MS medium containing 10 μM TFP and 100 μM CdCl2 to grow for 120 h. Figure 1 showed the most significant difference in root elongation growth compared to WT without Cd treatment (P < 0.01, only 22.2 ± 3.7 % of the control), which was even shorter than that treated with Cd alone. Moreover, there was no development of the lateral roots, indicating that CDPKs could enhance Cd tolerance in A. thaliana. Interestingly, when the plants were pretreated with H2S and subsequently treated with TFP and Cd, the growth of the roots was almost back to normal (77 ± 3 % of the control, no significant difference), suggesting that treatment with physiological concentration of H2S could enhance Cd tolerance when TFP present (Fig. 1).
CDPKs regulated H2S content and the activity of H2S-generating enzymes
In order to investigate the relationship between CDPKs and H2S during alleviate the toxicity of Cd, the activity of a key H2S-generating enzyme LCD and endogenous H2S content were measured after the application of TFP, a CDPKs inhibitor (Fig. 2a and b). In addition, the activity of LCD and H2S content were also analyzed in several CDPKs mutants (cdpk3, cdpk4, cdpk6 and cdpk32) (Fig. 2c and d). The results demonstrated that after TFP treatment, the activity of LCD and the endogenous H2S content showed the most significant difference compared to WT without TFP treatment (P < 0.01). Moreover, as compared to the WT control, the reduction in LCD activity and H2S content in cdpk3 mutant were even more significant (P < 0.05), indicating that CDPKs have beneficial effects on the increase in LCD activity and H2S content. Since cdpk3 mutant had more significant effect, it was used for further experiments. Figure 2e showed that the interaction of CDPK3 and LCD in vitro drastically raised LCD activity.
The measurements of endogenous H2S content and the activity of LCD. (a) The LCD activity after 6 h TFP treatment; (b) H2S content after 6 h TFP treatment; (c) The LCD activity in WT and different cdpk mutants; (d) Endogenous H2S content in WT and different cdpk mutants; (e) The effect of phosphorylation of LCD by CDPK3 on LCD activity. Each value is expressed as mean ± SE. * indicates P < 0.05 compared to WT and ** indicates P < 0.01 compared to WT
H2S and CDPKs alleviated the oxidative damages induced by Cd stress
In order to further explore the effect of H2S on Cd stress in A. thaliana, reactive oxygen species (ROS), oxidative damages and antioxidant enzymes were analyzed.
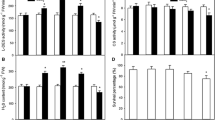
In normal conditions, ROS content was not obviously observed in WT, lcd, and cdpk3 plants, and MDA content and the activities of antioxidant enzymes do not vary significantly (Fig. 3a–e). After Cd treatment alone for 120 h, all the plants (WT, lcd and cdpk3) exhibited high ROS and MDA content as well as enhanced SOD activity; however, the activities of CAT and GR decreased sharply. As opposed to this phenomenon, all plants pretreated with H2S and then treated with Cd for 120 h showed the exact opposed results and all the parameters were almost back to the control level (Fig. 3a–e).
Analysis of reactive oxygen species (ROS), oxidative damages and antioxidant enzymes activities in WT, lcd and cdpk3 mutants under different treatments. (a) Detection of ROS using NBT method; (b) The MDA content in A. thaliana leaves; (c) Effect of Cd/H2S treatment on SOD activity. One unit of SOD activity is defined as the amount of enzymes that causes 50 % inhibition of NBT reduction; (d) Effect of Cd/H2S treatment on CAT activity. One unit of CAT is the amount of enzymes that oxidizes 1 nmol of H2O2 min−1 under the assay conditions; (e) Effect of Cd/H2S treatment on GR activity. One unit of GR is the amount of enzymes that oxidizes 1 mM of NADPH min−1 under the assay conditions. Each value is expressed as mean ± SE. * indicates P < 0.05 compared to WT
H2S up-regulated Cd stress associated genes in WT and cdpk3 plants
As is shown in Fig. S1, the expression of LCD was strongly induced by Cd stress, with a maximum accumulation at 3 h. The transcriptional expressions of the five Cd associated genes (MYB107, CAX3, POX1, MT3 and PCS1) were accumulated in WT plants under Cd stress. MYB107 responded faster than other genes. The expressions of LCD and Cd associated genes were all induced by Cd in a similar way, suggesting that H2S content was related to Cd tolerance in plants (Fig. 4a).
Expression analysis of Cd associated genes. (a) Expressions of MYB107, CAX3, POX1, MT3 and PCS1 under Cd stress at different time points. Total RNA was extracted from 14 day-old plants pretreated with 100 μM CdCl2 for 0, 1, 3, 6, 9, 12 h (b) Expressions of MYB107, CAX3, POX1, MT3 and PCS1 during H2S fumigation. 14 day-old seedlings were pretreated with 50 μM H2S for 0, 1, 3, 6, 9, 12 h respectively and RNA was extracted. (c) Expressions of MYB107, CAX3, POX1, MT3 and PCS1 in cdpk3 mutants with different treatments. Total RNA was extracted from 14 day-old plants pretreated with CdCl2 or H2S 6 h. The expression level of each gene was detected by RT-PCR, with ACTIN as an internal control
To determine the influence of H2S at the physiological concentration on Cd associated genes, the expressions of Cd associated genes in WT plants were analyzed under normal growth conditions after treatment with H2S. We observed that the maximum expressions following H2S treatment occurred at 3 h (Fig. 4b).
After 6 h treatment with 100 μM CdCl2 in cdpk3 mutant, the expression level of the five associated genes above did not change significantly. However, when the plants were pretreated with H2S for 6 h, the expression level of the five genes increased to various degrees (Fig. 4c). All these results indicated the significant effect of H2S on transcriptional level of Cd stress-related genes.
H2S and CDPKs enhanced S-sulfhydration level in A. thaliana
H2S, acting as a downstream molecule of CDPKs, plays a significant role in alleviation of Cd stress. It’s also been reported that H2S signal is related to protein S-sulfhydration. Therefore, GSSH content was measured in WT, lcd and cdpk3 mutants respectively. In Fig. 5, H2S content dramatically decreased in lcd and cdpk3 mutants and TFP treated WT plants under normal growth conditions as compared to WT. However, endogenous H2S content in WT treated with Cd showed the most significant difference compared to WT without Cd treatment (P < 0.01, increased by 95 ± 17 %), whereas there is no significant difference in lcd and cdpk3 mutants under Cd treatments (only slightly increased by 10 ± 1.2 % and 12 ± 1.5 % respectively). Similar to H2S content, the change in GSSH content exhibited a similar trend. This is to say, the contents of H2S and GSSH are correlated after Cd treatment, both contents would increase.
Protein S-sulfhydration is highly controlled by GSSH which is regulated by two enzymes, ETHE1 and STR1 (Fig. 6). Therefore, the expression level of ETHE1 and STR1 genes under Cd stress was quantified by q-RT PCR. In Fig. S2, a significant change in expression level was observed after 6 h Cd treatment. So duration of 6 h was used to treat plants. In Fig. 7, the expression level of both ETHE1 and STR1 with Cd treatment showed the significant compared with the control without Cd treatment in all three plants (P < 0.05, about 90 ± 1.5 % decrease in ETHE1 and about 110 ± 40 % increase in STR1).
Discussion
There have been few studies regarding the interaction between CDPKs and H2S in response to abiotic stresses in plants. In our study, an inhibitor of CDPKs decreased LCD activity and endogenous H2S content in Arabidopsis seedlings, which thereby led to reduced tolerance to Cd. Our results indicated that Cd treatment alone caused the increase in ROS and MDA contents; whereas H2S pretreatment alleviated the accumulation of ROS and MDA in all plants (WT, lcd and cdpk3). In Fig. 4c, we also observed that in cdpk3 mutants, Cd stress could not up regulate the expression of Cd-associated genes, which nevertheless could be up regulated by H2S treatment as compared to WT. Based on the fact that H2S could rescue the defective physiological indexes caused by cdpk3 mutants, we speculate that CDPKs prior to H2S respond to Cd stress and subsequently regulate the expressions of relevant genes and alter the activity of antioxidant enzymes, which as a result, enhances Cd tolerance in plants. Our in vitro experiments demonstrated that CDPKs could raise LCD activity. Therefore we further infer that CDPKs could boost LCD activity to generate more endogenous H2S and then enhance plants’ antioxidative capacity and at the same time alter the expressions of Cd-associated genes. Consequently, Cd tolerance in plants is elevated. The Kinase Phos website (http://kinasephos.mbc.nctu.edu.tw/) predicts that the C-terminal of LCD can be phosphorylated. We will further analyze its specific phosphorylation sites. Fang found that the expression level of CDPKs was up regulated and also the LCD activity was elevated when Setaria italica was treated with CaCl2, an exogenous donor of Ca2+ (Fang et al. 2014). In addition, Mori discovered that both CDPKs and H2S function in promotion of stomatal closure in plants (Mori et al. 2006). Stomatal closure can weaken transpiration and further inhibit the uptake and accumulation of Cd, leading to the alleviation of Cd stress (Hart et al. 1998), which is consistent with the phenotypes we observed.
H2S is mainly generated through cysteine desulfhydrases in plants (Wang 2012), and this signal can be transformed into a biological response through protein S-sulfhydration of the thiol residue of cysteines (Mustafa et al. 2009; Romeroa et al. 2013). During S-sulfhydration, reactive cysteines in the active site are sulfhydrated (−SSH) to fulfill its function. At physiological conditions, cysteine residues have a low pKa, thereby mainly existing in the form of thiolate anion (S-). When S- encounters a ROS, such as H2O2, it is easily oxidized to sulfenic, sulfinic or sulfonic species, which are subsequently attacked by GSSH to form -SSH. Therefore, the content of GSSH can to some extent reflect the level of in vivo protein S-sulfhydration (Finkel 2012).
S-sulfhydration takes place on cysteine residues at active site of target proteins. In mammals, the detection of protein S-sulfhydration has been well studied through the biotin switch method. However, this area has just become a topic of interest in plants. It has been proposed that several types of proteins have undergone S-sulfhydration, including proteins involved in electron transport and energy pathways, actins, glyceraldehyde 3 phosphate dehydrogenase, ATP synthase and so on (Aroca et al. 2015; Romeroa et al. 2013). According to Lu’s paper (Lu et al. 2013), in our study, MBB (monobrombimane method) was used to measure the content of GSSH, a reliable indicator of protein S-sulfhydration level.
Plants can respond to heavy metal toxicity in a variety of ways, which include immobilization, exclusion, phytochelatin-based sequestration and compartmentalization of the metal ions, etc. (Perfus-Barbeoch et al. 2002). During all these processes, sensing and transduction of the “metal signal” is critical since it can activate transcription factors and enable stress-associated genes to express, which consequently lead to the counteraction of heavy metal stress (DalCorso et al. 2010).
During heavy metal stresses, both ROS and H2S are generated which subsequently lead to the promotion of protein S-sulfhydration level. In Fig. 5, GSSH content was significantly increased in WT plants stressed with Cd indicative of high level of S-sulfhydration, whereas this content was much less in lcd and cdpk3 mutants than in WT. To our surprise, the expression level of two key genes STR1 and ETHE1 involved in the metabolism of GSSH did not vary significantly in both WT and mutants. Based on all these results, we speculate that the decrease in GSSH content in the mutants might mainly be due to the reduction in H2S, thereby leading to the sensitive response to Cd. This speculation further indicates the important function of H2S in response to Cd and this function is most likely to be implemented through protein S-sulfhydration modification. Interestingly it has been reported that sulfhydration can protect critical cysteine residues from being oxidized under oxidative stress, thus avoiding permanent damages and maintaining protein functions (Paul and Snyder 2012). This may be another means that H2S functions in Cd tolerance in plants. In this study, we observed that CDPKs could raise LCD activity and intensify H2S signal and we think that phosphorylation of LCD is taking place and we will study the post-translational modification of LCD in future.
CDPKs Calcium-Dependent Protein Kinases, Cd cadmium, LCD L-cystine desulfhydrase, GSSH glutathione persulfide, H 2 S hydrogen sulfide, HT hypotaurine, MDA malondialdehyde, ROS reactive oxygen species, TFP Trifluoroperazine.
References
Alvarez C, Calo L, Romero LC, Garcia I, Gotor C (2010) An O-acetylserine(thiol)lyase homolog with L-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiol 152:656–669
Aroca A, Serna A, Gotor C, Romero LC (2015) S-sulfhydration: a new post-translational modification in plant systems. Plant Physiol. doi:10.1104/pp. 15.00009
Becana M, Aparicio-Tejo P, Irigoyen JJ, Sanchez-Diaz M (1986) Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of medicago sativa. Plant Physiol 82:1169–1171
Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605
Chen J, Wu FH, Wang WH, Zheng CJ, Lin GH, Dong XJ, He JX, Pei ZM, Zheng HL (2011) Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J Exp Bot 62:4481–4493
Cheng SH, Willmann MR, Chen HC, Sheen J (2002) Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol 129:469–485
DalCorso G, Farinati S, Furini A (2010) Regulatory networks of cadmium stress in plants. Plant Signal Behav 5:663–667
Dawood M, Cao F, Jahangir MM, Zhang G, Wu F (2012) Alleviation of aluminum toxicity by hydrogen sulfide is related to elevated ATPase, and suppressed aluminum uptake and oxidative stress in barley. J Hazard Mater 209–210:121–128
Dooley FD, Nair SP, Ward PD (2013) Increased growth and germination success in plants following hydrogen sulfide administration. PLoS One 8, e62048
Fang H, Jing T, Liu Z, Zhang L, Jin Z, Pei Y (2014) Hydrogen sulfide interacts with calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium 56:472–481
Finkel T (2012) From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci Signal 5:pe10
Garcia-Mata C, Lamattina L (2013) Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci 201–202:66–73
Halliwell B, Chirico S (1993) Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr 57:715S–724S, discussion 724S-725S
Hart JJ, Welch RM, Norvell WA, Sullivan LA, Kochian LV (1998) Characterization of cadmium binding, uptake, and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiol 116:1413–1420
Jin Z, Shen J, Qiao Z, Yang G, Wang R, Pei Y (2011) Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem Biophys Res Commun 414:481–486
Jin Z, Xue S, Luo Y, Tian B, Fang H, Li H, Pei Y (2013) Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol Biochem 62:41–46
Krishnan N, Fu C, Pappin DJ, Tonks NK (2011) H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal 4:ra86
Krussel L, Junemann J, Wirtz M, Birke H, Thornton JD, Browning LW, Poschet G, Hell R, Balk J, Braun HP, Hildebrandt TM (2014) The mitochondrial sulfur dioxygenase ETHYLMALONIC ENCEPHALOPATHY PROTEIN1 is required for amino acid catabolism during carbohydrate starvation and embryo development in Arabidopsis. Plant Physiol 165:92–104
Li L, Rose P, Moore PK (2011) Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 51:169–187
Li L, Wang Y, Shen W (2012a) Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. Biometals 25:617–631
Li ZG, Gong M, Xie H, Yang L, Li J (2012b) Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L) suspension cultured cells and involvement of Ca(2+) and calmodulin. Plant Sci 185–186:185–189
Li ZG, Ding XJ, Du PF (2013a) Hydrogen sulfide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J Plant Physiol 170:741–747
Li ZG, Yang SZ, Long WB, Yang GX, Shen ZZ (2013b) Hydrogen sulphide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ 36:1564–1572
Lisjak M, Teklic T, Wilson ID, Whiteman M, Hancock JT (2013) Hydrogen sulfide: environmental factor or signalling molecule? Plant Cell Environ 36:1607–1616
Lu C, Kavalier A, Lukyanov E, Gross SS (2013) S-sulfhydration/desulfhydration and S-nitrosylation/denitrosylation: a common paradigm for gasotransmitter signaling by H2S and NO. Methods 62:177–181
Mori IC, Murata Y, Yang Y, Munemasa S, Wang YF, Andreoli S, Tiriac H, Alonso JM, Harper JF, Ecker JR, Kwak JM, Schroeder JI (2006) CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca(2+)-permeable channels and stomatal closure. PLoS Biol 4, e327
Munaron L, Avanzato D, Moccia F, Mancardi D (2013) Hydrogen sulfide as a regulator of calcium channels. Cell Calcium 53:77–84
Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH (2009) H2S signals through protein S-sulfhydration. Sci Signal 2:ra72
Papenbrock J, Riemenschneider A, Kamp A, Schulz-Vogt HN, Schmidt A (2007) Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants - from the field to the test tube and back. Plant Biol (Stuttg) 9:582–588
Paul BD, Snyder SH (2012) H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13:499–507
Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C (2002) Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J 32:539–548
Qiao Z, Jing T, Liu Z, Zhang L, Jin Z, Liu D, Pei Y (2015) H2S acting as a downstream signaling molecule of SA regulates Cd tolerance in Arabidopsis. Plant Soil 393:137–146
Riemenschneider A, Wegele R, Schmidt A, Papenbrock J (2005) Isolation and characterization of a D-cysteine desulfhydrase protein from Arabidopsis thaliana. Febs J 272:1291–1304
Romeroa LC, Ángeles Arocaa M, Sernab A, Gotora C (2013) Proteomic analysis of endogenous S-sulfhydration in Arabidopsis thaliana. Nitric Oxide 31:S23
Sambrook J, Manistis T (1987) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schaedle M (1977) Chloroplast glutathione reductase. Plant Physiol 59:1011–1012
Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH (2012) Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol Cell 45:13–24
Shi H, Ye T, Chan Z (2013) Exogenous application of hydrogen sulfide donor sodium hydrosulfide enhanced multiple abiotic stress tolerance in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol Biochem 71:226–234
Shi H, Ye T, Chan Z (2014a) Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol Biochem 74C:99–107
Shi H, Ye T, Han N, Bian H, Liu X, Chan Z (2014b) Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J Integr Plant Biol 57:628–640
Tuteja N, Mahajan S (2007) Calcium signaling network in plants: an overview. Plant Signal Behav 2:79–85
Vandiver MS, Paul BD, Xu R, Karuppagounder S, Rao F, Snowman AM, Ko HS, Lee YI, Dawson VL, Dawson TM, Sen N, Snyder SH (2013) Sulfhydration mediates neuroprotective actions of parkin. Nat Commun 4:1626
Wang R (2012) Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92:791–896
Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, Wang R (2013) Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 18:1906–1919
Zhang H, Hu LY, Hu KD, He YD, Wang SH, Luo JP (2008) Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol 50:1518–1529
Zhang H, Tang J, Liu XP, Wang Y, Yu W, Peng WY, Fang F, Ma DF, Wei ZJ, Hu LY (2009a) Hydrogen sulfide promotes root organogenesis in Ipomoea batatas, Salix matsudana and Glycine max. J Integr Plant Biol 51:1086–1094
Zhang H, Ye Y, Wang S, Luo J, Tang J, Ma D (2009b) Hydrogen sulfide counteracts chlorophyll loss in sweetpotato seedling leaves and alleviates oxidative damage against osmotic stress. Plant Growth Regul 58:243–250
Zhang H, Tan ZQ, Hu LY, Wang SH, Luo JP, Jones RL (2010) Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. J Integr Plant Biol 52:556–567
Zhu SY, Yu XC, Wang XJ, Zhao R, Li Y, Fan RC, Shang Y, Du SY, Wang XF, Wu FQ, Xu YH, Zhang XY, Zhang DP (2007) Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 19:3019–3036
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31400237 to Zhuping Jin; 31372085 to Yanxi Pei and 31300236 to Zhiqiang Liu).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barcelo.
Zengjie Qiao and Tao Jing contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Expressions of LCD and CDPK3 induced by Cd. Total RNA was extracted from 14 day-old plants treated with CdCl2 for 0, 1, 3, 6, 12 and 24 h. The expression level of each gene was detected by RT-PCR, with ACTIN as an internal control. (JPEG 10 kb)
Fig. S2
The expressions of ETHEI and STR1. The mRNAs were isolated from 14-day-old seedlings at 0, 1, 3, 6, 12, and 24 h of 100 μM Cd treatment. The expression level of each gene was detected by qRT-PCR. (JPEG 24 kb)
Rights and permissions
About this article
Cite this article
Qiao, Z., Jing, T., Jin, Z. et al. CDPKs enhance Cd tolerance through intensifying H2S signal in Arabidopsis thaliana . Plant Soil 398, 99–110 (2016). https://doi.org/10.1007/s11104-015-2643-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2643-x