Abstract
SBA-15-Pr–SO3H catalyzes the multi-component condensation of aromatic aldehydes, ketones and acetonitrile in the presence of acetyl chloride at 80°C to afford β-acetamido ketones in excellent yields. The catalyst can be recovered and recycled for subsequent reactions without any appreciable loss of efficiency.

SBA-15-Pr–SO3H as nanoreactor catalyzes the multicomponent condensation of aromatic aldehydes, ketones, and acetonitrile in the presence of acetyl chloride at 80°C to afford β-acetamido ketones in excellent yields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The demand for structure diversification to synthesize compound libraries for screening in drug discovery is the driving force behind the development of new methodologies and structural motifs.
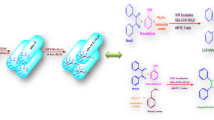
Multi-component reactions are a promising and vital field of chemistry because the synthesis of complex molecules can be accomplished in a very fast, efficient and time saving manner without the isolation of any intermediates and hence it has attracted organic chemists to develop new multi-component reactions[1](figure 1).
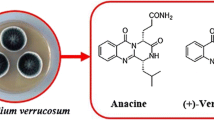
β−Acetamido ketones are versatile intermediates in organic synthesis due to their polyfunctional nature and also their presence in a number of biologically or pharmacologically important compounds.[2,3]These compounds are also useful in the synthesis of other important organic molecules like 1,3-amino alcohols[4,5]units common in natural nucleoside peptide antibiotics, e.g., nikkomycines or neopolyoxins[6,7](figure 2).
Owing to the versatile biological activity of β−acetamido ketones, development of an alternative synthetic methodology is of paramount importance. Earlier reports on the synthesis of β-acetamido ketones through multi-component reactions is mainly by using catalysts such as CoCl2, [8a] FeCl3.6H2O, [8b] montmorillonite K10 clay, [9a] selectfluor, [9b] silica sulphuric acid, [10a] CeCl3.7H2O, [10b] various metal chlorides, [11a] TMSCl, [11b] Borax/POCl3, [12a] solid acid H β-zeolite, [12b] and iodine.
However, in spite of their potential utility, some of these reported methods suffer from some drawbacks such as low yield, prolonged reaction time, use of hazardous and noxious reagents, use of excess amounts of reagents, and incompatibility of sensitive groups to the reaction conditions. In recent years, the development of more economical and environment-friendly conversion processes is gaining interest in the chemical community.
Mesoporous materials have attracted considerable attention since they possess potential applications as catalysts, catalysts supports, adsorbents as well as nanoreactors for making new materials.[13–18]
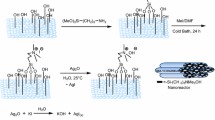
The SBA-15 is nanoporous silica with a hexagonal structure, large pore size, high surface area, high thermal stability and is also diffusion-free due to the thicker pore walls and larger pore size (figure 3). Integration of acidic functional groups (e.g., SO3H) into SBA-15 has been explored to produce promising solid acids. Surface functionalization with sulfonic acid groups was carried out according to the known literature procedure[19]with modification in the oxidation step[20] (scheme 1). Typically, the acid content of SBA-15-Pr-SO3H was measured by ion-exchange pH analysis. The acid amount of SBA-15-Pr-SO3H was determined to be 1 mmolg −1.
As a part of our continued activities in this area,[21] we have investigated a mild and suitable method for the preparation of β-acetamido ketones by multi-component reactions of an aromatic aldehyde, acetyl chloride, ketone and acetonitrile in the presence of sulfonic acid functionalized mesoporous SBA-15 as a heterogeneous recyclable nanoreactor catalyst (scheme 2).
2 Experimental
Products were known and characterized by comparison of their spectral data (NMR) and melting points with those reported in the literature. Monitoring of the reaction was accomplished by TLC on SIL G/UV 254 sheets. All yields refer to isolated products.
2.1 General procedure for synthesis of β-acetamido ketones
A mixture of aromatic aldehyde (1 mmol), acetophenone (1 mmol), acetyl chloride (2 mmol) and SBA-15-Pr–SO3H (0.6 g, 0.6 mmol, –SO3H groups) in acetonitrile (5 mL) was stirred at 80°C. After completion of the reaction as indicated by TLC, the reaction mixture was filtered, and the heterogeneous catalyst was separated. The organic portion was poured into cold water (10 mL). The solid was suction filtered, washed with cold water (20 mL × 2), filtered and recrystallized from ethyl acetate/n-hexane to afford pure β-acetamido ketone in good to excellent yields. The wet catalyst was recycled and no significant change in activity was observed after three cycles.
2.2 Spectral data of some of the products
2.2.1 N-(1-(4-Chlorophenyl)-3-oxo-3-phenylpropyl)acetamide (5g)
1H NMR (CDCl3, 200 MHz): δ 1.94 (s, 3H), 3.39 (dd, 2 J17.0 Hz and 3 J6.1 Hz, 1H), 3.65 (dd, 2 J17.0 Hz and 3 J5.7 Hz, 1H), 5.46 (m, 1H), 7.18–7.52 (m, 8H), 7.83–7.87 (m, 2H).
13C NMR (CDCl3, 50 MHz): δ 22.9, 43.8, 49.0, 127.9, 128.1, 128.4, 128.5, 132.6, 133.3, 136.3, 140.3, 169.7, 197.4.
2.2.2 N-(1-(2-Chlorophenyl)-3-oxo-3-phenylpropyl)acetamide (5h)
1H NMR (CDCl3, 200 MHz): δ 2.10 (s, 3H), 3.52 (dd, 2 J 16.7 Hz and 3 J 5 Hz, 1H), 3.82 (dd, 2 J 16.7 Hz and 3 J 5.8 Hz, 1H), 5.89 (m, 1H), 7.13–7.62(m, 9H), 7.96 (d, J7.4Hz, 2H).
13C NMR (CDCl3, 50 MHz): δ 23.3, 41.4, 48.1, 127.0, 128.1, 128.4, 128.7, 129.9, 132.5, 133.6, 136.4, 138.1, 198.8.
2.2.3 N-(3-(4-Chlorophenyl)-1-(4-chlorophenyl)-3- oxopropyl)acetamide (5i)
1H NMR (CDCl3, 200 MHz): δ 2.01 (s, 3H), 3.36 (dd, 2 J17.0 Hz and 3 J6.1 Hz, 1H), 3.7 (dd, 2 J17.0 Hz and 3 J5.0 Hz, 1H), 5.50 (m, 1H), 6.69 (d, J 7.9 Hz, 1H), 7.41(d, J8.6 Hz, 4H), 7.83 (d, J8.6 Hz, 4H).
13C NMR (CDCl3, 50 MHz): δ 23.4, 43.0, 49.3, 127.9, 128.8, 129.1, 129.5, 133.3, 134.7, 139.3, 140.2, 169.6, 197.0.
2.2.4 N-(1-(3-Nitrophenyl)-3-oxo-3-phenylpropyl)acetamide (5j)
1H NMR (CDCl3, 200 MHz): δ 1.98 (s, 3H), 3.42 (dd, 2 J17.6 Hz and 3 J5.2 Hz, 2H), 3.71 (dd, 2 J17.6 Hz and 3 J8.5 Hz, 2H), 5.54–563(m, 1H), 6.99–8.14 (m, 10H).
13C NMR (CDCl3, 50 MHz): δ 23.0, 42.4, 48.7, 120.9, 122.0, 128.0, 128.4, 129.1, 132.6, 133.5, 135.8, 143.1, 148.0, 169.4, 197.5.
2.2.5 N-(1-(4-Chlorophenyl)-3-(4-nitrophenyl)-3- oxopropyl)acetamide (5k)
1H NMR (CDCl3, 200 MHz): δ 1.70 (s, 3H), 3.40 (dd, 2 J17.0 Hz and 3 J6.0 Hz, 1H), 3.54 (dd, 2 J 17.0 Hz and 3 J 4.5Hz, 1H), 5.43 (m, 1H), 6.51 (s, 1H), 7.21(s, 4H), 8.00 (d, J8.5 Hz, 2H), 8.22 (d, J8.5 Hz, 2H).
13C NMR (CDCl3, 50 MHz): δ 22.9, 43.5, 49.0, 123.6, 127.6, 128.5, 128.8, 133.1, 138.5, 140.3, 150.1, 169.3, 196.1.
3 Results and Discussion
In order to carry out such a multi-component reaction in a more efficient way, some experimentation varying the molar ratio of the reactants, reaction temperature, reaction time and possible solvents were conducted. The optimized condition utiliziing a 1:1:2 ratio of aldehyde, ketone and acetyl chloride in a one-pot reaction employing refluxing acetonitrile (reagent as well as solvent), 0.6 equivalent of propylsulfonic acid functionalized mesoporous silica (SBA-15-Pr–SO3H) with respect to the aldehyde suffices for complete conversion of the starting materials.
The reaction remains incomplete if lower amounts of catalyst are used. The effect of temperature was studied by carrying out the model reaction in the presence of SBA-15-Pr–SO3H (0.6 g, 0.6 mmol, –SO3H groups) in acetonitrile at different temperatures (room temperature, 60 and 80°C). It was observed that the time reaction was decreased and the yield was increased as the reaction temperature was raised. From these results, 80°C is selected as the best temperature for all subsequent studies. It is noteworthy that, increasing the amount of SBA-15-Pr–SO3H and time did not increase the yield of product in the model reaction.
The used catalyst could be washed with dichloromethane and subjected to a second run of the reaction process with benzaldehyde and acetophenone. The results of the first experiment and subsequent experiments were almost consistent in yields (93, 94 and 91% after three runs). Thus, the catalyst can be recycled several times with negligible loss of catalytic activity and there is no need for regeneration of the catalyst.
To show the generality of this method, a wide range of aldehydes and acetophenones were subjected to optimized reaction conditions and the results are summarized in table 1. It is evident from table 1 that this method is effective for the preparation of β-acetamido ketones from both aldehydes as well as acetophenone derivatives. Aromatic aldehydes or acetophenones with activating and deactivating groups underwent smooth transformation to the corresponding β-acetamido ketones, without the formation of any side products, in high to excellent yields and reasonable reaction times, emphasizing the generality of our methodology. The results also showed that the reaction times for the substrates containing electron withdrawing groups are slightly longer. Several functionalities such as bromo, chloro, hydroxyl, methoxy and nitro were compatible with this procedure. The experiment for this reaction is remarkably simple and requires no inert atmosphere. Anyway, this protocol has its limitations, for example, it produces low yields for aliphatic aldehydes (table 1, entry 15).

The suggested mechanism for this reaction is presented in scheme 3. It is suggested that the first protonation of the aldehyde produces intermediate I which then reacts with acetonitrile to produce intermediate II. Next, the enol form of acetophenone derivative attacks II to give III. Hydrolysis of III with elimination of acetate gave the desired β-acetamido ketone.
A comparison of the efficiency of this method to previously selected methods is gathered in table 2. As can be seen, the present protocol is indeed superior to several other protocols.
4 Conclusion
We have described an efficient method for the one-pot synthesis of β-acetamido ketones using an environment friendly, heterogeneous nanoreactor catalyst. Simplicity, excellent yield of products, reusability of the catalyst, and clean reaction conditions are established. This methodology also overcomes the formation of unwanted by-product. Thus, we believe that the present methodology opens up new possibilities for medicinal chemistry and material sciences, and can prove to be an important addition to the existing methodologies. Further investigations into the scope and synthetic applications of this nanoreactor catalyst are currently under investigation in our laboratory.
References
(a) Terret N K, Gardner M, Gordon D W, Kobylecki, R J and Steele J 1995 Tetrahedron 51 8135; (b) Armstrong R W, Combs A P, Tempest P A, Brown S D and Keating T A 1996 Acc. Chem. Res. 29 123; (c) Thomson L A and Ellman J A 1996 Chem. Rev. 96 555; (d) Tietze L F and Lieb M E 1998 Curr. Opin. Chem. Biol. 2 363; (e) Domling A and Ugi I 2000 Angew. Chem. Int. Ed. 39 3168
Casimir J R, Turetta C, Ettouati L and Paris J 1995 Tetrehedron Lett. 36 4797
Godfrey A G, Brooks D A, Hay L A, Peters M, McCarthy J R and Mitchell D 2003 J. Org. Chem. 68 2623
Barluenga J, Viado A L, Aguilar E, Fustero S and Olano B 1993 J. Org. Chem. 58 5972
Enders D, Moser M, Geibel G and Laufer M C 2004 Synthesis 2040
Dahn U, Hagenmaier H, Hohne H, Konig W A, Wolf G and Zahner H 1976 Arch. Microbiol. 107
Kobinata K, Uramoto M, Nishii M, Kusakabe H, Nakamura G and Isono K 1980 Agric. Biol. Chem. 44 1709
(a) Mukhopadhyay M, Bhatiaand B and Iqbal J 1997 Tetrahedron Lett. 38 1083; (b) Khan A T, Parvin T and Choudhury L H 2007 Tetrahedron 63 5593
(a) Bahulayan D, Das S K and Iqbal J 2003 J. Org. Chem. 68 5735; (b) Shinu V S, Sheeja B, Purushothaman E and Bahulayan D 2009 Tetrahedron Lett. 50 4838
(a) Khodaei M M, Khosropour A R and Fattahpour P 2005 Tetrahedron Lett. 46 2105; (b) Khan A T, Choudhury L H, Parvin T and Ali M A 2006 Tetrahedron Lett. 47 8137
(a) Pandey G, Singh R P, Garg A and Singh V K 2005 Tetrahedron Lett. 46 2137; (b) Mao H, Wan J and Pan Y 2009 Tetrahedron 65 1026
(a) Gholivand K, Jafari H and Adibi H 2011 Synth. Commun. 41 1786; (b) Bhat R P, Vivek P R, Varghese M A, Sachin B P and Samant S D 2005 Tetrahedron Lett. 46 4801; (c) Das B, Reddy K R, Ramu R, Thirupathi P and Ravikanth B 2006 Synlett 1756
(a) Corma A 1997 Chem. Rev. 97 2373; (b) Karimi B and Vafaeezadeh M 2012 Chem. Commun. 48 3327
Li C 2004 Catal. Rev. 46 419
Taguchi A and Schuth F 2005 Microporous Mesoporous Mater. 77 1
Zhao X S, Bao X Y, Guo W P and Lee F Y 2006 Mater. Today 9 32
Wang Y J and Caruso F 2005 Chem. Mater. 17 953
(a) Liu C J, Li S J, Pang W Q and Che C M 1997 Chem. Commun. 65; (b) Kresge C T, Leonowicz M E, Roth W J, Vartuli J C and Beck J S 1992 Nature 359 710; (c) Lin V S-Y, Radu D R, Han M K, Deng W, Kuroki S, Shanks B H and Pruski M 2002 J. Am. Chem. Soc. 124 9040
Yang L M, Wang Y J, Luo G S and Dai Y Y 2005 Microporous Mesoporous Mater. 84 275
Bahrami K, Khodaei M M and Abbasi J 2012 Synthesis 316
Bahrami K, Khodaei M M and Fattahpour P 2011 Catal. Sci. Technol. 1 389
Bhatia B, Reddy M M and Iqbal J 1994 J. Chem. Res. (S) 713
Maiti S and Chakraborty A 2005 Synlett 115
Kumar A, Rao M S, Ahmad I and Khungar B 2009 Aust. J. Chem. 62 322
(a) Rafiee E, Tork F and Johaghani M 2006 Bioorg. Med. Chem. Lett. 16 1221; (b) Yakaiah T, Lingaiah, B P V, Reddy G V, Narsaiah B and Rao P S 2007 Arkivoc viii 227; (c) Momeni A R and Sadeghi M 2009 Appl. Catal. A: Gen. 357 100
Rafiee E, Shahbazi F, Joshaghani M and Tork F 2005 J. Mol. Catal. A: Chem. 242 129
Selvam N P and Perumal P T 2007 Arkivoc 10 265
Maghsoodlou M T, Hassankhani A, Shaterian H R, Habibi-Khorasani S M and Mosaddegh E 2007 Tetrahedron Lett. 48 1729
Tayebee R and Taizabi S 2011 Am. J. Chem. 1 22
Shaterian H R, Hosseinian A and Ghashang M 2008 Synth. Commun. 38 3766
Acknowledgments
We are thankful to the Razi University Research Council for partial support of this work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information
Complete experimental procedures and relevant spectra (1H NMR and 13C NMR spectra) for some compounds (figure S1–S8) are given in Supplementary Information, which is available free of charge in www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
BAHRAMI, K., KHODAEI, M.M. & FATTAHPOUR, P. SBA-15-Pr–SO3H: An efficient, environment friendly and recyclable heterogeneous nanoreactor catalyst for the one-pot multicomponent synthesis of β-acetamido ketones. J Chem Sci 127, 167–172 (2015). https://doi.org/10.1007/s12039-014-0762-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0762-1