Abstract
Preexisting diabetes increases risk of fractures after kidney transplantation (KT). However, little is known about mechanisms and prevention of increased fragility in these patients. Pathophysiology of osteoporosis after KT is complex and characterized by high prevalence of adynamic bone disease. Despite high prevalence of preexisting diabetes in KT recipients, diabetes patients were underrepresented in the studies that explored mechanisms and treatments of osteoporosis after KT. Therefore, caution should be exercised before considering conventional fracture prevention strategies in this unique group of patients. Many traditional osteoporosis medications reduce bone turnover and, hence, can be ineffective or even harmful in diabetic patients after KT. Contrary to predictions, evidence from the studies conducted in mostly non-diabetic subjects demonstrated that bisphosphonates failed to reduce fracture rates after KT. Therefore, bisphosphonates use should be limited in diabetic patients until more evidence supporting their post-transplant efficacy is available. We recommend the following strategies that may help reduce fracture risk in diabetes subjects after KT such as adequate management of calcium, parathyroid hormone, and vitamin D levels, optimization of glycemic control, use of steroid-sparing immunosuppressive regimens, and fall prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Kidney transplantation (KT) is the most effective treatment of end-stage renal disease (ESRD). In 2013, 17,600 kidney transplants were performed in the United States with the largest growth in deceased donor transplantation numbers that occurred among recipients with diabetes or hypertension [1]. Up to 30% of kidney transplant recipients (KTRs) have diabetes listed as one of the diagnosis [2]. With excellent rates of patient and graft survival measures, 193,262 KTRs had a functioning graft in 2012 in the United States [1]. Osteoporosis and fracture complicate the course of the post-transplant period. Prevalence of fractures in KTRs is 4–5-fold higher than in general population and, importantly, these fractures are associated with an increased hospital admission rate and all-cause mortality [3, 4]. While diabetes is highly prevalent in KTRs, little is known if fracture pathophysiology, prevention, and treatment strategies in diabetic patients are different from non-diabetic KTRs. In this review, we will discuss pathobiology of bone disease in patients with diabetes, ESRD and after kidney transplantation. Based on the available literature data, we will further attempt to formulate the approach for the evaluation and management of osteoporosis in KTRs with diabetes.
1.1 Diabetes as a risk factor for fractures
In patients with diabetes, fracture rates are increased compared with the general population [5]. Absolute fracture risk is much higher in type 1 than in type 2 diabetes patients when compared with general population [6]. However, given the high prevalence of type 2 diabetes in adults, fractures in these patients will have the highest clinical and socio-economic impact. The prevalence of osteoporotic fractures in type 2 diabetes varies depending on the study design. Analysis of medical claims of commercially insured type 2 diabetes patients in the US demonstrated non-vertebral fracture rate of approximately 4 per 1000 person-years [7]. In contrast, in prospective interventional trials the fracture rates were significantly higher at 14–15 per 1000 person-years in non-thiazolidinedione groups and up to 27 per 1000 person-years in the rosiglitazone-treated group in the ADOPT study [8, 9]. Overall, diabetes adds anywhere from 20 to 70% to the risk of hip and non-vertebral fractures, as compared with non-diabetic individuals [10, 11]. Of interest, diabetes may have significant impact on non-vertebral fracture risk in elderly non-Hispanic blacks and Mexican Americans [12].
Paradoxically, bone mineral density (BMD) is higher in diabetic patients, as compared to non-diabetics, suggesting reduced benefits of dual-energy x-ray absorptiometry (DXA) screening in predicting the fracture risk in diabetics [5]. Our understanding of bone histomorphometry changes in diabetes is limited due to low number of bone biopsy studies. It is thought that decreased bone cortical porosity, compromised vascular supply to cortical areas, inhibition of osteoblast functions, and dysregulation in collagen matrix attachment are present in diabetic bone [5, 10]. Assessment of bone turnover markers (BTMs) typically demonstrates decreased levels of markers of bone formation and resorption [13] consistent with early observations in bone biopsy studies that showed low bone turnover state in diabetes patients [14]. Therefore, the alterations in bone microarchitecture coupled with the low bone turnover state and accumulation of advanced glycation end products (AGEs) in collagen may reduce quality of diabetic bone and predispose to fracture.
Several clinical factors can also contribute to the increased fracture risk in diabetic patients. Anti-diabetic medications rosiglitazone and pioglitazone increase risk of mostly non-vertebral fractures [8, 15] likely via inhibition of osteoblast function and activation of osteoclastogenesis [16]. Other non-insulin hypoglycemic agents are mostly neutral in regards to fracture risk in diabetes except increased fracture incidence associated with medications that belong to classes of sulphonylurea and sodium-glucose co-transporter 2 (SGLT2)-inhibitors [16]. Patients with diabetes are also prone to falls that may predispose them to fragility fractures due to neuropathy, impaired vision, and/or use of medications that predispose to hypoglycemia such as sulphonylureas and insulin [5]. Uncontrolled hyperglycemia is another risk factor for fractures. It has been consistently demonstrated that A1c reduction to levels below 8.0% is associated with the lowest incidence of fractures [11]. It is likely that chronic elevation of blood glucose results in accumulation of AGEs in bone collagen which reduces bone quality [17]. Furthermore, in studies in type 1 diabetic mice, it was demonstrated that chronic hyperglycemia can contribute to bone loss via suppression of osteoblast function and increase in bone marrow adiposity [18]. Hypogonadism in women and men should be also considered as a factor reducing bone density and quality. Prevalence of hypogonadotropic hypogonadism can approach 50% in type 2 diabetes men [19]. Testosterone deficiency can exert a multitude of clinical manifestations that range from the direct effects on bone to associations with cardiovascular disease regardless of diabetes status [20, 21]. Therefore, careful assessment of clinical symptoms that can arise from sex hormone deficiency should be conducted in diabetic patients [21]. Finally, diabetes-related vascular complications are associated with higher fracture risk [11]. Diabetic kidney disease was shown to be an independent risk factor of fractures in type 1 diabetes in one large case-control study [22].
In summary, diabetes is a strong and independent risk factor for fractures due to diabetes-related bone changes, associated co-morbidities, and treatment-related complications. Osteoporosis in diabetes is characterized by low bone turnover and complex interaction of modifiable and non-modifiable clinical and pathophysiologic processes that predispose to fragility in spite of higher BMD values. The role of DXA in estimating initial fracture risk in diabetes patients remains to be studied.
1.2 Bone abnormalities and fracture risk in ESRD
Chronic kidney disease (CKD) is found in up to 45% of diabetic individuals [23]. As renal function declines, a unique combination of electrolyte and hormonal changes almost invariably develop. These abnormalities are called CKD-associated mineral and bone disorder (CKD-MBD) and include a reduction in serum calcium and active vitamin D (1,25(OH)2D3) levels and an increase in serum phosphorus, parathyroid hormone (PTH), and fibroblast growth factor 23 (FGF-23). Biochemical abnormalities in CKD-MBD start when glomerular filtration rate (GFR) is still fairly preserved. Hormonal changes such as reduction in 1,25(OH)2D3 and elevation of FGF-23 and PTH usually precede alterations in calcium and phosphorus and observed as early as estimated GFR (eGFR) below 80 ml/min/1.73m2. In CKD stages 4–5, elevated PTH and low 1,25(OH)2D3 were observed in 70% and 60% of patients, respectively [24]. Initial reduction in serum calcium and elevation in phosphorus are typically occur later in the course of CKD at eGFR below 40 ml/min/1.73m2. Calcium and phosphorus remain fairly stable until eGFR 20 ml/min/1.73m2 but after that they show additional progressive alterations.
Bone changes related to CKD-MBD are called renal osteodystrophy (ROD). ROD is not a single disease and is comprised of a heterogeneous group of disorders affecting both bone quantity and quality. Bone biopsy remains a gold standard in diagnosing ROD and its classification is based on measures of bone turnover (i.e., bone formation and resorption rates), mineralization of extracellular matrix, and trabecular bone volume [25]. High-bone turnover disease is a form of ROD that occurs due to excessive PTH-mediated bones changes resulting in concomitant augmentation of bone formation and resorption and accompanied by the formation of peritrabecular fibrosis, woven osteoid, and increased cortical porosity. It is usually associated with mild to moderate degree of secondary hyperparathyroidism (SHPT). A more severe form of bone disease that is associated with marked SHPT is called osteitis fibrosa cystica. Other forms of ROD include development of osteomalacia that is characterized by accumulation unmineralized osteoid and prolonged mineralization lag time, adynamic bone disease (ABD) that is characterized by severely diminished bone formation and resorption, and mixed uremic osteodystrophy that is characterized by combinations of abnormal bone turnover and mineralization. The prevalence of different ROD types varies depending on CKD stage and modality of renal replacement therapy (RRT) is presented in Table 1 [26].
Over last 2 decades, there has been an increase in the prevalence of ABD, both as a single ROD lesion, as well as a predominant finding in mixed lesions [27]. Diabetic patients with ESRD have especially high rates of ABD with reported prevalence of 67% [28]. Overall aging population, increase in AGEs, over suppression of circulating PTH levels via calcium-containing phosphate binders and high calcium dialysate, skeletal resistance to PTH action, hypogonadism, metabolic acidosis, malnutrition, and uremia-induced oxidative stress all contribute to the high prevalence of ABD in CKD patients with diabetes [29, 30].
The Kidney Disease Improving Global Outcomes (KDIGO) guidelines do not recommend evaluation of bone density in patients with CKD on dialysis [31] because BMD in this population predicts poorly the risk of bone fractures or type of ROD. Bone density measured by BMD cannot characterize bone volume at the same extend as bone biopsy. In high-bone turnover disease, mineralization of newly formed bone is decreased; while, there is reduction in the volume in low-bone turnover disease. Therefore, both high- and low-bone turnover states can result in lower than expected BMD. A recent prospective study involving 213 dialysis patients reported low bone density in osteoporotic range in 39% of patients measured by either DXA or quantitative computed tomography (QCT). Although, no bone biopsies were performed or fracture risk assessment was reported, low BMD was strongly associated with increased coronary artery calcifications [32]. Therefore, although the utility of BMD measurement to predict fracture risk is uncertain in dialysis patients, BMD values in osteoporosis range may suggest a need for comprehensive cardiovascular disease risk assessment in ESRD patients.
Biochemical testing has limited role in diagnosing variants of ROD. PTH is the most widely used surrogate marker of bone turnover and high PTH levels are associated with high-bone turnover disease [33, 34]. Nevertheless, elevated PTH levels may be also seen in ABD, especially in African-Americans [35, 36]. In contrast, low to normal intact PTH levels (< 50–100 pg/ml) in ESRD patients were consistently shown to be associated with biopsy proven ABD. Bone-specific alkaline phosphatase (BSAP) may be useful in determining bone turnover state in CKD patients [37]. Low and markedly high levels of BSAP predict bone turnover in ESRD patients treated with hemodialysis [33].
1.2.1 Fracture risk in ESRD
Abnormalities in bone quality and quantity coupled with general frailty of ESRD patients increase risk of fractures. Depending on the studied dialysis population, the reported fracture rates are 4.4–17.6 - fold higher among ESRD patients, as compared with aged-matched individuals from the general population [38, 39]. The incidence of hip fracture and any site fracture were 8.9 and 25.6 per 1000 patient-years, respectively, in the large international phase 2 Dialysis Outcomes and Practice Patterns Study (DOPPS) [40]. The incidence of fractures is particularly high in young female dialysis patients (~100-fold higher risk in women aged <40–50 years) [39]. Diabetes does not appear to augments risk of fracture in ESRD patients. For example, the DOPPS study that involved 12,782 dialysis patients from 12 countries found no increased fracture risk in diabetic versus non-diabetic dialysis patients [40]. The risk of vertebral and hip fractures in dialysis patients with suspected ABD showed association of incident vertebral and hip fractures with low to normal PTH levels regardless of diabetes diagnosis [39–41]. On the other hand, high PTH levels can lead to a higher fracture risk in ESRD. The fracture effects associated with PTH reduction following parathyroidectomy or initiation of calcimimetic drug cinacalcet were recently studied in dialysis patients [42, 43]. In the parathyroidectomy study, dialysis patients with diabetes had about 30% fracture reduction rate that was similar to non-diabetic ESRD subjects [43]. In contrast, when cinacalcet was given to ESRD patients, diabetic patients had unexpected 46% increase in fracture rate [42] suggesting that pharmacological reduction of elevated PTH in dialysis patients with diabetes can be detrimental.
In summary, dialysis patients have markedly elevated risk of fracture compared with age- and gender-matched patients with normal renal function. The risk of adynamic bone disease is high in ESRD patients and clinical strategies for diagnosis of osteoporosis in these patients are not well studied. Diabetes status does not appear to modulate fracture risk in dialysis patients. Unfortunately, no standardized clinical, radiographic and biochemical approaches exist to predict if diabetes patients could benefit from certain fracture preventive strategies in ESRD. Very low PTH levels were shown to be associated with increased fracture risk in diabetic ESRD patients, therefore, PTH over suppression should be avoided. Early evidence indicates that parathyroidectomy in patients with high PTH levels can diminish fracture risk in ESRD including diabetic patients.
1.3 Fracture risk and osteoporosis after kidney transplantation: focus on diabetes
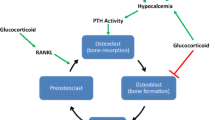
Osteoporosis is defined as a skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. In routine clinical practice, bone strength can only be estimated indirectly by the assessment of bone mineral density (BMD) by DXA. It is accepted that either low BMD measurement or history of low trauma fracture can independently be used to establish the diagnosis of osteoporosis. KTRs have multiple risk factors that can compromise bone strength arising from pre-transplant and post-transplant metabolic milieu (Fig. 1). Low BMD in the osteoporotic range is observed in up to 50% of KTRs [44], but the rates may vary depending on the frequency of steroids use for immunosuppression [44–47]. However, several studies suggested that the BMD itself cannot explain high risk of fractures in KTRs and improvements in the BMD may not result in the fracture risk reduction following KT [48, 49] suggesting that DXA use during early post-transplant period can be of limited value.
Overall, fracture rates in KTRs exceed that of ESRD patients [50]. The prevalence of fractures after the transplantation is almost 50% [44, 51, 52]. Fracture risk is highest during the first year after KT and is likely associated with the marked steroid exposure during that period; while in 2 years after transplantation the fracture incidence in KTRs becomes equal to dialysis patients [50]. It is important to emphasize, that in addition to the multiple other known osteoporosis risk factors (Fig. 1), diabetes in post-transplant patients was found to be independently associated with the higher risk of both low BMD and fracture [45, 53, 54]. Early studies in type 1 diabetes patients showed that almost 50% of the patients would sustain a fracture after combined kidney-pancreas transplantation [52, 55]. Pre-transplant type 2 diabetes is associated with 36–40% increase in risk of fracture after transplantation [51, 53]. However, molecular mechanisms responsible for increased bone fragility in diabetic patients after KT are poorly understood.
The clinical sequel of fractures in KTRs is not trivial. The most common fractures in KTRs are appendicular in nature and occur in ankle, foot, and hip [51, 54], which may diminish patient quality of life and mobility after fracture. The hip fracture in KTRs was shown to be associated with 1.6-fold increase in all-cause mortality according to the USRDS data [3] and 3-fold increase in all-cause mortality in the analysis from the United Kingdom [4]. Out of many contributors implicated in the rapid BMD reduction, glucocorticosteroids are the most important factor that affects bone health after KT [44, 56]. The pathobiology of the bone disease after transplantation is characterized by reduced osteoblast proliferation, accelerated osteoclastogenesis, and impaired bone mineralization process that can result in deterioration of trabecular and cortical thickness and density. In addition to the direct effects on the bone cells and matrix, glucocorticosteroids reduce growth hormone (GH) and insulin-like growth factor 1 production which, in turn, can inhibit collagen synthesis and decrease proliferation of chondrocytes thereby impairing bone formation [57]. This aspect of detrimental skeletal actions of steroids can be of particular importance in young post-transplant patients in whom GH actions are critical for optimal bone growth.
The rate of bone turnover can be high, low or mixed with the rate of ABD approaching 50% in the KTRs [44, 58]. In patients with high PTH following the transplantation, the risk of low BMD and fracture was directly associated with PTH concentration and bone turnover rate [59]. Within the first several months after KT, up to 40–50% of the patients may have persistent hyperparathyroidism and after the first year up to 20–30% of the patients will have persistent hypercalcemia and/or elevated PTH levels [60, 61]. Spontaneous resolution of hypercalcemia due to hyperparathyroidism after 1 year is uncommon [62]. Interestingly, in one prospective study, persistent elevation of PTH level over 5 years after KT was an independent risk factor for fractures [63]. It was shown, that PTH level > 130 ng/L at 3 months after KT was associated with 7.5-fold increase in subsequent fracture risk during 5 year follow up.
1.4 Prevention and treatment of osteoporosis in diabetes patients after kidney transplantation
Increased fracture risk after KT is multifactorial due to rapid and significant reduction in bone mass and bone quality in the setting of glucocorticosteroid exposure, particularly during the first year after KT, high prevalence of of both low and high PTH-associated bone abnormalities. Bisphosphonates is the only anti-resorptive drug class that was systematically evaluated in randomized controlled trials for the prevention and treatment of osteoporosis in patients after KT [44] based on the medications efficacy in fracture risk reduction, BMD accrual and improvements in bone mechanics in post-menopausal women [64–66]. Indeed, bisphosphonates prevent post-transplant BMD loss or can even improve BMD in lumbar spine and femoral neck when administered for at least 12 months after KT, mainly in non-diabetic KTR [67, 68]. However, surprisingly, intravenous (pamidronate, ibadronate) or oral (alendronate, risedronate) bisphosphonates failed to reduce the fracture risk during first 12 months after KT; of note, these studies were not powered to detect treatment effects on fracture incidence [49, 69–76].
In prospective interventional trials that assessed BMD changes after KT, diabetes patients were consistently underrepresented in spite of the high prevalence of pre-existing diabetes in the KTRs. We were able to identify only 4 studies that clearly identified diabetes as one of the causes of ESRD and that had >10% of diabetes patients in intervention groups (Table 2). Compared with routine care in the KTRs, the bisphosphonates in these trials led to either BMD accrual or prevented bone loss during first 12 months after KT. However, there were no differences in fracture rates between the usual care and the active intervention groups. Importantly, in a retrospective analysis of transplant clinic records, bisphosphonates were associated with a higher fracture risk [49].
At present, the evidence does not support the routine use of antiresorptive agents in KTRs because BMD improvement following bisphosphonates initiation does not necessarily translate into reduced fracture risk. There could be several reasons as to why bisphosphonates were ineffective in reducing bone fractures in the KTRs. As many as 50% of patients after KT have adynamic bone disease and inhibition of bone resorption with bisphosphonates may further worsen bone quality [44, 70]. Importantly, as was demonstrated before, diabetes patients with normal renal function are already characterized by low bone turnover [14]. The limited evidence also suggests that bisphosphonates can promote ABD in the KTRs. Coco et al. performed bone biopsies at the time of KT and after 6 months in patients treated with pamidronate and the control group [70]. It was found that 100% of the pamidronate-treated individuals developed ABD after 6 months compared with only about 40% patients in the control group.
Another important clinical consideration arguing against the prophylactic use of bisphosphonates after KT is that in patients with osteoporosis and normal renal function, the use of bisphosphonates is associated mainly with a reduction in vertebral fractures and bisphosphonates are much less effective against non-vertebral fractures. As was mentioned above, the majority of fractures after KT are non-vertebral in nature and occurred in foot, ankle, hand, humerus, and hip, as was shown in the studies from single center [77], systematic review of literature data [53], or using the U.S. Renal Data System data [51].
What could be offered to the KTRs including those with diabetes to prevent fragility during early post-transplant period? Pending the results of future studies aiming to assess bone histomorphometry in diabetes patients after KT, hyperglycemia should remain an important modifiable risk factor influencing bone health in the KTRs. Given complex pathophysiology of bone and mineral metabolism and high prevalence of elevated calcium, low phosphorus, and/or high PTH during post-transplant period, pre-transplant assessment and, if necessary, correction of disorders of calcium-PTH-vitamin D axis are imperative. Dynamic evaluation of calcium and PTH levels after KT will help to identify those patients at risk for fracture due to hyperparathyroidism [61, 63, 78]. Trends in markers of bone turnover over time in patients with unexplained bone loss can offer preliminary estimation of balance between bone formation and resorption, though there is no systematic evidence to support prognostic value of bone turnover markers after KT. The KDIGO recommends obtaining baseline BMD during the first year after KT in individuals with eGFR ≥ 30 ml/min/1.73m2 [31]. The biochemical evaluations coupled with the baseline BMD, and, if indicated, periodic follow up in BMD changes can help to identify a cohort of the patients with progressive bone loss who, in spite of routine management of calcium and vitamin D metabolism, could have increased fracture risk. The International Society for Clinical Densitometry recommends screening for vertebral fractures for those patients who will receive glucocorticoid therapy equivalent to ≥5 mg of prednisone daily for ≥3 months [79]. The DXA images of the thoracolumbar spine that are called vertebral fracture assessment (VFA) or plain X-ray before the KT will help to establish the baseline spine imaging. In KTRs with new onset back pain, one of these imaging approaches should be considered to rule out lumbar spine fractures.
As discussed above, fracture-prevention strategies should not include prophylactic use of bisphosphonates in any patient after KT and particularly in patients with diabetes given concerns of the high prevalence of ABD. On the other hand, combined use of low-dose calcitriol at 0.25 mcg/day dose and physiological calcium supplementation of 500–1000 mg/day daily appears to be safe and may preserve BMD during first year after KT [48, 73, 74, 80]. In our opinion, calcitriol can specifically benefit those patients who have persistent secondary hyperparathyroidism. Tertiary hyperparathyroidism after KT requires evaluation for subtotal/near-total parathyroidectomy as this treatment approach can improve post-transplant metabolic milieu and bone health and potentially reduce fragility [60, 81]. Additionally, routine screening for vitamin D deficiency and correction of hypovitaminosis D should be implemented [31]. It is important to note that the latter recommendation is not based on the evidence from randomized trials. However, nutritional vitamin D deficiency is extremely common with reported prevalence of vitamin D levels <30 ng/ml in over 95% of the KTRs [82]. Given a negative impact of low vitamin D on bone health in general population, it is will be reasonable to restore vitamin D levels in the normal range in the KTRs.
There are no prospective trials that addressed the question if an intensive hyperglycemia management program after KT would provide any skeletal benefits to the patients with known pre-transplant diabetes. There is, however, a rationale to provide adequate glycemic control in the KTRs. It was shown before that fracture risk increased in patients with uncontrolled diabetes having A1c 8.0% and above [11]. Anti-diabetes medication use should exclude regimens containing thiazolidinediones such as pioglitazone or rosiglitazone and aim to avoid hypoglycemia. In both women and men with pre-transplant gonadal dysfunction, KT can improve hypothalamic-pituitary-gonadal processes resulting in clinical or biochemical improvements of sex hormone functions [83]. In patients with persistent or newly diagnosed hypogonadism, if identified after KT, sex hormone deficiency should be therapeutically addressed if there are no contraindications to hormone replacement therapy. The fall prevention in the KTRs is very important as patients following KT are at high risk of non-vertebral fractures. Reduction of mobility, if limb fractured, can significantly affect patients` function and potentially lead to weight gain and further worsening of glycemic control. Glucocorticosteroid-sparing regimens can provide additional benefits for the bone health, though the prescription of steroid-free regimens should always be weighed carefully against the risk of graft rejection. Repeat DXA measurements 1 year after KT can identify the individuals with unexpected progressive bone loss in spite of adequate management of mineral metabolism who may then benefit from more comprehensive work up of other causes BMD loss.
2 Conclusion
Diabetes is independent risk factor for fractures after kidney transplantation. There are no descriptive, observational or interventional studies that focused specifically on pathophysiology, diagnosis and treatment of increased fragility in diabetes patients after KT. Based on available evidence, Fig. 2 proposes diagnostic and therapeutic considerations in the evaluation for and management of osteoporosis after KT. In the absence of definitive data, approaches for fracture prevention in these patients should involve development of strategies focusing on the optimization of glycemic control, correction of calcium, phosphorus, and PTH metabolism, vitamin D replacement, use of active vitamin D, and fall prevention. Bisphosphonate use for osteoporosis prevention in KTRs with diabetes should be avoided.
References
Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, et al. US renal data system 2015 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016;67(3 Suppl 1):S1–434. doi:10.1053/j.ajkd.2015.12.014.
OPTN/SRTR Annual Report. 2004 annual report of the US organ procurement and transplantation network and the scientific registry of transplant recipients: Transplant data 1994-2003 Department of Health and Human Services, Health Resources and Services Administration, healthcare systems bureau, division of transplantation, Rockville, MD; united network for organ sharing, Richmond, VA; university renal research and education association, Ann Arbor. MI. 1999-2008;2009
Abbott KC, Oglesby RJ, Hypolite IO, Kirk AD, Ko CW, Welch PG, et al. Hospitalizations for fractures after renal transplantation in the United States. Ann Epidemiol. 2001;11(7):450–7.
Ferro CJ, Arnold J, Bagnall D, Ray D, Sharif A. Fracture risk and mortality post-kidney transplantation. Clin Transpl. 2015;29(11):1004–12. doi:10.1111/ctr.12621.
Epstein S, Defeudis G, Manfrini S, Napoli N, Pozzilli P. Scientific Committee of the First International Symposium on D et al. diabetes and disordered bone metabolism (diabetic osteodystrophy): Time for recognition. Osteoporos Int. 2016;27(6):1931–51. doi:10.1007/s00198-015-3454-x.
Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18(4):427–44. doi:10.1007/s00198-006-0253-4.
Majumdar SR, Josse RG, Lin M, Eurich DT. Does Sitagliptin affect the rate of osteoporotic fractures in type 2 diabetes? Population-based cohort study. J Clin Endocrinol Metab. 2016;101(5):1963–9. doi:10.1210/jc.2015-4180.
Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, et al. Rosiglitazone-associated fractures in type 2 diabetes: An analysis from a diabetes outcome progression trial (ADOPT). Diabetes Care. 2008;31(5):845–51. doi:10.2337/dc07-2270.
Mosenzon O, Wei C, Davidson J, Scirica BM, Yanuv I, Rozenberg A, et al. Incidence of fractures in patients with type 2 diabetes in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38(11):2142–50. doi:10.2337/dc15-1068.
Leslie WD, Rubin MR, Schwartz AV, Kanis JA. Type 2 diabetes and bone. J Bone Miner Res. 2012;27(11):2231–7. doi:10.1002/jbmr.1759.
Schwartz AV. Epidemiology of fractures in type 2 diabetes. Bone. 2016;82:2–8. doi:10.1016/j.bone.2015.05.032.
Looker AC, Eberhardt MS, Saydah SH. Diabetes and fracture risk in older U.S. adults. Bone. 2016;82:9–15. doi:10.1016/j.bone.2014.12.008.
Starup-Linde J, Vestergaard P. Biochemical bone turnover markers in diabetes mellitus - a systematic review. Bone. 2016;82:69–78. doi:10.1016/j.bone.2015.02.019.
Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes. 1995;44(7):775–82.
Habib ZA, Havstad SL, Wells K, Divine G, Pladevall M, Williams LK. Thiazolidinedione use and the longitudinal risk of fractures in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95(2):592–600. doi:10.1210/jc.2009-1385.
Meier C, Schwartz AV, Egger A, Lecka-Czernik B. Effects of diabetes drugs on the skeleton. Bone. 2016;82:93–100. doi:10.1016/j.bone.2015.04.026.
Panza JA, Quyyumi AA, Brush Jr JE, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323(1):22–7.
Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. 2005;146(8):3622–31. doi:10.1210/en.2004-1677.
Dandona P, Dhindsa S. Update: Hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2011;96(9):2643–51. doi:10.1210/jc.2010-2724.
Ullah MI, Washington T, Kazi M, Tamanna S, Koch CA. Testosterone deficiency as a risk factor for cardiovascular disease. Hormone and metabolic research. 2011;43(3):153–64. doi:10.1055/s-0030-1270521.
Morgentaler A, Zitzmann M, Traish AM, Fox AW, Jones TH, Maggi M, et al. Fundamental concepts regarding testosterone deficiency and treatment: International expert consensus resolutions. Mayo Clin Proc. 2016;91(7):881–96. doi:10.1016/j.mayocp.2016.04.007.
Vestergaard P, Rejnmark L, Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif Tissue Int. 2009;84(1):45–55. doi:10.1007/s00223-008-9195-5.
Bailey RA, Wang Y, Zhu V, Rupnow MF. Chronic kidney disease in US adults with type 2 diabetes: An updated national estimate of prevalence based on kidney disease: Improving global outcomes (KDIGO) staging. BMC Res Notes. 2014;7:415. doi:10.1186/1756-0500-7-415.
Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–8. doi:10.1038/sj.ki.5002009.
Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from kidney disease: Improving global outcomes (KDIGO). Kidney Int. 2006;69(11):1945–53. doi:10.1038/sj.ki.5000414.
Chapter 3.1: Diagnosis of CKD-MBD: Biochemical abnormalities. Kidney Int. 2009;76113:S22–49. doi:10.1038/ki.2009.191.
Drueke TB, Massy ZA. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016;89(2):289–302. doi:10.1016/j.kint.2015.12.004.
Spasovski GB, Bervoets AR, Behets GJ, Ivanovski N, Sikole A, Dams G, et al. Spectrum of renal bone disease in end-stage renal failure patients not yet on dialysis. Nephrol Dial Transplant. 2003;18(6):1159–66.
Andress DL. Adynamic bone in patients with chronic kidney disease. Kidney Int. 2008;73(12):1345–54. doi:10.1038/ki.2008.60.
Brandenburg VM, Floege J. Adynamic bone disease-bone and beyond. NDT Plus. 2008;1(3):135–47. doi:10.1093/ndtplus/sfn040.
Kidney Disease. Improving Global Outcomes CKDMBDWG. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney international. 2009;113:S1–130. doi:10.1038/ki.2009.188.
Malluche HH, Blomquist G, Monier-Faugere MC, Cantor TL, Davenport DL. High parathyroid hormone level and osteoporosis predict progression of coronary artery calcification in patients on dialysis. J Am Soc Nephrol. 2015;26(10):2534–44. doi:10.1681/ASN.2014070686.
Gerakis A, Hutchison AJ, Apostolou T, Freemont AJ, Billis A. Biochemical markers for non-invasive diagnosis of hyperparathyroid bone disease and adynamic bone in patients on haemodialysis. Nephrol Dial Transplant. 1996;11(12):2430–8.
Torres A, Lorenzo V, Hernandez D, Rodriguez JC, Concepcion MT, Rodriguez AP, et al. Bone disease in predialysis, hemodialysis, and CAPD patients: Evidence of a better bone response to PTH. Kidney Int. 1995;47(5):1434–42.
Moore C, Yee J, Malluche H, Rao DS, Monier-Faugere MC, Adams E, et al. Relationship between bone histology and markers of bone and mineral metabolism in African-American hemodialysis patients. Clin J Am Soc Nephrol. 2009;4(9):1484–93. doi:10.2215/CJN.01770408.
Sawaya BP, Butros R, Naqvi S, Geng Z, Mawad H, Friedler R, et al. Differences in bone turnover and intact PTH levels between African American and Caucasian patients with end-stage renal disease. Kidney Int. 2003;64(2):737–42. doi:10.1046/j.1523-1755.2003.00129.x.
Cavalier E, Bergmann P, Bruyere O, Delanaye P, Durnez A, Devogelaer JP, et al. The role of biochemical of bone turnover markers in osteoporosis and metabolic bone disease: A consensus paper of the Belgian bone Club. Osteoporos Int. 2016;27(7):2181–95. doi:10.1007/s00198-016-3561-3.
Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58(1):396–9. doi:10.1046/j.1523-1755.2000.00178.x.
Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115–21. doi:10.1053/ajkd.2000.19812.
Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int. 2006;70(7):1358–66. doi:10.1038/sj.ki.5001754.
Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis. 2006;47(1):149–56. doi:10.1053/j.ajkd.2005.09.024.
Moe SM, Abdalla S, Chertow GM, Parfrey PS, Block GA, Correa-Rotter R, et al. Effects of cinacalcet on fracture events in patients receiving hemodialysis: The EVOLVE trial. J Am Soc Nephrol. 2015;26(6):1466–75. doi:10.1681/ASN.2014040414.
Rudser KD, de Boer IH, Dooley A, Young B, Kestenbaum B. Fracture risk after parathyroidectomy among chronic hemodialysis patients. J Am Soc Nephrol. 2007;18(8):2401–7. doi:10.1681/ASN.2007010022.
Alshayeb HM, Josephson MA, Sprague SM. CKD-mineral and bone disorder management in kidney transplant recipients. Am J Kidney Dis. 2013;61(2):310–25. doi:10.1053/j.ajkd.2012.07.022.
Dounousi E, Leivaditis K, Eleftheriadis T, Liakopoulos V. Osteoporosis after renal transplantation. Int Urol Nephrol. 2015;47(3):503–11. doi:10.1007/s11255-014-0862-3.
Rocha A, Martins LS, Malheiro J, Dores J, Santos C, Henriques C. Changes in bone mineral density following long-term simultaneous pancreas-kidney transplantation. J Bone Miner Metab. 2016;34(2):209–15. doi:10.1007/s00774-015-0657-3.
Pereira S, Pedroso S, Martins L, Santos P, Almeida M, Freitas C, et al. Bone mineral density after simultaneous kidney-pancreas transplantation: Four years follow-up of 57 recipients. Transplant Proc. 2010;42(2):555–7. doi:10.1016/j.transproceed.2010.01.046.
Palmer SC, Strippoli GF, McGregor DO. Interventions for preventing bone disease in kidney transplant recipients: A systematic review of randomized controlled trials. Am J Kidney Dis. 2005;45(4):638–49.
Conley E, Muth B, Samaniego M, Lotfi M, Voss B, Armbrust M, et al. Bisphosphonates and bone fractures in long-term kidney transplant recipients. Transplantation. 2008;86(2):231–7. doi:10.1097/TP.0b013e318176b40f.
Mainra R, Elder G. Review article: Managing bone complications after kidney transplantation. Nephrology (Carlton). 2009;14(4):437–42. doi:10.1111/j.1440-1797.2009.01156.x.
Nikkel LE, Hollenbeak CS, Fox EJ, Uemura T, Ghahramani N. Risk of fractures after renal transplantation in the United States. Transplantation. 2009;87(12):1846–51. doi:10.1097/TP.0b013e3181a6bbda.
Nisbeth U, Lindh E, Ljunghall S, Backman U, Fellstrom B. Increased fracture rate in diabetes mellitus and females after renal transplantation. Transplantation. 1999;67(9):1218–22.
Naylor KL, Li AH, Lam NN, Hodsman AB, Jamal SA, Garg AX. Fracture risk in kidney transplant recipients: A systematic review. Transplantation. 2013;95(12):1461–70. doi:10.1097/TP.0b013e31828eead8.
Vautour LM, Melton 3rd LJ, Clarke BL, Achenbach SJ, Oberg AL, McCarthy JT. Long-term fracture risk following renal transplantation: A population-based study. Osteoporos Int. 2004;15(2):160–7. doi:10.1007/s00198-003-1532-y.
Smets YF, van der Pijl JW, de Fijter JW, Ringers J, Lemkes HH, Hamdy NA. Low bone mass and high incidence of fractures after successful simultaneous pancreas-kidney transplantation. Nephrol Dial Transplant. 1998;13(5):1250–5.
Bouquegneau A, Salam S, Delanaye P, Eastell R, Khwaja A. Bone disease after kidney transplantation. Clin J Am Soc Nephrol. 2016; doi:10.2215/CJN.11371015.
Kamenicky P, Mazziotti G, Lombes M, Giustina A, Chanson P. Growth hormone, insulin-like growth factor-1, and the kidney: Pathophysiological and clinical implications. Endocr Rev. 2014;35(2):234–81. doi:10.1210/er.2013-1071.
Monier-Faugere MC, Mawad H, Qi Q, Friedler RM, Malluche HH. High prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. J Am Soc Nephrol. 2000;11(6):1093–9.
Roe SD, Porter CJ, Godber IM, Hosking DJ, Cassidy MJ. Reduced bone mineral density in male renal transplant recipients: Evidence for persisting hyperparathyroidism. Osteoporos Int. 2005;16(2):142–8. doi:10.1007/s00198-004-1653-y.
Gosmanova EO, Tangpricha V, Gosmanov AR. Endocrine-metabolic pathophysiologic conditions and treatment approaches after kidney transplantation. Endocrine practice: Official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2012;18(4):579–90. doi:10.4158/12016.RA.
Wolf M, Weir MR, Kopyt N, Mannon RB, Von Visger J, Deng H, et al. A prospective cohort study of mineral metabolism after kidney transplantation. Transplantation. 2016;100(1):184–93. doi:10.1097/TP.0000000000000823.
Torres A, Rodriguez AP, Concepcion MT, Garcia S, Rufino M, Martin B, et al. Parathyroid function in long-term renal transplant patients: Importance of pre-transplant PTH concentrations. Nephrol Dial Transplant. 1998;13(Suppl 3):94–7.
Perrin P, Caillard S, Javier RM, Braun L, Heibel F, Borni-Duval C, et al. Persistent hyperparathyroidism is a major risk factor for fractures in the five years after kidney transplantation. Am J Transplant. 2013;13(10):2653–63. doi:10.1111/ajt.12425.
Borah B, Dufresne TE, Ritman EL, Jorgensen SM, Liu S, Chmielewski PA, et al. Long-term risedronate treatment normalizes mineralization and continues to preserve trabecular architecture: Sequential triple biopsy studies with micro-computed tomography. Bone. 2006;39(2):345–52. doi:10.1016/j.bone.2006.01.161.
Keaveny TM, Hoffmann PF, Singh M, Palermo L, Bilezikian JP, Greenspan SL, et al. Femoral bone strength and its relation to cortical and trabecular changes after treatment with PTH, alendronate, and their combination as assessed by finite element analysis of quantitative CT scans. J Bone Miner Res. 2008;23(12):1974–82. doi:10.1359/jbmr.080805.
Ward J, Wood C, Rouch K, Pienkowski D, Malluche HH. Stiffness and strength of bone in osteoporotic patients treated with varying durations of oral bisphosphonates. Osteoporos Int. 2016;27(9):2681–8. doi:10.1007/s00198-016-3661-0.
Kan SL, Ning GZ, Chen LX, Zhou Y, Sun JC, Feng SQ. Efficacy and safety of bisphosphonates for low bone mineral density after kidney transplantation: A meta-analysis. Medicine (Baltimore). 2016;95(5):e2679. doi:10.1097/MD.0000000000002679.
Wang J, Yao M, Xu JH, Shu B, Wang YJ, Cui XJ. Bisphosphonates for prevention of osteopenia in kidney-transplant recipients: A systematic review of randomized controlled trials. Osteoporos Int. 2016;27(5):1683–90. doi:10.1007/s00198-015-3465-7.
Ahn HJ, Kim HJ, Kim YS, Kim MS, Huh KH, Kim JH, et al. Risk factors for changes in bone mineral density and the effect of antiosteoporosis management after renal transplantation. Transplant Proc. 2006;38(7):2074–6. doi:10.1016/j.transproceed.2006.06.106.
Coco M, Glicklich D, Faugere MC, Burris L, Bognar I, Durkin P, et al. Prevention of bone loss in renal transplant recipients: A prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol. 2003;14(10):2669–76.
Coco M, Pullman J, Cohen HW, Lee S, Shapiro C, Solorzano C, et al. Effect of risedronate on bone in renal transplant recipients. J Am Soc Nephrol. 2012;23(8):1426–37. doi:10.1681/ASN.2011060623.
Grotz W, Nagel C, Poeschel D, Cybulla M, Petersen KG, Uhl M, et al. Effect of ibandronate on bone loss and renal function after kidney transplantation. J Am Soc Nephrol. 2001;12(7):1530–7.
Jeffery JR, Leslie WD, Karpinski ME, Nickerson PW, Rush DN. Prevalence and treatment of decreased bone density in renal transplant recipients: A randomized prospective trial of calcitriol versus alendronate. Transplantation. 2003;76(10):1498–502. doi:10.1097/01.TP.0000092523.30277.13.
Smerud KT, Dolgos S, Olsen IC, Asberg A, Sagedal S, Reisaeter AV, et al. A 1-year randomized, double-blind, placebo-controlled study of intravenous ibandronate on bone loss following renal transplantation. Am J Transplant. 2012;12(12):3316–25. doi:10.1111/j.1600-6143.2012.04233.x.
Walsh SB, Altmann P, Pattison J, Wilkie M, Yaqoob MM, Dudley C, et al. Effect of pamidronate on bone loss after kidney transplantation: A randomized trial. Am J Kidney Dis. 2009;53(5):856–65. doi:10.1053/j.ajkd.2008.11.036.
Yamamoto S, Suzuki A, Sasaki H, Sekiguchi-Ueda S, Asano S, Shibata M, et al. Oral alendronate can suppress bone turnover but not fracture in kidney transplantation recipients with hyperparathyroidism and chronic kidney disease. J Bone Miner Metab. 2013;31(1):116–22. doi:10.1007/s00774-012-0391-z.
Smets YF, de Fijter JW, Ringers J, Lemkes HH, Hamdy NA. Long-term follow-up study on bone mineral density and fractures after simultaneous pancreas-kidney transplantation. Kidney Int. 2004;66(5):2070–6. doi:10.1111/j.1523-1755.2004.00986.x.
Cayco AV, Wysolmerski J, Simpson C, Mitnick MA, Gundberg C, Kliger A, et al. Posttransplant bone disease: Evidence for a high bone resorption state. Transplantation. 2000;70(12):1722–8.
Rosen HN, Vokes TJ, Malabanan AO, Deal CL, Alele JD, Olenginski TP, et al. The official positions of the International Society for Clinical Densitometry: Vertebral fracture assessment. J Clin Densitom. 2013;16(4):482–8. doi:10.1016/j.jocd.2013.08.003.
De Sevaux RG, Hoitsma AJ, Corstens FH, Wetzels JF. Treatment with vitamin D and calcium reduces bone loss after renal transplantation: A randomized study. J Am Soc Nephrol. 2002;13(6):1608–14.
Cruzado JM, Moreno P, Torregrosa JV, Taco O, Mast R, Gomez-Vaquero C, et al. A randomized study comparing parathyroidectomy with cinacalcet for treating hypercalcemia in kidney allograft recipients with hyperparathyroidism. J Am Soc Nephrol. 2015; doi:10.1681/ASN.2015060622.
Stavroulopoulos A, Cassidy MJ, Porter CJ, Hosking DJ, Roe SD. Vitamin D status in renal transplant recipients. Am J Transplant. 2007;7(11):2546–52. doi:10.1111/j.1600-6143.2007.01978.x.
Palmer BF, Clegg DJ. Gonadal dysfunction in chronic kidney disease. Rev Endocr Metab Disord. 2016; doi:10.1007/s11154-016-9385-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
No potential conflicts of interest relevant to this article are reported.
Support
None received.
Financial disclosure
Authors declare no competing interests. E.O. G and A.R.G. are employees of the US Department of Veterans Affairs and opinions expressed in this manuscript is authors` personal opinions and do not necessarily represent the opinion of the Department of Veterans Affairs.
Rights and permissions
About this article
Cite this article
Gosmanova, E.O., Gosmanov, A.R. Osteoporosis in patients with diabetes after kidney transplantation. Rev Endocr Metab Disord 18, 97–106 (2017). https://doi.org/10.1007/s11154-016-9397-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-016-9397-5