Abstract
Gold nanoparticles were deposited into the mesoporous aminopropylsylane functionalized SBA-15 by impregnation. The catalyst was characterized by XRD, UV–Vis, BET, MET, and IR. The characterization demonstrates that the gold nanoparticles situated in the SBA-15 pores with small sizes in an average of 1–8 nm. The catalyst exhibited a highest catalytic performance in the synthesis of β-acetamido ketones molecules with a short time reaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The SBA-15 is a highly ordered two-dimensional hexagonal structure synthesized in 1998 by stucky et al. [1] which has attracted particular attention because of its properties as a high specific surface [2], large pore size, thick silica walls favoring thermal and hydrothermal stability [3, 4], and a high density of hydroxyl on the surface. It is easily synthesized in acidic medium, using as structural agent as the amphiphilic triblock copolymer [1]. Indeed, based on these typical characteristics, SBA-15 has an exciting field of research of great scientific importance and different applications, as a heterogeneous catalyst in waste water treatment, hydro-cracking, biodiesel production and drug delivery [5, 6].
Many researchers have been carried out in order to develop SBA-15 where it was necessary to modify it in order to widen its application for the design of new catalysts. However, various metals were used such as Al-SBA-15 [7], Vx-SBA-15 [8] Zr-SBA-15 [9], Ag/SBA-15 [10], Au/SBA-15 [11], and MoO3/SBA-15 [12].
Mesoporous silica functionalized by introduction of organic group is one of the approaches achieved by scientists; the organic active species are oriented towards the surface and facilitate the modification and introduction of new physicochemical characteristics. The organo-functionalization on the surface of mesoporous silica can be carried out generally either by integrating functionalities directly by co-condensation also called direct synthesis [13,14,15], or by the grafting method also called post-synthesis [16], these methods present advantages for example the control of morphology, functionalities, and the dimension of the pores but also disadvantages such as the reduction of the structural properties and the manufacture on a large scale. The grafting method consists in modifying the surface of silica with organic groups by creating covalent bonds between the active function and the internal surface of the pores by means of silanol (Si–OH) groups by using trichloro or trialkoxy-organosilane or silyamines as organic precursors [17]. On the other hand, co-condensation is a simpler and faster synthesis and allows the obtain a more homogeneous distribution of the organic groups in the material compared to grafting method, in addition it avoids the possibility of pore blocking. Nevertheless, this method also has some disadvantages because there is an antagonistic effect between the degree of mesoscopic order and the concentration of the organosilanes in the reaction medium. For this purpose, in the presence of a structuring agent, an organosilane precursor (R′O)3SiR is combined with a silica precursor salt of the type Si(OR′)4, generally tetraethylorthosilicate TEOS (R′ = ethyl group) was used [18]. However, organo-functionalized SBA-15 has shown very promising results in various applications such as adsorption [19, 20], biodiesel production [21], drug delivery [22], water decontamination [23], and corrosion inhibitor [24]. In addition, supported SBA-15 catalysts were also used in organic synthesis such as the synthesis of propargylamines [25, 26], imidazoles [27], and pyrroles [28].
Acetamido ketones derivatives are suitable mediators for a variety of pharmaceutically and biologically essential compounds [29]. Also, they have an important moiety used as precursors in organic synthesis like 1,3-amino alcohols [30], 1,3-aminoacids [31] as well as their for the preparation of diverse bioactive molecules such as the antibiotics nikkomycins or neopolyxins [32] and other different antibiotic drugs [33]. Generally, the Dakin-West reaction is one of the most popular tools used for the synthesis of β‐acetamido ketones [34]. In 1994, a simple procedure for preparation of β-acetamido ketone was developed by Bhatia et al. [35], which represents a one-pot multi-component coupling condensation of an enolizable ketone, aromatic aldehyde, and acetonitrile in the presence of acetyl chloride and catalyst, so this transformation became a model where different catalysts was developed like H7SiV3W9O40 [36], Fe3O4@SiO2-GA-M(Pc) [37], NiFe2O4@SiO2–PPA [38], SBA-15-Pr–SO3H [39], IL [(PS)2pi][OTf]2 [40], H5PW10V2O40 [41], Cellulose sulfuric acid [42], and Mg(HSO4)2 [43]
Gold is known to have an exceptionally rich and exciting chemistry especially as homogeneous [44] and heterogeneous [45] catalysts. However, gold nanoparticles catalysts were used in organic synthesis several times due to their capacity to activate C–H and C–C bands. Their activity is directly related to the particles size, dispersion, and the characteristics of the support, which can control the metal-support interaction [26, 46, 47]
The development of solid heterogeneous catalysts with various organic functionalities is considered to be an attractive topic due to the advantage of easier separation, recovery and environmental improvement compared with homogeneous catalyst. In this context, this paper demonstrates for the first time, the synthesis and characterization of heterogeneous catalyst; amine-modified mesoporous silica SBA-15 (Au/NH2-SBA-15) and their activity in the synthesis of β-acetamido ketones via one pot condensation of an enolizable ketone,an aromatic aldehyde,, and acetyl chloride at room temperature.
Experimental
Preparation of SBA-15
The synthesis of SBA-15 was realized using the Zhao’s method reported previously [48]. At room temperature under stirring,a total of 4 g of block copolymer surfactant Pluronic 123 (EO20PO70EO20) used as a template, was dissolved in a mixture of ultra pure water (120 mL, 6.67 mol) and 7.5 mL of HCl (0.24 mol, pH = 0.83). Next, the solution was heated up to 40 °C followed by addition of 9.15 mL of tetraethyl orthosilicate TEOS. The mixture stirred for 24 h at the same temperature. Then, this later was placed into a Teflon-lined autoclave for 48 h at 100 °C. Finally, the solid product was filtered, dried at room temperature overnight, and calcined in air at 500 °C for 4 h.
SBA-15 functionalization with aminopropylsylane
Before the SBA-15 surface functionalization, 3 g of calcined SBA-15 was refluxed in 100 mL of distilled water for 6 h, and then the mixture was centrifuged and dried at room temperature for 24 h. The product was mixed with 1.5 g of n-octadecyl-trimetoxysilane and 100 mL of chloroform under stirring at 25 °C for 24 h. The solid was washed abundantly with chloroform and dried at room temperature. Next, 1,5 g of APTES was slowly added to the obtained sample diluted by 100 mL of chloroform under stirring for 24 h under. The mixture was then filtered and washed several times with chloroform and finally the product was dried overnight at 80 °C. The catalyst was denoted SBA-15-NH2.
Preparation of 1 wt% Au/SBA-15-NH2:
1 g of the functionalized SBA-15-NH2 support was stirred for 3 h in 139 mL of chloroauric acid (HAuCl4·3H2O). After filtration and washing with deionized water of mixture product, the recovered solid was reduced under hydrogen atmosphere at 100 °C for 2 h.
General procedure for synthesis of β-acetamido ketones
In a roud-bottomed flask, 2 mmol of acetyl chloride, 1 mmol of aromatic aldehyde, and 1 mmol of acetophenone derivative in the presence of 0.03 g of Au-SBA-15-NH2 were stirred in 3 mL of acetonitrile at room temperature for appropriate time. Progress of the reaction was monitored by TLC. After the reaction was completed, the catalyst was separated and the reaction mixture was extracted with dichloromethane and water and dried over Na2SO4. After evaporation of the solvent under reduced pressure, the crude product was purified by column chromatography (n-hexane/n-ethyl acetate).
Results and discussions
Catalyst characterization
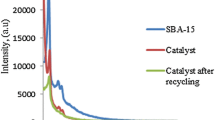
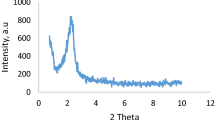
Wide-angle XRD of support and final catalyst are described in (Fig. 1). A broad peak is observed at 2θ = 23° corresponding to the formation of amorphous silica mesopores [49]. No visible diffraction peaks attributed to the Au NPs could be identified, which reveals that gold Au nanoparticles are well dispersed on the surface of the support or are in the form of non-crystal phases [50]. Also, the samples present the same diffraction peaks even after the organo-functionalization. In addition, the low-angle powder X-ray diffraction of the catalyst Au/SBA-15-NH2 gave 2θ values of 1.03°, 1.65° and 1.88° which are indexed to the (100), (110) and (200) reflections of an ordered hexagonallysba-15 materials [51]. The highest peak refers to the periodic inter-space area between the walls of mesoporous channels and the two lower peaks which is due to the elevated order of SBA-15 structure channels [52]
Fig. S1 shows the UV–Vis spectrum of Au/SBA-15-NH2. From 200 to 350 nm spectral zone represents the structure of the support. Moreover, the surface plasmon resonance (SPR) band at ~ 520 nm thus identifying small gold nanoparticle with spherical shape and size superior than 2 nm. Also, that certifying that Au NPs have been incorporated in the support [53, 54]
Fig. 2 demonstrates the FTIR spectrum of the support and the catalyst, a strong peak situated around 1087 cm−1 and 803 cm−1 assigned to the asymmetric vibrations of the Si–O–Si bond. Another peaks reveals at 1640 cm−1 could be attributed to H2O adsorbed in mesoporous structure [55]. This spectrum exhibits bending and stretching vibrations of the Si–OH bond as a small band at about 956 cm−1 [51]. Furthermore,the appearance of the C–H stretching of methyl groups containing amino silane at 2800–3000 cm−1, indicates that the NH2 groups have been incorporated into the backbones of the mesoporous silica (–NH2) to the SBA-15 support. Moreover, the presence of –NH2 after the APTES modification can also be confirmed by the vibration peak of N–H stretching at 500–750 cm−1 in the pattern of SBA-15-NH2. At the same time, the N–H stretching vibration peak at 500–750 cm−1 was no visible in Au/SBA-15-NH2, which assigned to the coordination interaction between –NH2 and Au [56].
In order to understand the structure of the catalysts, low temperature adsorption–desorption isotherms at 77 K and pore size distribution analyses (Fig. 3) were done. The analysis showed that all samples belong to type IV with H1 type hysteretic loop (P/P0 > 0.4), which represents capillary condensation of the ordered mesoporous structure. The resulting textural properties of these isotherms are summarized in (Table 1).We note a decrease in specific surface area which can be explained by the partial obstruction of the mesopores by the gold particles.
Fig. 4 shows the HRTEM images and particle size histogram for supported gold nanoparticles. The image of the support (Fig. 4a) shows well-ordered hexagonal pore structure and regular periodicity of SBA-15, this indicating that the addition of organic function group to the SBA-15 materials has no distinct influence on their morphology. The TEM analysis reveals also a highly dispersed and uniformly sized of Au NPs. The structure of SBA-15 mesoporous silica is retained after gold deposition and the Au NPs were clearly observed mainly inside porous channels of SBA-15 (Fig. 4c). As can be seen, the histogram of the particles size distribution (Fig. 4d) indicates that particle size of gold in Au/SBA-15-NH2 range from 2 to 8 nm with an average size of 3 nm.
Catalytic activity
In order to find the optimal reaction condition for the synthesis of β-acetamido ketones, the reaction of benzaldehyde, acetophenone, and acetyl chloride was selected as a model reaction (Table 2). Initially, the reaction was performed in the absence of catalyst Au/SBA-NH2 for 3 h. Result indicates that the reaction could not bring about any yield product and no reaction occurred even after an extended reaction time to 24 h (Table 2, entries 1 and 2). Then, the supports SBA-15, SBA-15-NH2 were used with amount equal to 0.01 g, but no product was afforded (Table 2, entries 3 and 4). After introduction of gold NPs, the reaction achieved with a yield of 58% (Table 2, entry 5). This result revealed that gold nanoparticles were responsible on the catalyst activity.
To increase the product yield, the reaction was carried for 6 h and 24 h, with yields equal to 42 and 39 (Table 2, entries 6 and 7). The increase of catalyst amount to 0.08 g, leads to the decreases of yield to 94%. Using these conditions, several β-acetamido ketones derivatives were prepared with high yields 80–90% (Scheme S1).
The result above demonstrates that gold nanoparticles are responsible on the catalyst activity, this due to their contribution in the reaction as Lewis acid [57], so according to this information and the literature [38, 58], a proposed mechanism presented in Scheme 1 for the β-acetamido ketone synthesis via aldehyde, acetophenone, and acetylchloride condensation in acetonitril. In the first, the gold nanoparticles activate the C–H band of aldehyde and ketone by enolization of the acetophenone producing a proton (H +). Also, the gold nanoparticles were known for the C–Cl band activation, so the reaction between acetylchloride and intermediate given by the reaction between aldehyde and ketone will be faster to produce intermediate I. Then, this later reacts with acetonitrile to give the final desired product.
Conclusion
In the present paper we demonstrate for the first time that gold nanoparticles supported on modified SBA-15 is an effective catalyst for the one pot synthesis of the β-acetamido carbonyl compounds in short time. However, the use of SBA -15 or modified SBA-15 does not give product because the reaction needs an acidic cites which were given by supported gold nanoparticles. Also, the characterization reveals that gold nanoparticles were supported with good dispersion and small sizes and affect directly on the catalyst activity.
Data Availability
Data available for this research in Supplementary information.
References
Zhao D, Feng J, Huo Q et al (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279(5350):548–52
Zhu J, Kailasam K, Xie X et al (2011) High-surface-area SBA-15 with enhanced mesopore connectivity by the addition of poly(vinyl alcohol). Chem Mater 23(8):2062–2067
Wang J, Ge H, Bao W (2015) Synthesis and characteristics of SBA-15 with thick pore wall and high hydrothermal stability. Mater Lett 145:312–315
Margolese D, Melero JA, Christiansen SC et al (2000) Direct syntheses of ordered SBA-15 mesoporous silica containing sulfonic acid groups. Chem Mater 12(8):2448–2459
Jin M, Guo Z, Lv Z (2019) Immobilization of tungsten chelate complexes on functionalized mesoporous silica SBA-15 as heterogeneous catalysts for oxidation of cyclopentene. J Mater Sci 54(9):6853–6866
Aboelfetoh EF, Zain Elabedien ME, Ebeid E-ZM (2021) Effective treatment of industrial wastewater applying SBA-15 mesoporous silica modified with graphene oxide and hematite nanoparticles. J Environ Chem Eng 9(1):104817
Bhange P, Bhange DS, Pradhan S et al (2011) Direct synthesis of well-ordered mesoporous Al-SBA-15 and its correlation with the catalytic activity. Appl Catal A Gen 400(1):176–184
Luan Z, Bae JY, Kevan L (2000) Vanadosilicate mesoporous SBA-15 molecular sieves incorporated with N-alkylphenothiazines. Chem Mater 12(10):3202–3207
Thunyaratchatanon C, Luengnaruemitchai A, Chaisuwan T et al (2017) Synthesis and characterization of Zr incorporation into highly ordered mesostructured SBA-15 material and its performance for CO2 adsorption. Microporous Mesoporous Mater 253:18–28
Qu Z, Shen S, Chen D et al (2012) Highly active Ag/SBA-15 catalyst using post-grafting method for formaldehyde oxidation. J Mol Catal 356:171–177
Zhao J, Yuan H, Qin X et al (2020) Au nanoparticles confined in SBA-15 as a highly efficient and stable catalyst for hydrogenation of quinoline to 1,2,3,4-tetrahydroquinoline. Catal Lett 150(10):2841–2849
Bibak F, Moradi G (2020) Oxidative desulfurization of model oil and oil cuts with MoO3/SBA-15: experimental design and optimization by Box-Behnken method. React Kinet Mech Catal 131(2):935–951
Badiei A, Goldooz H, Ziarani GM et al (2011) One pot synthesis of functionalized SBA-15 by using an 8-hydroxyquinoline-5-sulfonamide-modified organosilane as precursor. J Colloid Interface Sci 357(1):63–69
Pham XN, Tran DL, Pham TD et al (2018) One-step synthesis, characterization and oxidative desulfurization of 12-tungstophosphoric heteropolyanions immobilized on amino functionalized SBA-15. Adv Powder Technol 29(1):58–65
Barczak M (2018) Synthesis and structure of pyridine-functionalized mesoporous SBA-15 organosilicas and their application for sorption of diclofenac. J Solid State Chem 258:232–242
Nandi M, Mondal J, Sarkar K et al (2011) Highly ordered acid functionalized SBA-15: a novel organocatalyst for the preparation of xanthenes. Chem Commun 47(23):6677–6679
Melero JA, van Grieken R, Morales G (2006) Advances in the synthesis and catalytic applications of organosulfonic-functionalized mesostructured materials. Chem Rev 106(9):3790–3812
Yadav R, Baskaran T, Kaiprathu A et al (2020) recent advances in the preparation and applications of organo-functionalized porous materials. Chem Asian J 15(17):2588–2621
Wu H, Xiao Y, Guo Y et al (2020) Functionalization of SBA-15 mesoporous materials with 2-acetylthiophene for adsorption of Cr(III) ions. Microporous Mesoporous Mater 292:109754
Maryam Hafezian S, Biparva P, Bekhradnia A et al (2021) Amine and thiol functionalization of SBA-15 nanoparticles for highly efficient adsorption of sulforaphane. Adv Powder Technol 32(3):779–790
Usai EM, Sini MF, Meloni D et al (2013) Sulfonic acid-functionalized mesoporous silicas: microcalorimetric characterization and catalytic performance toward biodiesel synthesis. Microporous Mesoporous Mater 179:54–62
Hwang DH, Lee D, Lee H et al (2010) Surface functionalization of SBA-15 particles for ibuprofen delivery. Korean J Chem Eng 27(4):1087–1092
Dindar MH, Yaftian MR, Rostamnia S (2015) Potential of functionalized SBA-15 mesoporous materials for decontamination of water solutions from Cr(VI), As(V) and Hg(II) ions. J Environ Chem Eng 3(2):986–995
Amini M, Naderi R, Mahdavian M et al (2020) Effect of piperazine functionalization of mesoporous silica type SBA-15 on the loading efficiency of 2-mercaptobenzothiazole corrosion inhibitor. Ind Eng Chem Res 59(8):3394–3404
Sadjadi S, Heravi MM, Ebrahimizadeh M (2018) Synthesis of Cu@Fur-SBA-15 as a novel efficient and heterogeneous catalyst for promoting A3-coupling under green and mild reaction conditions. J Porous Mater 25(3):779–788
Berrichi A, Bailiche Z, Bachir R (2022) Mesoporous Au/Fe2O3 catalyst for propargylamines synthesis via CH2Cl2 under visible light irradiation. Res Chem Intermed 48(10):4119–4134
Hajjami M, Ghorbani F, Yousofvand Z (2017) Copper(I) complex of 1,3-dimethylbarbituric acid modified SBA-15 and its catalytic role for the synthesis of 2,3-Dihydroquinazolin-4(1H)-ones and Imidazoles. Appl Organomet Chem 31(12):e3843
Rezanejade Bardajee G, Mahmoodian H, Boraghi SA et al (2023) A facile and efficient synthesis of highly functionalized pyrroles via a four-component one-pot reaction in the presence of Ni(II) Schiff base/SBA-15 heterogeneous catalyst. Res Chem Intermed 49(5):1959–1982
Ziadi Chibane A, Boulcina R, Boulebd H et al (2020) Green one-pot multicomponent synthesis, biological evaluation and theoretical investigations of some novel β-acetamido ketone derivatives as potent cholinesterase inhibitors. Tetrahedron 76(25):131260
Enders D, Moser M, Geibel G et al (2004) (2004) Diastereo-and enantioselective synthesis of differently N, O-protected 1, 3-amino alcohols with three neighbouring stereogenic centers. Synthesis 12:2040–2046
Mukhopadhyay M, Bhatia B, Iqbal J (1997) Cobalt catalyzed multiple component condensation route to β-acetamido carbonyl compound libraries. Tetrahedron Lett 38(6):1083–1086
Fiedler H-P, Kurth R, Langhärig J et al (1982) Nikkomycins: microbial inhibitors of chitin synthase. J Chem Technol Biotechnol 32(1):271–280
Tiwari AK, Kumbhare RM, Agawane SB et al (2008) Reduction in post-prandial hyperglycemic excursion through α-glucosidase inhibition by β-acetamido carbonyl compounds. Bioorganic Med Chem Lett 18(14):4130–4132
Dakin HD, West R (1928) A general reaction of amino acids. II. J Biol Chem 78(3):745–756
Bhatia B, Reddy MM, Iqbal J (1994) Cobalt-catalysed three-component coupling involving ketones or ketoesters, aldehydes and acetonitrile: a novel one-pot synthesis of β-acetamido ketones. J Chem Soc Chem Commun 6:713–714
Tayebee R, Pejhan A (2020) Studying catalytic activity of ternary mixed-metal Keggin H7SiV3W9O40 as a versatile acid catalyst for the synthesis of β-acetamido ketones. Appl Organomet Chem 34(2):e5350
Naeimi H, Rahmatinejad S (2018) Nano magnetite supported phthalocyanine complexes of Cu(II) and Fe(II) as new heterogeneous effective catalysts for synthesis of β-amido ketones. J Coord Chem 71(24):4210–4227
Khojastehnezhad A, Moeinpour F, Javid A (2019) NiFe2O4@SiO2–PPA nanoparticle: a green nanocatalyst for the synthesis of β-acetamido ketones. Polycycl Aromat Compd 39(5):404–412
Bahrami K, Khodaei MM, Fattahpour P (2015) SBA-15-Pr–SO3H: An efficient, environment friendly and recyclable heterogeneous nanoreactor catalyst for the one-pot multicomponent synthesis of β-acetamido ketones. J Chem Sci 127(1):167–172
Wang Y, Zhou J, Liu K et al (2013) Novel bi-SO3H-functionalized ionic liquids based on piperazinium: Highly efficient and recyclable catalysts for the synthesis of β-acetamido ketones. J Mol Catal 366:195–201
Tayebee R, Tizabi S (2012) One-pot four-component dakin-west synthesis of β-acetamido ketones catalyzed by a vanadium-substituted heteropolyacid. Chin J Catal 33(4):923–932
Oskooie HA, Heravi MM, Tahershamsi L et al (2010) Synthesis of new β-acetamido carbonyl derivatives using cellulose sulfuric acid as an efficient catalyst. Synth Commun 40(12):1772–1777
Momeni AR, Sadeghi M (2009) Zr(HSO4)4 and Mg(HSO4)2 as mild and efficient catalysts for the one-pot multicomponent synthesis of β-acetamido carbonyl compounds. Appl Catal A Gen 357(1):100–105
Narayanan KB, Park HH (2015) Homogeneous catalytic activity of gold nanoparticles synthesized using turnip (Brassica rapa L.) leaf extract in the reductive degradation of cationic azo dye. Korean J Chem Eng 32(7):1273–7
Schröder F, Ojeda M, Erdmann N et al (2015) Supported gold nanoparticles as efficient and reusable heterogeneous catalyst for cycloisomerization reactions. Green Chem 17(6):3314–3318
de la Serna VR, Agúndez J, Márquez-Álvarez C et al (2020) Immobilization of gold on short-channel mesoporous SBA-15 functionalized with thiol and hydrophobic groups for oxidation reactions. Catal Today 354:77–89
Bensaad M, Berrichi A, Bachir R et al (2021) Nano and sub-nano gold-cobalt particles as effective catalysts in the synthesis of propargylamines via AHA coupling. Catal Lett 151(4):1068–1079
Zhao D, Huo Q, Feng J et al (1998) Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J Am Chem Soc 120(24):6024–6036
Wang Z, Hao X, Hu D et al (2017) PdAu bimetallic nanoparticles anchored on amine-modified mesoporous ZrSBA-15 for dehydrogenation of formic acid under ambient conditions. Catal Sci Technol 7(11):2213–2220
Liu M, Zhang Q, Shi Y et al (2020) AuPd bimetal immobilized on amine-functionalized SBA-15 for hydrogen generation from formic acid: The effect of the ratio of toluene to DMF. The Canadian Journal of Chemical Engineering 98(4):879–891
Pourhassan F, Khalifeh R, Eshghi H (2021) Well dispersed gold nanoparticles into the multi amine functionalized SBA-15 for green chemical fixation of carbon dioxide to cyclic carbonates under solvent free conditions. Fuel 287:119567
Wisniewska J, Sobczak I, Ziolek M (2021) Gold based on SBA-15 supports—promising catalysts in base-free glucose oxidation. J Chem Eng 413:127548
Didó CA, Caneppele CDG, Schneid AC et al (2018) Small gold nanoparticles with narrow size distribution achieved in SBA-15 pores by using ionic silsesquioxane instead of thiol group as stabilizer and adhesion agent. Microporous Mesoporous Mater 270:48–56
Logunov SL, Ahmadi TS, El-Sayed MA et al (1997) Electron dynamics of passivated gold nanocrystals probed by subpicosecond transient absorption spectroscopy. J Phys Chem B 101(19):3713–3719
Wang C, Shang F, Yu X et al (2012) Synthesis of bifunctional catalysts Al-SBA-15-NH2 with high aluminum content and the catalytic application for different one-pot reactions. Appl Surf Sci 258(18):6846–6852
Li H, She T, Chen G et al (2021) Pd nanoparticles supported on amine-functionalized SBA-15 for the selective hydrogenation of phenol. Mol Catal 504:111493
Berrichi A, Bachir R, Benabdallah M et al (2015) Supported nano gold catalyzed three-component coupling reactions of amines, dichloromethane and terminal alkynes (AHA). Tetrahedron Lett 56(11):1302–1306
Heravi MM, Behbahani FK, Daraie M et al (2009) Fe(ClO4)3· 6H2O: a mild and efficient catalyst for one-pot three component synthesis of β-acetamido carbonyl compounds under solvent-free conditions. Mol Divers 13(3):375–378
Acknowledgements
We thank DGRDST and the University of Tlemcen for the financial of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Conflict of interest the authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hind, Y., Zahra, B., Amina, B. et al. The first gold nanoparticles supported SBA 15 functionalized aminopropylsylane as an efficient catalyst for the synthesis of β-acetamido ketone derivatives. Reac Kinet Mech Cat 137, 813–823 (2024). https://doi.org/10.1007/s11144-023-02552-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02552-3