Abstract
Herein, we report the synthesis of sulfonic acid functionalized SBA-15 by post-synthetic functionalization of mesoporous SBA-15. The successful incorporation of sulfonic acid moiety into the SBA-15 framework could be confirmed by physico-chemical characterization. The nature of acidic sites was confirmed using temperature-programmed desorption of ammonia. A simple synthetic route for the synthesis of 2-aryl benzimidazoles and benzothiazoles using SBA-15-SO3H as a green heterogeneous catalyst at room temperature was investigated. 100% conversion and an isolated yield of 70–85% could be obtained. The green synthetic approach offers reaction under ambient conditions, a simple work-up procedure, good to excellent yield and easy product isolation along with good recyclability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Green chemistry (also known as sustainable chemistry) is an emerging area of current research targeting the greening of chemical synthesis in the chemical industry. It advocates the invention of environmentally benign chemical processes involving the consumption of renewable resources and eliminating/reducing the generation of waste at the source emphasizing atom/circular economy [1,2,3,4]. Heterogeneous catalysis can go a long way in realizing the goals of sustainable chemistry by providing alternate synthetic strategies involving reusable and recyclable solid catalysts for fine chemical synthesis [5]. Solid acids form eco-friendly substitutes for commonly used hazardous and corrosive homogeneous liquid acid catalysts such as HCl, HNO3, H2SO4, HF etc. [6]. Replacing conventional production with sustainable routes alleviates environmental and economic concerns.
N-containing heterocyclics especially benzimidazoles and benzothiazoles have gained significant interest on account of their broad spectrum of biological activities and potential applications [7,8,9,10,11,12,13,14,15,16,17,18,19,20]. The myriad pharmaceutical applications have contributed to the considerable research focus on the development of new synthetic routes for these compounds. The most common method of synthesis involves the condensation of an arylene diamine or 2-aminothiophenol with a carboxylic acid [21, 22] or its derivatives [23, 24] under vigorous reaction conditions or under microwave irradiation. Another method is the condensation of an aldehyde with arylene diamine or 2-aminothiophenol [25,26,27] and is the extensively followed synthetic approach due to the easy availability of aryl aldehydes. A detailed literature survey of the synthetic approaches for benzimidazoles and benzothiazoles reveals diverse catalytic systems and methodologies employed for their synthesis [7, 22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45].
The discovery of ordered mesoporous materials (pore size 2–50 nm) has been a breakthrough in the field of material chemistry. The versatility of porous silica-based organic–inorganic hybrids catalysts is attributed to the structural robustness of the inorganic framework and functional characteristics of organic moieties [46]. These multi-functional silica-based hybrid catalysts possess diverse types of active sites (acid, base, redox) enabling them to facilitate a whole spectrum of organic transformations [47,48,49,50,51]. SBA-15 is a class of mesoporous silica material having a uniform hexagonal array of mesoporous with narrow pore size distribution. The large surface area in combination with the tunability of pore size and easy functionalization of the silanol groups with acidic, basic and redox moieties renders them widely used materials in heterogeneous catalysis. The introduction of SO3H moieties is reported to lend strong Brønsted acidity assisting the catalytic activity [52, 53]. Sulfonic acid functionalized SBA-15 has been explored extensively for a wide range of catalytic conversions [54,55,56,57,58,59,60,61,62,63,64].
2-Aryl benzimidazole synthesis over mesoporous silica-based materials has been reported in the literature [35, 36, 65,66,67,68,69,70,71,72,73]. Solvent selective formation of di and monosubstituted benzimidazoles over mesoporous silica supported ytterbium catalyst was demonstrated by Samanta et al. [65]. Mesoporous silica supported samarium and CuO was reported as recyclable heterogeneous catalyst for the synthesis 2-substituted benzothiazole [72]. Cobalt-anchored SBA-15 [66] and Al-MCM-41 [70] have been tried for benzimidazole synthesis. The coupling reaction of 2-iodoaniline, potassium sulfide and benzaldehyde over bimetallic Cu-Al supported on SBA-15 could produce benzothiazoles in excellent yields [67]. An organometallic type catalyst prepared through surface modification of silica nanoparticles with 3-chloropropyltriethoxysilane (CPTES) and thiocarbohydrazide (TCH) followed by metal–ligand coordination with Ni (II) for benzimidazole synthesis was reported by Kalhor et al. [64]. Functionalized MCM-41 [67, 71, 74], hexagonal mesoporous silica [69] as catalysts for benzimidazole and thiazole synthesis has also been reported. Further, sulfonic acid functionalized materials have also been explored for benzimidazole synthesis [75,76,77,78,79]. Only isolated reports are available regarding synthesis of 2-aryl benzimidazoles over sulfonic acid functionalized SBA-15. However, to the best of our knowledge, synthesis of benzothiazoles over SBA-15-SO3H has not been reported yet. Herein, we present the catalytic activity of sulfonic acid functionalized mesoporous SBA-15 for the synthesis of 2-arylbenzimidazoles and benzothiazoles. A high yield of isolated products could be obtained under optimized reaction conditions in ethanol with considerable scope for catalyst recyclability.
Experimental
Materials and methods
Pluronic 123, Tetraethyl orthosilicate (TEOS) and 3-mercaptopropyltrimethoxysilane (MPTMS) were purchased from Sigma-Aldrich. Sulfuric acid (H2SO4, 95%), Hydrochloric acid (HCl, 35%) were purchased from Merck. All other chemicals, benzaldehyde, 2-aminothiophenol, o-phenylenediamine, toluene, hydrogen peroxide (30 wt%) were supplied by Spectrochem. All reagents and solvents were of analytical grade and used as such without any further purification.
Synthesis of SBA-15
SBA-15 was prepared based on a slight modification of the procedure reported by Zhao et al. [80]. As reported in our previous work [81], 4.4 g of triblock copolymer P123 was dispersed in 30 mL distilled water and stirred for 1.5 h. To the dispersed solution, 120 g 2 M HCl was added and the stirring was continued for 2 h. This was followed by dropwise addition of 9 g of TEOS under continued stirring for 1 h. The resulting solution was aged for 24 h followed by hydrothermal treatment at 100 °C for 48 h. The precipitate was then filtered, washed with distilled water, dried overnight at 70 °C in a hot air oven and calcined at 450 °C for 8 h.
Functionalization of SBA-15
The functionalization of SBA-15 was done by the post-grafting method. To 0.5 g SBA-15, 5 mL MPTMS and 10 mL toluene were added and refluxed for 6 h at 60 °C. The precipitate was filtered, thoroughly washed with methanol and distilled water and dried overnight at 70 °C to get thiol functionalized SBA-15. The oxidation of thiol functionality was achieved by treatment with 5 mL H2O2 and a drop of H2SO4. The solid obtained was filtered, washed, and dried to get SBA-15-SO3H.
Catalyst characterization
The synthesized catalysts were subjected to structural and morphological characterization. Small angle XRD was recorded on a Bruker AXS D8 Advance powder diffractometer using Cu Kα radiation (wavelength 1.54 Å) source. Fourier transform infrared spectroscopy (FTIR) spectra were obtained from JASCO model 4100 FTIR spectrometer. Thermal stability of the catalytic systems was tested in a Perkin Elmer Diamond TG/DTA analyzer at a heating rate of 10 °C/min under nitrogen atmosphere. Morphological characterization was obtained from SEM and TEM analyses. The TEM images were recorded using a JEOL JEM 2100 microscope with a resolution of 0.24 nm operated under the voltage of 200 kV. SEM–EDX analysis was done in JEOL JSM-6390LV with magnification 5×to 300,000×. The surface area and pore volume characteristics were determined by N2 adsorption/desorption isotherms in a Micromeritics Gemini VI version 3.03 analyser after degassing at 200 °C for 4 h. XPS analysis was conducted on PHI 5000 Versa Probe II, ULVAC-PHI Inc., USA equipped with micro-focused (200 μm, 15 kV) monochromatic Al Kα X-ray source (hν = 1486.6 eV). Both survey spectra and narrow scans (high-resolution spectra) were recorded. Survey scans were recorded with an X-ray source power of 50W and pass energy of 187.85 eV. High-resolution spectra of the major elements were recorded at 46.95 eV pass energy. XPS data were processed using PHI's Multipack software.
General procedure for the synthesis of 2-phenyl benzimidazole/thiazoles
For a typical run, a mixture of o-phenylenediamine (OPD)/2-aminothiophenol (1 mmol) (for benzimidazole/benzothiazole) and benzaldehyde (1 mmol) in 10 ml solvent was taken in a 50 mL flask and stirred at room temperature in presence of catalyst (50 mg) for a definite time interval (Scheme 1). The progress of the reaction was monitored by TLC and the filtrate was subjected to GC–MS analysis in an Agilent 7890A system. The product isolation was achieved by pouring the reaction mixture into chilled water containing ice. The precipitate formed was then filtered, washed, dried and recrystallized from ethanol.
Results and discussion
Characterization of the catalyst
The results of the BET surface area analysis (Table 1) imply the successful incorporation of sulfonic acid moieties into the framework. SBA-15 exhibited a surface area of 697 m2/g which upon functionalization reduced to 568 m2/g. A slight reduction in pore volume and pore diameter was also observed in SBA-15-SO3H indicating the partial filling of the mesopores. This is in line with the previous literature reports [58, 82, 83]. The decrease may also be attributed to the occlusion of pores by propyl sulfonic acid groups inhibiting N2 diffusion. [58]. The enhancement in wall thickness may be attributed to the incorporation of sulfonic acid moieties onto the surface. The N2 adsorption–desorption isotherms (Fig. S1) can be correlated to Type IV with H1 hysteresis loop at relative pressures 0.6 to 0.8 which are characteristics of mesoporous materials. The retention of the hysteresis loop in SBA-15-SO3H indicates the preservation of the mesoporous framework after functionalization.
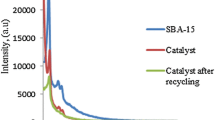
The small angle XRD patterns of the pure SBA-15 (Fig. 1) exhibited three well-resolved peaks at 2θ values 0.99°, 1.55° and 1.9° corresponding to the reflections from (100), (110) and (200) planes. These peaks are the characteristics of mesoporous materials with p6mm hexagonal space group [81]. The XRD pattern remained essentially unchanged in SBA-15-SO3H emphasising the stability of the hexagonally closed-packed mesoporous framework. A reduction in the peak intensity after functionalization is evocative of a small disruption in the long-range order caused by post-grafting treatment and oxidation [55, 56, 59]. Such a lowering of intensity has been reported during the incorporation of other species also [65]. The reduction in intensity may also be a consequence of the lowering of crystallinity during post grafting [57]. It has to be specially mentioned that no considerable alterations were observed in the lattice parameters (Table 1) once again confirming the retention of the mesoporous framework.
The FTIR spectra of SBA-15, thiol functionalized SBA-15 and sulfonic acid functionalized SBA-15 (Fig. 2) exhibited a broad peak around 3450 cm−1 assigned to the O–H stretching vibration of the surface silanol groups and adsorbed moisture. The characteristic vibrations of mesoporous silica could be identified: band at 960 cm−1 (Si–O bending vibration), 800 cm−1 (symmetric stretching vibrations of Si–O–Si) and wide band between 1000 and 1300 cm−1 (asymmetric stretching vibrations of Si–O–Si) [81]. The attachment of the mercaptopropyl group on the silica surface was identified by the bands at 2940 cm−1 and 2850 cm−1 which are due to the asymmetric and symmetric stretching peaks of methylene group. A weak peak around 2600 cm−1 is due to the S–H stretching vibrations [84].
The thermal stability of the as-prepared samples was investigated by TG-DTG analysis (Fig. S2). The weight loss below 130 °C in both cases may be assigned to the removal of physisorbed water. The absence of a significant weight loss in 150–270 °C region confirms the complete removal of the surfactant during calcination process. In the case of sulfonic acid functionalized SBA-15, the weight loss observed beyond 400 °C may be ascribed to the decomposition of the sulfonic acid moiety [54, 55].
The textural and morphological characteristics of the samples were obtained from SEM–EDX (Fig. S3) and TEM analysis (Fig. S4). TEM images reveal the hexagonally aligned mesopores and parallel pore channels which are retained after sulfonic acid functionalization [58, 60, 75]. This is in alignment with the preservation of mesoscopic structure as evidenced by BET and XRD data. EDX analysis reveals a sulfur content of 1.1 wt% in the functionalized sample confirming the inclusion of SO3H moiety into SBA-15 framework.
Effective functionalization of SBA-15 could be further confirmed by XPS analysis (Fig. 3 and S5). The C1s spectra indicate the presence of the propyl group while that of S 2p establishes the inclusion of sulfonic acid moiety. The O1s peak at 532.3 eV may be assigned to Si–O–Si linkage. The binding energy for Si 2p was found to be at 103.7 eV consistent with previous reports [75]. The single peak at a binding energy of 169 eV observed for S 2p confirms the + 6 oxidation state of S which in turn indicates the complete oxidation of the thiol group [56].
Catalytic activity
Synthesis of 2-aryl benzimidazole
The selection of a proper solvent is a crucial step in organic synthesis. A preliminary scanning of the solvent effect was done at room temperature for a time interval of 24 h (Fig. S6). Less polar and aprotic solvents like dichloroethane, tetrahydrofuran and toluene were found to be unsuitable for the reaction whereas polar solvents like ethanol, acetonitrile gave the highest yield of product. Ethanol being a green solvent was selected for further studies. In order to assess the catalytic performance, parallel runs were conducted over unfunctionalized SBA and also para-toluene sulfonic acid (PTSA). Without catalyst, the product yield was negligible while pristine SBA-15 gave a yield of around 40%. The activity of SBA-15 could be assigned to the silanol groups contributing towards acidity. For PTSA and SBA-15-SO3H, the yields obtained were 78% and 85%. The higher activity of SBA-SO3H relative to the PTSA may be attributed to the enhanced surface area and exposed active sites provided by the SBA-15 support. Such an enhancement in activity upon heterogenization is reported in the literature [85,86,87]. To have a more quantitative perspective of the enhancement in activity in terms of the sulfonic acid functionalities, an estimation of sulfonic acid groups was carried out (Table S1). Higher activity of SBA-15-SO3H despite its lesser amount of sulfonic acid moieties may be a consequence of easy access of the reactants to the active sites as compared to PTSA.
The scope and efficiency of the protocol were tested using substituted aldehydes containing both electron releasing and withdrawing groups and a series of 2-aryl benzimidazole derivatives were synthesized. The product formation was confirmed by GCMS analysis (representative chromatograms are provided in Fig. S7) and the isolated product yields are reported in Table 2. In all cases, corresponding 2-aryl benzimidazoles were obtained in 70 to 80% yield indicating that substituent functionality did not have a pronounced impact. The recyclability of the catalyst was also tested and reuse up to five successive runs was observed without significant loss in activity (Fig. 4).
Synthesis of 2-aryl benzothiazole
As in the previous case, the reaction between 2-aminothiophenol and benzaldehyde was carried out with 1:1 molar ratio over 0.05 g catalyst using a series of solvents for a duration of 3 h (Fig. S8). As in the case of benzimidazoles, nonpolar solvents gave poor yields. Acetonitrile was found to be the best solvent giving a yield of 90% while ethanol gave a yield of around 84%. So further reactions were carried out in acetonitrile. The progress of the reaction with time was monitored using acetonitrile as solvent. The product yield increased with time up to 3 h and thereafter no significant change could be observed (Fig. S9).
The scope of the methodology was screened by using a series of substituted aldehydes and the result is tabulated (Table 3). The product formation was confirmed by GCMS analysis and representative chromatograms are included in (Fig. S7). Irrespective of the nature of the substituent a yield in the range 78 to 85% was obtained indicating the generality of the synthetic route. The catalyst could be reused without a significant loss in activity up to five successive runs (Fig. 5).
Before probing into the mechanistic aspects driving the reaction, the acidity of the neat and functionalized catalysts was evaluated temperature programmed desorption of ammonia. The samples were saturated with ammonia gas at room temperature and then subjected to a progressive temperature rise. The categorization of acid sites into weak, moderate and strong depends on the temperature range at which ammonia is desorbed [88, 89]. Weakly adsorbed ammonia is easily desorbed at relatively low temperatures (below 150 °C). Desorption in the interval 150–300 °C may be assigned to moderate acid sites while high temperature desorption indicates strong acidity. The signal obtained in the present case (Fig. 6) reveals the presence of weak acid sites in SBA-15 which contributes towards its mild activity. Incorporation of sulfonic acid groups imparts strong acidic character boosting its activity.
Based on the literature reports [90], a plausible mechanism for SBA-15-SO3H catalyzed formation of 2-aryl benzimidazole or benzothiazole may be sketched (Scheme 2). Sulfonic acid group can facilitate the protonation of the carbonyl oxygen of the aromatic aldehyde. The consequent enhancement in the electrophilicity of the carbonyl carbon improves the ability of aromatic aldehyde to form a Schiff base intermediate by reacting with amino group of o-phenylenediamine or 2-aminothiophenol. The second NH2 group of o-phenylenediamine or the SH group of 2-amino thiophenol then donates a lone pair of electron to the intermediate resulting in the formation of a five-membered ring via intramolecular ring closing. The deprotonation of positively charged N or S by the negatively charged catalyst species regenerates the catalyst. On air oxidation, the intermediate thus formed yields the corresponding 2-aryl benzimidazole or benzothiazole. A comparative evaluation of the activity of our catalytic system with available literature reports for the synthesis of 2-aryl benzimidazoles and thiazoles is provided (Table S2).
Conclusion
To summarize, we present here a simple synthetic route for the 2-aryl benzimidazoles and benzothiazoles catalyzed by sulfonic acid functionalized SBA-15 under ambient conditions. The catalyst was found to be highly stable and could be reused up to five successive runs without a significant loss in activity. The product isolation was quite simple avoiding any tedious work up strategy.
References
Rogers L, Jensen KF (2019) Continuous manufacturing–the green chemistry promise? Green Chem 21(13):3481–3498. https://doi.org/10.1039/c9gc00773c
Zimmerman JB, Anastas PT, Erythropel HC, Leitner W (2020) Designing for a green chemistry future. Science 367(6476):397–400. https://doi.org/10.1126/science.aay3060
Loste N, Chinarro D, Gomez M, Roldan E, Giner B (2020) Assessing awareness of green chemistry as a tool for advancing sustainability. J Clean Prod 256:120392. https://doi.org/10.1016/j.jclepro.2020.120392
Sheldon RA (2018) Metrics of green chemistry and sustainability: past, present and future. ACS Sustain Chem Eng 6(1):32–48. https://doi.org/10.1021/acssuschemeng.7b03505
Clark JH (2001) Catalysis for green chemistry. Pure Appl Chem 73(1):103–111. https://doi.org/10.1351/pac200173010103
Gupta P, Paul S (2014) Solid acids: green alternatives for acid catalysis. Catal Today 236:153–170. https://doi.org/10.1016/j.cattod.2014.04.010
Bai YB, Zhang AL, Tang JJ, Gao JM (2013) Synthesis and antifungal activity of 2-chloromethyl-1 H-benzimidazole derivatives against phytopathogenic fungi in vitro. J Agric Food Chem 61(11):2789–2795. https://doi.org/10.1021/jf3053934
Raut CN, Bharambe SM, Pawar YA, Mahulikar PP (2011) Microwave-mediated synthesis and antibacterial activity of some novel 2- (substituted biphenyl) benzimidazoles via Suzuki-Miyaura cross coupling reaction and their N-substituted derivatives. J Heterocycl Chem 48(2):419–425. https://doi.org/10.1002/jhet.610
Al-Mohammed NN, Alias Y, Abdullah Z, Shakir RM, Taha EM, Hamid AA (2013) Synthesis and antibacterial evaluation of some novel imidazole and benzimidazole sulfonamides. Molecules 18(10):11978–11995. https://doi.org/10.3390/molecules181011978
Arora RK, Kaur N, Bansal Y, Bansal G (2014) Novel coumarin–benzimidazole derivatives as antioxidants and safer anti-inflammatory agents. Acta Pharm Sinica B 4(5):368–375. https://doi.org/10.1016/j.apsb.2014.07.001
Kuş C, Ayhan-Kılcıgil G, Özbey S, Kaynak FB, Kaya M, Çoban T, Can-Eke B (2008) Synthesis and antioxidant properties of novel N-methyl-1, 3, 4-thiadiazol-2-amine and 4-methyl-2H-1, 2, 4-triazole-3 (4H)-thione derivatives of benzimidazole class. Bioorg Med Chem 16(8):4294–4303. https://doi.org/10.1016/j.bmc.2008.02.077
Alasmary FA, Snelling AM, Zain ME, Alafeefy AM, Awaad AS, Karodia N (2015) Synthesis and evaluation of selected benzimidazole derivatives as potential antimicrobial agents. Molecules 20(8):15206–15223. https://doi.org/10.3390/molecules200815206
Camacho J, Barazarte A, Gamboa N, Rodrigues J, Rojas R, Vaisberg A, Gilman R, Charris J (2011) Synthesis and biological evaluation of benzimidazole-5-carbohydrazide derivatives as antimalarial, cytotoxic and antitubercular agents. Bioorg Med Chem 19(6):2023–2029. https://doi.org/10.1016/j.bmc.2011.01.050
Huang ST, Hsei IJ, Chen C (2006) Synthesis and anticancer evaluation of bis (benzimidazoles), bis (benzoxazoles) and benzothiazoles. Bioorg Med Chem 14(17):6106–6119. https://doi.org/10.1016/j.bmc.2006.05.007
Hutchinson I, Chua MS, Browne HL, Trapani V, Bradshaw TD, Westwell AD, Stevens MF (2001) Antitumor benzothiazoles. 14. Synthesis and in vitro biological properties of fluorinated 2-(4-aminophenyl) benzothiazoles. J Med Chem 44(9):1446–1455. https://doi.org/10.1021/jm001104n
Singh M, Singh SK, Gangwar M, Nath G, Singh SK (2014) Design, synthesis and mode of action of some benzothiazole derivatives bearing an amide moiety as antibacterial agents. RSC Adv 24(36):19013–19023. https://doi.org/10.1039/C4RA02649G
Moreno-Díaz H, Villalobos-Molina R, Ortiz-Andrade R, Díaz-Coutiño D, Medina-Franco JL, Webster SP, Binnie M, Estrada-Soto S, Ibarra-Barajas M, Leon-Rivera I, Navarrete-Vázquez G (2008) Antidiabetic activity of N-(6-substituted-1, 3-benzothiazol-2-yl) benzenesulfonamides. Bioorg Med Chem Lett 18(9):2871–2877. https://doi.org/10.1016/j.bmcl.2008.03.086
Singh M, Singh SK, Gangwar M, Nath G, Singh SK (2015) Design, synthesis and mode of action of novel 2-(4-aminophenyl) benzothiazole derivatives bearing semicarbazone and thiosemicarbazone moiety as potent antimicrobial agents. Med Chem Res. https://doi.org/10.1007/s00044-015-1479-5
Nagarajan SR, De Crescenzo GA, Getman DP, Lu HF, Sikorski JA, Walker JL, McDonald JJ, Houseman KA, Kocan GP, Kishore N, Mehta PP (2003) Discovery of novel benzothiazolesulfonamides as potent inhibitors of HIV-1 protease. Bioorg Med Chem 11(22):4769–4777. https://doi.org/10.1016/j.bmc.2003.07.001
Su X, Vicker N, Ganeshapillai D, Smith A, Purohit A, Reed MJ, Potter BV (2006) Benzothiazole derivatives as novel inhibitors of human 11β-hydroxysteroid dehydrogenase type 1. Mol Cell Endocrinol 248(1–2):214–217. https://doi.org/10.1016/j.mce.2005.10.022
Wang R, Lu XX, Yu XQ, Shi L, Sun Y (2007) Acid-catalyzed solvent-free synthesis of 2-arylbenzimidazoles under microwave irradiation. J Mol Catal A Chem 266(1–2):198–201. https://doi.org/10.1016/j.molcata.2006.04.071
Wen X, El Bakali J, Deprez-Poulain R, Deprez B (2012) Efficient propylphosphonic anhydride (® T3P) mediated synthesis of benzothiazoles, benzoxazoles and benzimidazoles. Tetrahedron Lett 53(19):2440–2443. https://doi.org/10.1016/j.tetlet.2012.03.007
Nadaf RN, Siddiqui SA, Daniel T, Lahoti RJ, Srinivasan KV (2004) Room temperature ionic liquid promoted regioselective synthesis of 2-aryl benzimidazoles, benzoxazoles and benzthiazoles under ambient conditions. J Mol Catal A Chem 214(1):155–160. https://doi.org/10.1016/j.molcata.2003.10.064
Matsushita H, Lee SH, Joung M, Clapham B, Janda KD (2004) Smart cleavage reactions: the synthesis of benzimidazoles and benzothiazoles from polymer-bound esters. Tetrahedron Lett 45(2):313–316. https://doi.org/10.1016/j.tetlet.2003.10.168
Yang D, Zhu X, Wei W, Sun N, Yuan L, Jiang M, You J, Wang H (2014) Magnetically recoverable and reusable CuFe2O4 nanoparticle-catalyzed synthesis of benzoxazoles, benzothiazoles and benzimidazoles using dioxygen as oxidant. RSC Adv 4(34):17832–17839. https://doi.org/10.1039/C4RA00559G
Bahrami K, Khodaei MM, Nejati A (2010) Synthesis of 1, 2-disubstituted benzimidazoles, 2-substituted benzimidazoles and 2-substituted benzothiazoles in SDS micelles. Green Chem 12(7):1237–1241. https://doi.org/10.1039/C000047G
Chen YX, Qian LF, Zhang W, Han B (2008) Efficient aerobic oxidative synthesis of 2-substituted benzoxazoles, benzothiazoles and benzimidazoles catalyzed by 4-methoxy-TEMPO. Angew Chem Int Ed 47(48):9330–9333. https://doi.org/10.1002/anie.200803381
Ghosh P, Subba R (2015) MgCl2 6H2O catalyzed highly efficient synthesis of 2-substituted-1H-benzimidazoles. Tetrahedron Lett 56(21):2691–2694. https://doi.org/10.1016/j.tetlet.2015.04.001
Brahmachari G, Laskar S, Barik P (2013) Magnetically separable MnFe2O4 nano-material: an efficient and reusable heterogeneous catalyst for the synthesis of 2-substituted benzimidazoles and the extended synthesis of quinoxalines at room temperature under aerobic conditions. RSC Adv 3(34):14245–14253. https://doi.org/10.1039/C3RA41457D
Naeimi H, Babaei Z (2016) MnO2 nanoparticles as efficient oxidant for ultrasound-assisted synthesis of 2-substituted benzimidazoles under mild conditions. Polycyclic Aromat Compd 36(4):490–505. https://doi.org/10.1080/10406638.2015.1014970
Azizian J, Torabi P, Noei J (2016) Synthesis of benzimidazoles and benzoxazoles using TiCl3OTf in ethanol at room temperature. Tetrahedron Lett 57(2):185–188. https://doi.org/10.1016/j.tetlet.2015.11.092
Shingalapur RV, Hosamani KM (2010) An efficient and eco-friendly tungstate promoted zirconia (WO x/ZrO2) solid acid catalyst for the synthesis of 2-aryl benzimidazoles. Catal Lett 137:63–68. https://doi.org/10.1007/s10562-010-0340-1
Yang ZJ, Gong QT, Yu Y, Lu WF, Wu ZN, Wang N, Yu XQ (2021) Fast and high-efficiency synthesis of 2-substituted benzothiazoles via combining enzyme catalysis and photoredox catalysis in one-pot. Bioorg Chem 107:104607. https://doi.org/10.1016/j.bioorg.2020.104607
Asatkar A, Lambat TL, Mahmood S, Mondal A, Singh M, Banerjee S (2020) Facile protocol for the synthesis of benzothiazole, benzoxazole and N-benzimidazole derivatives using rice husk derived chemically activated carbon. Mater Today Proc 29:738–742. https://doi.org/10.1016/j.matpr.2020.04.510
Sharghi H, Mashhadi E, Aberi M, Aboonajmi J (2021) Synthesis of novel benzimidazoles and benzothiazoles via furan-2-carboxaldehydes, o-phenylenediamines, and 2-aminothiophenol using Cu (II) Schiff-base@ SiO2 as a nanocatalyst. Appl Organomet Chem 35(9):e6330. https://doi.org/10.1002/aoc.6330
Zakeri M, Moghadam M, Mirkhani V, Tangestaninejad S, Mohammadpoor-Baltork I, Pahlevanneshan Z (2018) Copper containing nanosilica thioalated dendritic material: a recyclable catalyst for synthesis of benzimidazoles and benzothiazoles. Appl Organomet Chem 32(1):e3937. https://doi.org/10.1002/aoc.3937
Nasr-Esfahani M, Mohammadpoor-Baltork I, Khosropour AR et al (2013) Synthesis and characterization of Cu(II) containing nanosilica triazine dendrimer: a recyclable nanocomposite material for the synthesis of benzimidazoles, benzothiazoles, bis-benzimidazoles and bis-benzothiazoles. J Mol Catal A Chem 379:243–254. https://doi.org/10.1016/j.molcata.2013.08.009
Prajapti SK, Nagarsenkar A, Guggilapu SD, Babu BN (2015) B(C6F5)3 as versatile catalyst: an efficient and mild protocol for the one-pot synthesis of functionalized piperidines and 2-substituted benzimidazole derivatives. Tetrahedron Lett 56:6795–6799. https://doi.org/10.1016/j.tetlet.2015.10.074
Nguyen TT, Nguyen XTT, Nguyen TLH, Tran PH (2019) Synthesis of benzoxazoles, benzimidazoles and benzothiazoles using a brønsted acidic ionic liquid gel as an efficient heterogeneous catalyst under a solvent-free condition. ACS Omega 4:368–373. https://doi.org/10.1021/acsomega.8b02932
Karthik M, Suresh P (2018) Brønsted acidic reduced graphene oxide as a sustainable carbocatalyst: a selective method for the synthesis of C-2-substituted benzimidazole. New J Chem 42:17931–17938. https://doi.org/10.1039/C8NJ03257B
Senapak W, Saeeng R, Jaratjaroonphong J, Sirion U (2018) Brönsted acid-surfactant-combined ionic liquid catalyzed green synthesis of 2-alkyl and 2-arylbenzothiazoles in water: reusable catalyst and metal-free conditions. Mol Catal 458:97–105. https://doi.org/10.1016/j.mcat.2018.06.017
Mobinikhaledi A, Moghanian H, Ghazvini SMBH, Dalvand A (2018) Copper containing poly(melamine-terephthaldehyde)-magnetite mesoporous nanoparticles: a highly active and recyclable catalyst for the synthesis of benzimidazole derivatives. J Porous Mater 25:1123–1134. https://doi.org/10.1007/s10934-017-0524-9
Sharma P, Gupta M, Kant R, Gupta VK (2015) Formation of a nanorod shaped ionogel and its high catalytic activity for one-pot synthesis of benzothiazoles. New J Chem 39:5116–5120. https://doi.org/10.1039/C5NJ00454C
Sankar V, Karthik P, Neppolian B, Sivakumar B (2020) Metal–organic framework mediated expeditious synthesis of benzimidazole and benzothiazole derivatives through an oxidative cyclization pathway. New J Chem 44:1021–1027. https://doi.org/10.1039/C9NJ04431K
Garazhian Z, Rezaeifard A, Jafarpour M (2019) A nanoscopic icosahedral Mo 72 Fe 30 cluster catalyzes the aerobic synthesis of benzimidazoles. RSC Adv 9:34854–34861. https://doi.org/10.1039/C9RA06581D
Erigoni A, Diaz U (2021) Porous silica-based organic-inorganic hybrid catalysts: a review. Catalysts 11(1):79. https://doi.org/10.3390/catal11010079
Berdini F, Otalvaro JO, Avena M, Brigante M (2022) Photodegradation of doxycycline in water induced by TiO2-MCM-41. Kinetics, TOC evolution and reusability. Result Eng 16:100765. https://doi.org/10.1016/j.rineng.2022.100765
Kandasamy T, Banu M, Shanthi RV, Sivasanker S (2022) Suitability of different supported Ru, Pt and Ni catalysts for the hydrogenolysis of sorbitol. Result Eng 15:100594. https://doi.org/10.1016/j.rineng.2022.100594
Thahir R, Irwan M, Alwathan A, Ramli R (2021) Effect of temperature on the pyrolysis of plastic waste using zeolite ZSM-5 using a refinery distillation bubble cap plate column. Result Eng 11:100231. https://doi.org/10.1016/j.rineng.2021.100231
Diaz U, Brunel D, Corma A (2013) Catalysis using multifunctional organosiliceous hybrid materials. Chem Soc Rev 42(9):4083–4097. https://doi.org/10.1039/C2CS35385G
Fernandes AE, Jonas AM (2019) Design and engineering of multifunctional silica-supported cooperative catalysts. Catal Today 334:173–186. https://doi.org/10.1016/j.cattod.2018.11.040
Verma P, Kuwahara Y, Mori K, Raja R, Yamashita H (2020) Functionalized mesoporous SBA-15 silica: recent trends and catalytic applications. Nanoscale 12(21):11333–11363. https://doi.org/10.1039/D0NR00732C
Zhao H, Han H (2020) Synthesis and characterization of functionalized SBA-15 silica through template removal. J Solid-State Chem 282:121074. https://doi.org/10.1016/j.jssc.2019.121074
Manayil JC, Inocencio CV, Lee AF, Wilson K (2016) Mesoporous sulfonic acid silicas for pyrolysis bio-oil upgrading via acetic acid esterification. Green Chem 18(5):1387–1394. https://doi.org/10.1039/C5GC01889G
Posada JA, Cardona CA, Giraldo O (2010) Comparison of acid sulfonic mesostructured silicas for 1-butylacetate synthesis. Mater Chem Phys 121(1–2):215–222. https://doi.org/10.1016/j.matchemphys.2010.01.027
Testa ML, La Parola V, Venezia AM (2014) Transesterification of short chain esters using sulfonic acid-functionalized hybrid silicas: effect of silica morphology. Catal Today 223:115–121. https://doi.org/10.1016/j.cattod.2013.09.029
González MD, Cesteros Y, Llorca J, Salagre P (2012) Boosted selectivity toward high glycerol tertiary butyl ethers by microwave-assisted sulfonic acid-functionalization of SBA-15 and beta zeolite. J Catal 290:202–209. https://doi.org/10.1016/j.jcat.2012.03.019
Machado J, Castanheiro JE, Matos I, Ramos AM, Vital J, Fonseca IM (2012) SBA-15 with sulfonic acid groups as a green catalyst for the acetoxylation of α-pinene. Microporous Mesoporous Mater 163:237–242. https://doi.org/10.1016/j.micromeso.2012.07.028
Shah KA, Parikh JK, Maheria KC (2014) Biodiesel synthesis from acid oil over large pore sulfonic acid-modified mesostructured SBA-15: process optimization and reaction kinetics. Catal Today 237:29–37. https://doi.org/10.1016/j.cattod.2014.04.028
Crisci AJ, Tucker MH, Lee MY, Jang SG, Dumesic JA, Scott SL (2011) Acid-functionalized SBA-15-type silica catalysts for carbohydrate dehydration. ACS Catal 1(7):719–728. https://doi.org/10.1021/cs2001237
Sasidharan M, Bhaumik A (2013) Selective conversion of nitroalcohols to nitroolefins over sulfonic acid functionalized mesoporous SBA-15 material. J Mol Catal A Chem 367:1–6. https://doi.org/10.1016/j.molcata.2012.11.006
Oyola-Rivera O, He J, Huber GW, Dumesic JA, Cardona-Martínez N (2022) Catalytic conversion of cellulose to levoglucosenone using propylsulfonic acid functionalized SBA-15 and H2SO4 in tetrahydrofuran. Biomass Bioenergy 156:106315. https://doi.org/10.1016/j.biombioe.2021.106315
Cheng X, Feng Q, Ma D, Chen H, Zeng X, Xing F, Teng J (2021) Efficient catalytic production of levulinic acid over hydrothermally stable propyl sulfonic acid functionalized SBA-15 in γ-valerolactone-water system. J Environ Chem Eng 9(4):105747. https://doi.org/10.1016/j.jece.2021.105747
Wang L, Zhang L, Li H, Ma Y, Zhang R (2019) High selective production of 5-hydroxymethylfurfural from fructose by sulfonic acid functionalized SBA-15 catalyst. Compos B Eng 156:88–94. https://doi.org/10.1016/j.compositesb.2018.08.044
Samanta PK, Banerjee R, Richards RM, Biswas P (2018) Mesoporous silica supported ytterbium as catalyst for synthesis of 1, 2-disubstituted benzimidazoles and 2-substituted benzimidazoles. Appl Organomet Chem 32(10):e4507. https://doi.org/10.1002/aoc.4507
Rajabi F, De S, Luque R (2015) An efficient and green synthesis of benzimidazole derivatives using SBA-15 supported cobalt nanocatalysts. Catal Lett 145:1566–1570. https://doi.org/10.1007/s10562-015-1546-z
Pei M, Luo X, Tang Q, Huang N, Wang L (2022) The application research on Cu-Al@ SBA-15 bimetallic synergistic effect in the CX bond sequential assembly. Catal Commun 172:106548. https://doi.org/10.1016/j.catcom.2022.106548
Pourhasan-Kisomi R, Shirini F, Golshekan M (2021) Synthetic applications of a new magnetic mesoporous nanocomposite catalyst Fe3O4@ MCM-41@ NH-SO3H. Org Prep Proced Int 53(2):166–175. https://doi.org/10.1080/00304948.2020.1870398
Gholamian F, Hajjami M (2019) Functionalization of hexagonal mesoporous silicas (HMS) for the synthesis of efficient catalyst and investigation of its catalytic activity in the synthesis of 1-amidoalkyl-2-naphthols and 2-substituted benzimidazoles. React Kinet Mech Catal 128:867–884. https://doi.org/10.1007/s11144-019-01663-0
Vasu A, Naresh M, Sai GK, Rohini YD, Murali B, Ramulamma M, Ramunaidu A, Narender N (2021) A heterogeneous catalytic strategy for facile production of benzimidazoles and quinoxalines from primary amines using the Al-MCM-41 catalyst. Green Chem 23(23):9439–9446. https://doi.org/10.1039/D1GC02627E
Mahdavinia GH, Rostamizadeh S, Amani AM, Sepehrian H (2012) Fast and efficient method for the synthesis of 2-arylbenzimidazoles using MCM-41-SO3H. Heterocycl Commun 18(1):33–37. https://doi.org/10.1515/hc-2011-0056
Samanta PK, Biswas R, Das T, Nandi M, Adhikary B, Richards RM, Biswas P (2019) Mesoporous silica supported samarium as recyclable heterogeneous catalyst for synthesis of 5-substituted tetrazole and 2-substituted benzothiazole. J Porous Mater 26:145–155. https://doi.org/10.1007/s10934-018-0626-z
Kalhor M, Rezaee-Baroonaghi F, Dadras A, Zarnegar Z (2019) Synthesis of new TCH/Ni-based nanocomposite supported on SBA-15 and its catalytic application for preparation of benzimidazole and perimidine derivatives. Appl Organomet Chem 33(5):e4784. https://doi.org/10.1002/aoc.4784
Pesyan NN, Batmani H, Havasi F (2019) Copper supported on functionalized MCM-41 as a novel and a powerful heterogeneous nanocatalyst for the synthesis of benzothiazoles. Polyhedron 158:248–254. https://doi.org/10.1016/j.poly.2018.11.005
Yadav P, Kakati P, Singh P, Awasthi SK (2021) Application of sulfonic acid fabricated cobalt ferrite nanoparticles as effective magnetic nanocatalyst for green and facile synthesis of benzimidazoles. Appl Catal A Gen 612:118005. https://doi.org/10.1016/j.apcata.2021.118005
Swami MB, Jadhav AH, Mathpati SR, Guge HG, Patil SG (2017) Eco-friendly highly efficient solvent free synthesis of benzimidazole derivatives over sulfonic acid functionalized graphene oxide in ambient condition. Res Chem Intermed 43:2033–2053. https://doi.org/10.1007/s11164-016-2745-y
Goswami M, Dutta MM, Phukan P (2018) Sulfonic-acid-functionalized activated carbon made from tea leaves as green catalyst for synthesis of 2-substituted benzimidazole and benzothiazole. Res Chem Intermed 44:1597–1615. https://doi.org/10.1007/s11164-017-3187-x
Zareyee D, Tuyehdarvary SR, Allahgholipour L, Hossaini Z, Khalilzadeh MA (2016) Catalytic performance of hydrophobic sulfonated nanocatalysts CMK-5-SO3H and SBA-15-Ph-PrSO3H for ecofriendly synthesis of 2-substituted benzimidazoles in water. Synlett 27(08):1251–1254. https://doi.org/10.1055/s-0035-1561354
Mohammadi Ziarani G, Badiei A, Shakiba Nahad M, Ghadim Alizadeh S (2012) Synthesis of 1, 2-disubstituted benzimidazoles in the presence of SBA-Pr-SO3H as a nano solid acid catalyst. J Nanostructures 2(2):213–220. https://doi.org/10.7508/JNS.2012.02.009
Zhao D, Huo Q, Feng J, Chmelka BF, Stucky GD (1998) Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J Am Chem Soc 120(24):6024–6036. https://doi.org/10.1021/ja974025i
Soumini C, Sugunan S, Haridas S (2019) Copper oxide modified SBA-15 for the selective vapour phase dehydrogenation of cyclohexanol to cyclohexanone. J Porous Mater 26:631–640. https://doi.org/10.1007/s10934-018-0658-4
Lourenço JP, Macedo MI, Fernandes A (2012) Sulfonic-functionalized SBA-15 as an active catalyst for the gas-phase dehydration of glycerol. Catal Commun 19:105–109. https://doi.org/10.1016/j.catcom.2011.12.029
Jeenpadiphat S, Björk EM, Odén M, Tungasmita DN (2015) Propylsulfonic acid functionalized mesoporous silica catalysts for esterification of fatty acids. J Mol Catal A: Chem 410:253–259. https://doi.org/10.1016/j.molcata.2015.10.002
Shi X, Wu Y, Yi H, Rui G, Li P, Yang M, Wang G (2011) Selective preparation of furfural from xylose over sulfonic acid functionalized mesoporous Sba-15 materials. Energies 4(4):669–684. https://doi.org/10.3390/en4040669
Van Grieken R, Melero JA, Morales G (2005) Fries rearrangement of phenyl acetate over sulfonic modified mesostructured SBA-15 materials. Appl Catal A 289(2):143–152. https://doi.org/10.1016/j.apcata.2005.04.059
Van Grieken R, Melero JA, Morales G (2006) Etherification of benzyl alcohols with 1-hexanol over organosulfonic acid mesostructured materials. J Mol Catal A: Chem 256(1–2):29–36. https://doi.org/10.1016/j.molcata.2006.04.040
Karimi B, Zareyee D (2008) Design of a highly efficient and water-tolerant sulfonic acid nanoreactor based on tunable ordered porous silica for the von Pechmann reaction. Org Lett 10(18):3989–3992. https://doi.org/10.1021/ol8013107
Wang Y, Wang D, Tan M, Jiang B, Zheng J, Tsubaki N, Wu M (2015) Monodispersed hollow SO3H-functionalized carbon/silica as efficient solid acid catalyst for esterification of oleic acid. ACS Appl Mater Interfaces 7(48):26767–26775
Hermida L, Abdullah AZ, Mohamed AR (2011) Synthesis of monoglyceride through glycerol esterification with lauric acid over propyl sulfonic acid post-synthesis functionalized SBA-15 mesoporous catalyst. Chem Eng J 174(2–3):668–676. https://doi.org/10.1016/j.cej.2011.09.072
Yadav P, Kakati P, Singh P, Awasthi SK (2015) Application of sulfonic acid fabricated cobalt ferrite nanoparticles as effective magnetic nanocatalyst for green and facile synthesis of benzimidazoles. Appl Catal A General 612:118005. https://doi.org/10.1016/j.apcata.2021.118005
Acknowledgements
The authors gratefully acknowledge the financial support from UGC in the form of fellowship to Arun.R. The analysis services provided by SAIF-STIC, CUSAT is gratefully acknowledged. The funding from UGC-SAP, DST-FIST, SMNRI and RUSA 2.0 grants for general facility creation and upgradation is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arun, R., Athira, M.P., Remello, S.N. et al. SBA-15-SO3H catalysed room temperature synthesis of 2-aryl benzimidazoles and benzothiazoles. Reac Kinet Mech Cat 136, 2277–2294 (2023). https://doi.org/10.1007/s11144-023-02464-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02464-2