Abstract
The symptoms of chronic renal disease-related mineral and bone disease improve significantly in patients after successful simultaneous pancreas-kidney transplantation (SPKT); however, bone pathology is still present even after many post-transplant years. The aim of this study was to analyze the bone densitometry in different periods after SPKT. Three-point densitometry was performed with the dual-energy X-ray absorptiometry (DXA) technique. Serum levels of alkaline phosphatase (ALP), total serum calcium, phosphate and parathyroid hormone were analyzed as markers of mineral metabolism. The study population consisted of 48 patients (28 females, 20 males) with a mean age of 35 ± 6 years and mean 24 ± 6 years of prior diabetes. Mean period of maintenance dialysis was 36 ± 26 months. The median time from SPKT and DXA measurement was 0.53, 26.2 and 41.9 months, respectively. Based on the DXA technique, 35.4 % of patients were categorized as having osteoporosis at the lumbar spine and 39.6 % at the femoral neck. Patients with diagnosed osteoporosis had significantly higher levels of ALP (OR = 1.5; 95 % CI = 1.1–2.2; p < 0.05 at the lumbar spine; OR = 1.4; 95 % CI = 1.0–1.9; p < 0.05 at the femoral neck). In addition, subjects with lumbar osteoporosis were characterized by a significantly lower body mass index (BMI) (OR = 0.5; 95 % CI = 0.3–0.9; p < 0.05). In the long-term follow-up, BMD increased significantly at the lumbar spine (T-score −1.86 ± 1.07 to −1.08 ± 0.89) and femoral neck (T-score −2.12 ± 0.78 to −1.63 ± 0.65). A multivariate linear model identified a BMI increase as a significant factor associated with improvement in BMD. Results of our study led us to conclude that, according to three-point densitometry, BMD among patients with functioning kidney and pancreas grafts improved. Increased serum levels of ALP were significantly associated with a decrease in BMD, suggesting a higher risk of osteoporosis. BMI gain was predictive of BMD improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Simultaneous pancreas-kidney transplantation (SPKT) is the treatment of choice for selected patients with type 1 diabetes and end-stage renal disease (ESRD).
Despite the ability to reverse many complications following transplantation, namely chronic renal disease-related mineral and bone disease (CKD-MBD), bone mineral loss with subsequent development of osteopenia and osteoporosis remains a frequent and serious complication. Although it has been recognized, the routine application of adequate diagnostic tools and preventive or treatment strategies to correct bone loss or mineral disarrays may often be suboptimal [1].
The guidelines published by Kidney Disease Improving Global Outcomes (KDIGO) in 2009 recommend measuring the bone mineral density (BMD) in the first 3 months after kidney transplant in patients with an estimated glomerular filtration rate (GFR) greater than ~30 ml/min per 1.73 m2 if they receive corticosteroids or have risk factors for osteoporosis as in the general population [2].
The magnitude of pre-existing CKD-MBD, hypophosphatemia and disturbances in the fibroblastic growth factor 23-parathyroid-vitamin D axis before transplant, degree of kidney function recovery, and effects of immunosuppressive and other therapies create a heterogeneous patient population in the first year after transplantation. Therefore, the KDIGO guidelines indicate that it is probably useful to distinguish the time period immediately after the kidney transplant, with rapidly changing GFR and concomitantly given therapies, from the subsequent time period when a more stable graft function has been achieved [2].
Data characterizing bone loss after SPKT, particularly in the long term, are scarce. The aim of our study was to evaluate serum markers of mineral metabolism, grafts function, and lumbar and femoral BMD determined by dual-energy X-ray absorptiometry (DXA) over at least 3 years of follow-up among 48 patients who underwent SPKT.
Materials and methods
From May 2000 to December 2009, 107 type 1 diabetic patients underwent SPKT at the Transplantation Department of our unit.
All the procedures were performed using grafts from deceased donors, both grafts from the same donor, and using systemic-enteric drainage (venous drainage to the iliac vein; exocrine drainage through an enteric anastomosis of the pancreatic-duodenal arch).
The BMD was determined by DXA using the same Hologic QDR® 4500 X-ray Bone Densitometer over the study period. Instrument quality control on the DXA scanner was performed by daily scanning of a spine phantom. The coefficients of variation for BMD measurements were 1 % (spine) and 2 % (femoral neck). The results were expressed as T-scores (the number of standard deviations a person’s BMD is below the mean BMD for the young healthy population). The osteoporotic label cutoff was –2, 5 or lower, in accordance with World Health Organization guidelines.
A prospective, cross-sectional, single-center study was performed with three serial DXA examinations.
Forty-eight patients fulfilled the following criteria: a first DXA measurement in the first 3 months after SPKT and two other DXA measurements performed at least 2 years after transplant and 1 year apart, respectively.
Biological markers of mineral metabolism (total serum calcium, phosphate, intact parathyroid hormone (PTH), alkaline phosphatase) were regularly measured during follow-up. Blood was drawn after an overnight fast. Serum calcium, phosphorus and alkaline phosphatase were determined by colorimetric methods. The binding reagent was o-cresolphtalein, acid molybdate and para-nitrophenylphosphate, respectively. Serum PTH levels were measured using electrochemiluminescence immunoassay (ECLIA) performed on the Elecsys® 2010 fully automated immunoanalyzer. Hyperparathyroidism was defined as an intact PTH level higher than 70 pg/ml.
Body weight was recorded at each examination.
Kidney graft function was determined by measuring serum creatinine and estimated GFR levels using the modification of diet in renal disease (MDRD) formula. Pancreas graft function was determined by measuring fasting glycemia, glycosylated hemoglobin (HbA1C) and C-peptide.
Because of the small number of patients initiating bone active medications, which included vitamin D with or without a calcium supplement, we were unable to assess whether they had effects on the changes reported in this investigation. Exclusion of participants who were taking these medicines from the analysis did not change our conclusions.
The data were analyzed with SPSS 18.0 (Statistical Package for the Social Sciences, Evanston, IL, USA). Results were expressed as mean values and standard deviations for continuous, normally distributed variables and as percentages for categorical data. Paired and unpaired t tests, chi-square tests and Kruskal-Wallis one-way analysis of variance were performed to assess the significance of differences between groups by using continuous and categorical variables. For significant risk factors in univariate analysis, multivariate logistic models were built using the stepwise forward elimination of nonsignificant factors.
To determine clinical predictors of changes in bone densitometry—∆ BMD—a multivariate linear regression model was used. Statistical significance was considered when P < 0.05.
Results
Baseline evaluation
Forty-eight patients had 24 ± 6 years (range 11–38) of prior diabetes, 58.3 % were female, and the overall mean age was 35 ± 6 (range 20–47) years at transplantation date. The dialysis vintage (hemodialysis or peritoneal dialysis) was 36 ± 26 months (range 5–98), but two patients underwent a preemptive procedure.
Induction immunosuppressive therapy included glucocorticoids, tacrolimus, mycophenolate mofetil and antithymocyte globulin.
At the time of transplantation, the average BMD was low. The lumbar spine and femoral neck T-scores were −1.86 ± 1.07 and −2.12 ± 0.78, respectively. Osteoporosis was reported in 35.4 % of recipients in the lumbar spine and in 39.6 % of recipients in the femoral neck. Genders did not differ regarding T-scores.
In univariate analysis, higher alkaline phosphatase values were associated with osteoporosis at both regions, while low BMI and high PTH were associated, respectively, with lumbar spine and femoral neck osteoporosis (Table 1).
No correlation was found between BMD and diabetes and dialysis duration, calcium and phosphate values, kidney and pancreas function.
In multivariate analysis, high alkaline phosphatase levels were found to significantly increase the risk of low BMD. Another risk factor for lumbar spine osteoporosis was low BMI (Table 2).
Sequential changes post-transplantation
Glucocorticoid tapering below the daily dose of 10 mg was normally initiated after month 6 with complete withdrawal achieved in 60.4 % of patients at the end of the second year. No rejection episodes were registered. At the end of follow-up, 81.2 % of patients did not receive glucocorticoids in the immunosuppressive regimen.
Their mean serum creatinine and creatinine clearance remained stable. All were insulin-independent.
After SPKT, a decrease in serum PTH levels occurred (Table 3). Serum phosphate, alkaline phosphatase and calcium levels did not change significantly after transplantation.
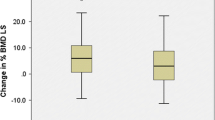
Bone densitometry enhanced gradually through the follow-up (Figs. 1, 2). The Kruskal-Wallis test revealed a significant difference between evaluations. There was a good correlation between the femoral neck and lumbar spine BMD at three-point densitometry (r = 0.673, P < 0.001; r = 0.394, P = 0.006; r = 0.439; P = 0.002, respectively).
After 2 years of SPKT, 10.4 and 18.8 % of patients remained osteoporotic in the lumbar and femoral regions, respectively. At the end of follow-up, only two and four osteoporosis cases at the lumbar and femoral regions, respectively, were verified.
In a model adjusted for gender, age, diabetes duration, ∆ BMI and cumulative glucocorticoid dose, higher ∆ BMI values predicted a significant improvement in ∆ BMD. Each 10 % increase in ∆ BMI was associated with an improvement of 3.62 and 3.53 % in ∆ BMD at the lumbar spine [β = 3.620 (95 %CI 0.155–7.086) P = 0.041] and femoral neck [β = 3.533 (95 %CI 0.430–6.636) P = 0.027], respectively.
Discussion
Combined kidney-pancreas transplantation is a recognized risk factor for osteoporosis and consequently fractures [3, 4]. Beyond the mechanisms associated with transplantation, type I diabetes per se is an independent risk factor. The anabolic effects of insulin on bone, alteration of bone metabolism by advanced glycation end products or vascular complications of diabetes such as neuropathy, visual impairment and amputation are possible explanations [5].
A study in SPKT demonstrated a prevalence of vertebral and nonvertebral fractures of 45 % and a high incidence of osteoporosis 1 year following transplantation [4]. In a population of 31 patients, 23 % had a significant decrease in bone mass (T score <−2.5) at the predominantly trabecular lumbar spine sites, and 58 % demonstrated a similarly low bone mass at the femoral neck, where cortical bone is prevalent [4].
A group recently evaluated the rate of hospitalization for fracture among 6,212 patients with type I diabetes who received a kidney transplant alone compared to 4,933 patients who received an SPKT and came to an interesting conclusion. They found that a significant difference between both a 5.9 versus 4.7 % rate of hospitalization, respectively, persisted after adjustment for many fracture risk factors [5], challenging the higher risk of osteoporosis in SPKT patients.
In most published scientific research studies, the follow-up period may have been too short; therefore, the results should be interpreted carefully. In the first year following transplantation, it is likely that at least two major factors influence BMD: hyperparathyroidism and use of corticosteroids. Normalization of PTH secretion and, consequently, of bone remodeling could be responsible for prevention of further deterioration of the BMD over time post-transplantation [6]. Furthermore, persistent hyperparathyroidism is an independent risk factor for fractures [7]. Moreover, glucocorticoid withdrawal in transplant recipients results in an increase in BMD [8].
Previous prospective studies evaluating BMD in consecutive SPKT recipients before and at 3, 6 and 12 months after establishment of graft function [9] revealed significant bone loss within 6 months of transplantation at both trabecular and cortical sites, mainly due to glucocorticoid therapy and the high doses required early after transplantation [10]. Steroids can be directly toxic to osteoblasts and lead to increased osteoclast activity. Other steroid effects include decreased calcium absorption in the gut, reduced gonadal hormone production, diminished insulin-like growth factor-1 production, decreased sensivity to PTH, an increase in receptor activator of NF-kappa beta ligand and increased osteoclastogenesis [1, 24]. The effect of other immunosuppressive drugs appears to be limited [1, 4].
In our study, in the baseline evaluation, low BMI, a traditional risk factor for low BMD in the general population, was a risk factor for osteoporosis of the lumbar spine in univariate and multivariate analysis. This correlation between loss of BMD and low BMI values was identified in kidney transplant recipients [11, 12]. The putative mechanisms are several, such as the effect of adipokines in bone remodeling and conversion of androgen to estrogen in the adipose tissue [13]. The World Health Organization fracture risk assessment tool (FRAX) includes BMI in the evaluation [14].
In our survey, the patients who remained osteoporotic at the lumbar level after the third evaluation had BMIs lower than 20.5 kg/m2, in one of them being lower than 18.5 kg/m2. In the long term, the only clinical predictor of BMD improvement was an increase in BMI.
A risk factor for accelerated BMD loss at the femoral region following transplantation was hyperparathyroidism, as revealed by other evaluations [15–17]. A novel and interesting finding was the significant association between the decrease in T-score in both sites of the lumbar spine and femoral neck and an increase in the alkaline phosphatase value from 10 units the upper limit of the reference interval.
An increase in serum alkaline phosphatase, known as hyperphosphatasemia or hyperphosphatasia, in patients with otherwise intact liver and biliary systems usually results from an excess of the bone isoforms of the enzyme [18]. Maruyama et al. examined the baseline data of 185,277 prevalent hemodialysis patients in Japan and related them to 1-year mortality and incident hip fracture events through calendar year 2010. They found that patients in the highest quartile of serum alkaline phosphatase had 46 and 25 % higher all-cause and cardiovascular death risks, respectively, as well as a 71 % higher incidence of hip fracture events, than those within the lowest quartile [18].
Data from the Scientific Registry of Transplant Recipients relative to 11,776 patients also concluded that recipients with pretransplant serum alkaline phosphatase of 120–160 and ≥160 U/l had 49 and 64 % higher graft failure censored all-cause mortality in multivariate adjusted models [19]. Several studies revealed that the alkaline phosphatase level appears to be at least as tightly linked to bone histomorphometry as the PTH concentration and to be more closely linked to clinical outcomes than the PTH concentration [20].
Osteoporosis results from complex interactions between genetic and environmental factors, and the association of a single nucleotide polymorphism in the alkaline phosphatase gene with BMD has been demonstrated [21]. Studies in the general population and in patients with chronic renal disease have recognized the elevation of alkaline phosphatase levels as a possibly more efficient method to detect patients with fast bone turnover rates, helpful in predicting osteoporosis [22, 23]. In our study, bone loss occurred in patients with higher levels of alkaline phosphatase.
As expected [6, 24], we verified a higher incidence of osteoporosis in the femoral neck, which contains more cortical bone than the spine. It is well known that most fragility fractures occur at nonvertebral sites where bone is composed mainly by compact (or cortical) tissue, since it accounts for 80 % of the total bone mass of an adult skeleton [25, 26].
The development and implementation of a comprehensive bone health protocol in posttransplant SPK patients is important for screening but certainly also for prevention of osteopenia and osteoporosis. In a protocol applied in 76 posttransplant kidney and SPK patients, a DXA scan was performed within 2 weeks of transplant. Prevention of bone disease was considered appropriate if the patient was educated on the reduction of modifiable risk factors and received at least 1,000 mg of elemental calcium and 400 IU of vitamin D daily. Treatment of bone disease was considered appropriate if the patient was receiving either an oral or intravenous bisphosphonate, oral raloxifene, or nasal or subcutaneous calcitonin for documented osteoporosis. According to the T-score, the DXA scan was repeated in 6 months, 1 year or every 2 years [27], with beneficial results in prevention and treatment. Data on the use of these medications in solid organ transplantation are scarce, and there are no well-established therapeutic approaches with a high degree of certainty [1]. Whether they are beneficial in increasing BMD in SPK recipients remains to be explored. In our study without bone active medications, we observed a good long-term evolution of bone density, bringing into question the role of antiosteoporotic therapy on a long-term basis.
Although the present study has limitations due to a relatively small and heterogeneous sample, it leads to the reasonable conclusion that bone density is enhanced following SPKT.
Conclusion
From this survey we obtained several relevant data: first we found an improvement in BMD present in the long-term follow-up of SPKT recipients. Another important aspect of this study was the identification of alkaline phosphatase as an independent risk factor of BMD in these patients. A multivariate clinical linear model determined that the change in BMD was positively associated with an increase in BMI.
This study supports that evaluation of SPK recipient bone disease requires the use of BMD measurements together with alkaline phosphatase and BMI to make the diagnosis, risk evaluation and probably therapy of osteoporosis more effective.
References
Kalantar-Zadeh K, Molnar MZ, Kovesdy CP, Mucsi I, Bunnapradist S (2012) Management of minerals and bone disorders after kidney transplantation. Curr Opin Nephrol Hypertens 21:389–403
KDIGO Guideline for Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) (2009) Chapter 5: Evaluation and treatment of kidney transplant bone disease. Kidney Int 76:S100–S110
NIH Consensus Development Panel (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Smets YF, van der Pijl JW, de Fijter JW, Ringers J, Lemkes HH, Hamdy NA (1998) Low bone mass and high incidence of fractures after successful simultaneous pancreas-kidney transplantation. Nephrol Dial Transpl 13:1250–1255
LE Nikkel, Iyer SP, Mohan S, Zhang A, McMahon DJ, Tanriover B, Cohen DJ, Ratner L, Hollenbeak CS, Rubin, Shane E, Nickolas TL, CURE Group (The Columbia University Renal Epidemiology Group). (2013) Pancreas-kidney transplantation is associated with reduced fracture risk compared with kidney-alone transplantation in men with type 1 diabetes. Kidney Int 83:471–478
Kusec V, Smalcelj R, Cvijetic S, Rozman B, Sfreb K (2000) Determinants of reduced bone mineral density and increased bone turnover after kidney transplantation: cross-sectional study. Croat Med L 41:396–400
Perrin P, Caillard S, Javier RM, Braun L, Heibel F, Borni-Duval C, Muller C, Olagne J, Moulin B (2013) Persistent hyperparathyroidism is a major risk factor for fractures in the 5 years after kidney transplantation. Am J Transpl 13:2653–2663
Farmer CK, Hampson G, Abbs IC, Hilton RM, Koffman CG, Fogelman I, Sacks SH (2006) Late low-dose steroid withdrawal in renal transplant recipients increases bone formation and bone mineral density. Am J Transpl 6:2929–2936
Smets YF, de Fijter JW, Ringers J, Lemkes HH, Hamdy NA (2004) Long-term follow-up study of bone mineral density and fractures after simultaneous pancreas-kidney transplantation. Kidney Int 66:2070–2076
Casez JP, Lippuner K, Horber FF, Montandon A, Jaeger P (2002) Changes in bone mineral density over 18 months following kidney transplantation: the respective roles of prednisone and parathyroid hormone. Nephrol Dial Transpl 17:1318–1326
Unal A, Kocyigit I, Sipahioglu MH, Tokgoz B, Kavuncuoglu F, Oymak O, Utas C (2010) Loss of bone mineral density in renal transplantation recipients. Transpl Proc 42:3550–3553
Sezer S, Ozdemir FN, Ibis A, Sayin B, Haberal M (2005) Risk factors for osteoporosis in young renal transplant recipients. Transpl Proc 37:3116–3118
Salamat MR, Salamat AH, Abedi I, Janghorbani M (2013) Relationship between weight, body mass index, and bone mineral density in men referred for dual-energy x-ray absorptiometry scan in Isfahan, Iran. J Osteoporos 2013:20596
Fujiwara S, Nakamura T, Orimo H, Hosoi T, Gorai I, Oden A, Johansson H, Kanis JA (2008) Development and application of a Japanese model of the WHO fracture risk assessment tool (FRAX). Osteoporos Int 19:429–435
Kokado Y, Takahara S, Ichimaru N, Toki K, Kyo M, Permpongkosol S, Kojima Y, Inoue T, Wang JD, Okuyama A (2000) Factors influencing vertebral bone density after renal transplantation. Transpl Int 13:S431–S435
Roe SD, Porter CJ, Godber IM, Hosking DJ, Cassidy MJ (2005) Reduced bone mineral density in male renal transplant recipients: evidence for persisting hyperparathyroidism. Osteoporos Int 16:142–148
Akaberi S, Lindergard B, Simonsen O, Nyberg G (2006) Impact of parathyroid hormone on bone density in long-term renal transplant patients with good graft function. Transplantation 82:749–752
Wei Ling Lau WL, Kalantar-Zadeh K (2014) Towards the revival of alkaline phosphatase for the management of bone disease, mortality and hip fractures. NDT 29:1450–1452
Molnar MZ, Kovesdy CP, Mucsi I, Salusky IB, Kalantar-Zadeh K (2012) Association of pre-kidney transplant markers of mineral and bone disorder with post-transplant outcomes. Clin J Am Soc Nephrol 7:1859–1871
Sardiwal S, Magnusson P, Goldsmith DJ, Lamb EJ (2013) Bone alkaline phosphatase in CKD-mineral bone disorder. Am J Kidney Dis 62:810–822
Sogabe N, Tanabe R, Haraikawa M, Maruoka Y, Orimo H, Hosoi T, Goseki-Sone M (2013) Associations between serum bone-specific alkaline phosphatase activity, biochemical parameters, and functional polymorphisms of the tissue-nonspecific alkaline phosphatase gene in a Japanese population. Asia Pac J Clin Nutr 22:160–165
Atalay S, Elci A, Kayadibi H, Onder CB, Aka N (2012) Diagnostic utility of osteocalcin, undercarboxylated osteocalcin, and alkaline phosphatase for osteoporosis in premenopausal and postmenopausal women. Ann Lab Med 32(1):23–30
Haarhaus M, Fernström A, Magnusson M, Magnusson P (2009) Clinical significance of bone alkaline phosphatase isoforms, including the novel B1x isoform, in mild to moderate chronic kidney disease. Nephrol Dial Transpl 24(11):3382–3389
Iyer SP, Nikkel LE, Nishiyama KK, Dworakowski E, Cremers S, Zhang C, McMahon DJ, Boutroy S, Liu XS, Ratner LE, Cohen DJ, Guo XE, Shane E, Nickolas TL (2014) Kidney transplantation with early corticosteroid withdrawal: paradoxical effects at the central and peripheral skeleton. J Am Soc Nephrol 25:1331–1341
Iolascon G, Napolano R, Gioia M, Moretti A, Riccio I, Gimigliano F (2013) The contribution of cortical and trabecular tissues to bone strength: insights from denosumab studies. Clin Cases Miner Bone Metab 10:47–51
Farr JN, Khosla S, Miyabara Y, Miller VM, Kearns AE (2013) Effects of estrogen with micronized progesterone on cortical and trabecular bone mass and microstructure in recently postmenopausal women. J Clin Endocrinol Metab 98:E249–E257
Taber DJ, Ashcraft EA, Baillie GM, Lawrence DB, Chavin KD, Baliga PK (2007) Use of bone health protocol to identify and prevent bone disease in kidney and pancreas transplant recipients. Ann Pharmacother 41:944–950
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Rocha, A., Martins, L.S., Malheiro, J. et al. Changes in bone mineral density following long-term simultaneous pancreas-kidney transplantation. J Bone Miner Metab 34, 209–215 (2016). https://doi.org/10.1007/s00774-015-0657-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-015-0657-3