Abstract
Propylsulfonic acid functionalized Santa Barbara Amorphous-15 (SBA-15–Pr–SO3H) catalyst has been synthesized using a surface modification of mesoporous SBA-15 via the one-pot co-condensation method. The synthesized SBA-15–Pr–SO3H has been characterized by peculiar characterization techniques such as small- and wide-angle XRD, SEM–EDX, TEM, TG–DTA, acidity, FT-IR, Py-FT-IR and BET surface area analysis. The catalytic activity of synthesized catalyst has been studied towards solvent-free MW irradiation for the green and rapid synthesis of multi-substituted imidazoles, [2,4,5-triphenyl-1(H)-imidazole (tri-imidazole) and 1-benzyl-2,4,5-triphenyl-1H-imidazole (tetra-imidazole)]. The SBA-15–Pr–SO3H catalyst was found to be an efficient and recyclable solid acid catalyst and this solvent-free MW protocol afforded products in good to excellent yields of both, tri and tetra imidazoles (> 95%) within shorter reaction time (3 min) at 600 W as compared to the SBA-15 and other existing protocols. The applicability of this protocol was further explored by conducting the experiments in the presence of varied solvents and substituted aldehydes to generate a library of both, tri- and tetra-imidazole scaffolds. The catalyst was found to be reusable up to several runs without loss of its catalytic activity. This report allows the rapid and scalable access to a variety of multi-substituted imidazoles using SBA-15–Pr–SO3H, as heterogeneous catalyst.
Graphical abstract
SBA-15–Pr–SO3H catalyzed solvent-free MW assisted green synthesis of multi-substituted imidazoles via MCRs.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the twenty-first century, microwave (MW) technique is a promising green tool, which has significant benefits in the science arena. MW assisted organic synthesis (MAOS) [1] is an interesting alternative to conventional organic synthesis for its operational simplicity, practicability, higher yields, improved selectivity, clean reaction profiles, etc. MAOS coupled with solvent-free techniques perform an effective and greener alternative to conventional synthesis. Combination of organic synthesis with solvent-free MW technique is one of the most eye-catching developments in environmentally benign organic synthesis, and it contributes even more in terms of “Green Chemistry” aspects. This solvent-free MW technique is used to reduce the reaction time and amount of waste generation. Another advantage is that one can avoid the use of all toxic reagents and/or solvents and can obtain the corresponding ~ 100% conversions and/or yield.
Organic transformations producing heterocyclic (especially N-containing) scaffolds are gaining increasing attention in recent years because of their pharmaceutical significance. Multicomponent reactions (MCRs) [2] are simple condensation reactions and are extensively used in organic transformations for the production of biologically active drug-like molecules, which exhibit a heterocyclic ring system in their ultimate scaffolds. The research on N-containing heterocyclic imidazole core ring system chemistry is quickly expanding because of the vital contribution in biochemical processes and biological properties in diverse areas, such as pharmaceutical drugs, agrochemicals, synthetic materials, supramolecular ligands and bio-mimetic catalysts [3]. Imidazole moiety is a largely used sub-structure and is found in a large number of natural products and pharmacologically active compounds. Multi-substituted imidazole scaffolds are known to maintain NO synthase inhibition and anti-fungal, anti-bacterial, anti-ulcerative, anti-biotic, anti-tumor, anti-mycotic, CB1 receptor antagonistic activities. [4]. They are also well known for their use in photography as photosensitive materials [5].
The synthesis of imidazole scaffolds through MCRs was first reported by Radziszewski, Japp and Robinson, [6, 7] in 1882 for the 3-component reaction (3-CR) starting from 1, 2-dicarbonyl compounds, aldehydes and ammonia to obtain 2,4,5-triphenyl imidazoles, and later, this synthetic approach was modified using amine by Drefahl and Herma [8] for the 4-component reaction (4-CR) to obtain 1,2,4,5-tetrasubstituted imidazoles. During the last decade, there have been several methods reported in the literature for the synthesis of multi-substituted imidazoles, such as hetero-cope rearrangement synthesis, four-component condensation of arylglyoxals, primary amines, carboxylic acids and isocyanides on Wang resin, reaction of N-(2-oxo)-amides with ammonium trifluoroacetate and reaction of N-alkyl-N-(β-keto)-amides with ammonium acetate [9,10,11,12]. The number of high-yielding and efficient protocols have been established for the synthesis of highly substituted imidazoles, which constitute the use of varied protonic/Lewis/Bronsted acids [4, 13], use of microwaves [14, 15], ionic liquids [16, 17], ultrasound irradiation [18], refluxing in acetic acid [19], silica sulfuric acid [20], etc. Many synthetic approaches have been developed for mobilizing and decorating the imidazole core ring with distinct functional groups. Many of these reported protocols have more than one drawback, such as longer reaction times, harsh reaction conditions, expensive reagents and catalysts, low selectivity, tedious workup procedure and purification, strongly acidic condition, low to moderate yield, use of large amounts of catalysts and formation of side products, which ultimately produce the large amounts of toxic waste. Therefore, the development of a catalytic system to overcome these problems and fulfill the criteria of a mild, efficient, and environmentally benign protocol for the synthesis of multi-substituted imidazoles is desirable and is in demand.
In recent years, different mesoporous materials are used in the field of catalysis [4, 13, 21,22,23,24,25]. Among them, Santa Barbara Amorphous-15 (SBA-15) and organically modified SBA-15 are well ordered hexagonal mesoporous silica materials and have been used as efficient solid acid catalysts for a variety of organic transformations because of their acidic properties, high surface area, low toxicity, ease of handling, low cost, high thermal stability and its reusability. Functionalization of the surface of SBA-15 with a –Pr–SO3H group has proven to be a promising alternative to the grafting procedures and is receiving great attention. Their broad applications ranging from adsorbents [26], gas separation [27], drug delivery [28] and catalysis [29] to biological uses are well reported.
In continuation of our interests for development in green synthesis of N-containing heterocyclic scaffolds via MCRs catalyzed by solid acids as a heterogeneous catalyst [4, 13, 21,22,23,24,25], we herein report, the use of –Pr–SO3H functionalized SBA-15 for the preparation of multi-substituted imidazoles under the solvent-free MW irradiation. The aim of this work is to find catalytic activity of SBA-15–Pr–SO3H towards the synthesis of multi-substituted imidazole derivatives under solvent-free MW irradiation via one-pot, 3-CR condensation of benzil, substituted aldehydes and ammonium acetate (NH4OAc) to produce 2,4,5-triphenyl-1(H)-imidazoles (Scheme 1) and 4-CR condensation of benzil, substituted aldehydes, NH4OAc and amine to produce 1-benzyl-2,4,5-triphenyl-1H-imidazoles (Scheme 2). To the author’s knowledge, such a novel synthetic protocol is not reported so far for the synthesis of tri- and tetra-substituted imidazole scaffolds.
Results and discussion
XRD analysis
The small angle X-ray scattering (SAXS) and wide X-ray diffraction (WXRD) patterns of SBA-15 and SBA-15–Pr–SO3H are depicted in Fig. 1. The SAXS of SBA-15 sample showed three well-resolved characteristic diffraction peaks of SBA-15 in the 2θ regions, 0.78°, 1.33° and 1.55° corresponding to the (100), (110) and (200) reflections, respectively. This indicates that the samples have typical two-dimensional hexagonal mesostructures (space group p6mm). The SAXS patterns for SBA-15–Pr–SO3H catalyst did not change after the functionalization process of SBA-15 indicating that the introduction of mercaptopropyl groups and H2O2 oxidation had no significant influence on the structural aspects of the material. The SBA-15–Pr–SO3H also exhibited these three characteristic diffraction peaks of SBA-15 in the low-angle 2θ region, indicating that the ordered mesoporous structure of SBA-15 remained intact after the functionalization with organic compounds. This indicated the stability of two-dimensional hexagonal mesostructure during the functionalization process. Additionally, the negligible decrease in the intensity of d(100) peak of the SBA-15–Pr–SO3H catalyst was found after the functionalization, which may be due to the blocking of pores with organic moieties. Moreover, the WXRD patterns of both materials show a broad peak between the 2θ region, 20°–30°, which reveals the amorphous nature of the catalysts.
N2 sorption and acidity analysis of the catalysts
Figure 2 depicts the textural parameters determined from N2 sorption isotherms and the corresponding BJH pore-size distribution curve of calcined SBA-15 and SBA-15–Pr–SO3H. The catalysts showed the typical Type-IV isotherms and a sharp capillary condensation at higher relative pressure with an H1 hysteresis loop, which is a characteristic of mesoporous materials with uniform cylindrical pores in a narrow pore size distribution. This indicates the 2D hexagonal symmetry of the mesoporous materials with large pore diameters and narrow pore size distribution. Figure 2B shows the corresponding BJH pore size distribution curves and exhibits maximum diameters of 3.25 nm and 3.36 nm for SBA-15 and SBA-15–Pr–SO3H, respectively (Table 1). The BET surface area, pore size and total pore volume of SBA-15 were found to be 514.76 m2 g−1, 3.81 nm, 0.34 cm3 g−1, respectively, and that of SBA-15–Pr–SO3H were found to be 464.14 m2 g−1, 3.74 nm, 0.68 cm3 g−1, respectively. The BET surface area, pore size and a pore wall thickness of functionalized SBA-15 decreased upon grafting it with –Pr–SO3H groups due to the partial accumulation of certain pores of SBA-15 with (3-Mercaptopropyl)-trimethoxysilane (MPTMS) and H2O2. However, the pore volume, pore diameter and unit cell parameter were found to be increased upon functionalization (Table 1), the values being 0.68 cm3 g−1, 5.86 nm, 129.32 nm, respectively.
The total acid sites in catalyst samples were determined by acid–base titration using molar solutions of NaOH and NaCl. In this procedure, 0.2 g of catalyst was added into 50 mL aqueous solution of 2 M NaCl as exchange reagents. The resulting suspension was allowed to equilibrate at room temperature (R. T.) with stirring for 3 h. After separating the catalyst, the solid was separated out, then a few drops of phenolphthalein indicator were added in the liquid portion. The resultant solution titrated against 0.1 M NaOH. The acidity was found using the formula, \( \frac{v\, \times \,N}{W} \), where \( v \) = volume of NaOH, \( N \) = normality of NaOH, \( W \) = wt. of catalyst. As a result, the acidity of the functionalized SBA-15–Pr–SO3H catalyst found higher than the pure SBA-15 due to the accumulation of –Pr–SO3H groups on the surface of SBA-15.
TG-DT analysis
The TG–DTA curves of the SBA-15 and SBA-15–Pr–SO3H are depicted in Fig. 3. The SBA-15 and SBA-15–Pr–SO3H show the obvious two weight losses. The first weight loss of 1.99 and 6.17% occurred up to 200 °C, which is associated with the desorption of surface water present in the mesopores. Then, the second weight loss of 3.20% and 15.23% was observed between 200 and 900 °C, which is attributed to the removal of some unsolvable fraction of Pluronic-123 (P-123) in SBA-15 and the decomposition of the –Pr–SO3H acid group anchored on to SBA-15. In addition, in SBA-15–Pr–SO3H catalyst shows higher % weight loss than SBA-15 due to the presence of a hydrophilic group of –SO3H. Moreover, the absence of any exothermic peak in the DTA curve of SBA-15 and SBA-15–Pr–SO3H beyond 600 °C, confirms the complete removal of organic groups or surfactants. The total weight loss of the SBA-15 and SBA-15–Pr–SO3H up to 900 °C is found to be 5.20 and 21.40%, respectively, and residue remains is 94.74% and 78.58%, respectively, as shown in their corresponding TGA curves (Fig. 3).
FT-IR and pyridine FT-IR analysis
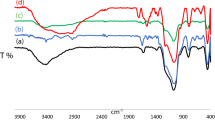
The FT-IR spectrum of SBA-15 and SBA-15–Pr–SO3H are shown in Fig. 4A. A large broadband is observed in the range 3250 cm−1 and 3500 cm−1 for both the samples, corresponding to –OH stretching vibrations due to the presence of surface silanol groups. The band at ~ 1630 cm−1 is observed due to the –OH deformation vibrations and the adsorbed water molecules on to the surface of the materials also show a peak at the same wave number. The peak observed at 1086 cm−1 corresponds to the asymmetric stretching of Si–O–Si bonds of silica walls. The sharp bands at 808 cm−1 and 462 cm−1 are associated with Si–OH and Si–O bond. The several new bands were observed after grafting SBA-15 with –Pr–SO3H acid groups which are as shown in Fig. 4A. The characteristic band of propyl group observed at 1599 cm−1 which is assigned to –CH2 stretching vibrations. Additionally, the band observed at 1174 cm−1 is attributed to –SO3H vibrations which show the presence of sulfonic acid groups. These vibrations of –Pr–SO3H chain indicate the successful anchoring of organic groups on to SBA-15. Further, the bands observed at 2895 cm−1 and 2600 cm−1 are associated with some unreacted –OCH3 and –SH groups of MPTMS. The peaks at 956 cm−1 and 797 cm−1 are observed corresponding to Si–OH and Si–CH3 vibrations. The FT-IR study of SBA-15–Pr–SO3H confirms the presence of essential additional functional groups after the modification of –Pr–SO3H acid on to the surface of SBA-15.
The FT-IR study of the pyridine adsorbed catalysts was also carried out in order to investigate the acidic properties of the catalyst materials. The bands observed at 1540 cm−1 and 1447 cm−1 are assigned to pyridinium ions adsorbed onto Bronsted acidic sites (BAS) and Lewis acidic sites (LAS), respectively, while the band at 1490 cm−1 is attributed to the pyridinium ion adsorbed onto both sites (B + L). Figure 4B shows that the intensities of SBA-15 material are very low, which proves the negligible acidic properties of the material. The result obtained from pyridine FT-IR study of SBA-15–Pr–SO3H has also proved the presence of LAS, BAS and (B + L) with enhanced intensities of their bands. The SBA-15–Pr–SO3H sample exhibits more intense bands at about 1540 cm−1, 1490 cm−1 and 1450 cm−1, indicating higher acidity of the SBA-15–Pr–SO3H than SBA-15 sample. This fact is also evidenced by the results obtained from acidity measurement of these materials by acid–base titration.
SEM–EDX analysis
The morphology of SBA-15 and SBA-15–Pr–SO3H was examined by SEM and the resultant images are shown in Fig. 5A, B. The SEM image of commonly siliceous SBA-15 catalyst was found to consist of rod or cylindrical like morphology. The same morphology was found to be retained in SEM of SBA-15–Pr–SO3H indicating no change in SBA-15 morphology after surface modification. The EDX analysis was also performed for both the materials, and the data are shown in Fig. 5C, D, which confirms the incorporation of –SO3H groups on the surface of SBA-15. The weight and atom percentage of sulfur in SBA-15–Pr–SO3H are found to be 4.21% and 2.28%, respectively, as shown in Fig. 5D (inset).
TEM analysis
The shape and surface properties of both solid acid catalysts were also characterized by TEM technique. The TEM images of SBA-15 and SBA-15–Pr–SO3H materials are shown in Fig. 6. The TEM images of the SBA-15 sample exhibited the well-ordered hexagonal arrays of mesostructures in one-dimensional channels (Fig. 6B). After the surface modification, –Pr–SO3H acid groups are incorporated into the SBA-15 silica while keeping the highly-ordered hexagonal mesostructure of SBA-15 intact. The TEM image shows a well-defined and ordered mesoporosity with highly parallel channels, which is similar to the pore structure of SBA-15 (Fig. 6B). This indicates the retention of the pore structure of SBA-15–Pr–SO3H during one-pot co-condensation reactions [30].
Synthesis of 3-CR and 4-CR of imidazole scaffolds
Initially, to determine the role of synthesized catalysts, an attempt has been made to carry out the imidazole synthesis in the absence of any catalyst under ethanol reflux conditions (at 75 °C). However, this attempt has not yielded the desired product even after a prolonged reaction time (Table 2, entry 1). To demonstrate the essential effect of SBA-15–Pr–SO3H on the reaction progress, in the next experiment, the catalytic performances of pure SBA-15 was also checked for the model reaction under the same reaction conditions (Table 2, entry 2). In this case, very poor yields were obtained, even after a prolonged reaction time. Then the reactions were carried out in the presence of SBA-15–Pr–SO3H catalyst and in this case, we succeeded in getting the desired product with a higher 90% yield (Table 2, entry 3).
In addition, to optimize the reaction conditions, the one-pot synthesis of 3-CR of imidazoles (4a) was investigated as a model reaction using the reaction mixture of benzil (1 mmol), benzaldehyde (1 mmol), NH4OAc (4 mmol) with varied stoichiometric amounts of SBA-15–Pr–SO3H (1, 3, 5 up to 10 wt%) catalyst under ethanol reflux and thermal solvent-free conditions. The best result was found with 3 wt% of SBA-15–Pr–SO3H under thermal solvent-free conditions (Table 2, entry 4). It can be seen that increasing in catalyst loading beyond 3 wt% resulted in the negligible decrease in the % yield, as some amount of the products may adsorb on the active catalytic sites, thereby leading to a decrease in active sites, for the new reagent to be adsorbed (Table 2, entry 5–7).
In the subsequent study, the reaction temperature range varied from the medium (70 °C) to the high (130 °C) reflux temperature (Table 2, entries 8–13) to find the effect of temperature on the reaction under solvent-free conditions. The result shows that as the temperature is increased, the % yield of the product is increasing within a reduced time period. Finally, the best result was obtained with 1:4:1 molar ratio between benzil, NH4OAc, benzaldehyde and 3 wt% of SBA-15–Pr–SO3H under the solvent-free conditions at 120 °C with 95% yield within 10 min (Table 2, entry 4). The reaction was also carried out with different polar and non-polar solvents to assess the solvent effect on the reaction rate. The use of different solvents afforded the desired product in lower yields (Table 2, entries 14–21) as compared to ethanol (Table 2, entry 4). In addition, it has been also observed that the solvents such as water, tetrahydrofuran and dichloromethane do not give reaction because NH4OAc was not dissolved in the reaction even after 120 min. The best result was in case of ethanol refluxing conditions (at 75 °C) in the presence of 3 wt% of SBA-15–Pr–SO3H for the reaction medium. Thus, the results clearly revealed that the efficiency of the reaction is mainly affected by the amount of catalyst, temperature and solvent.
To further explore the potential of this protocol for heterocyclic synthesis, an attempt was also made to carry out synthesis of 4-CR imidazoles (5a), to generate model compound using the reaction mixture of benzil (1 mmol), benzaldehyde (1 mmol), NH4OAc (4 mmol), amine (1 mmol) and SBA-15–Pr–SO3H following the reaction condition of 3-CR. The best result was obtained with 1:1:4:1 (benzil: benzaldehyde: NH4OAc: amine) molar ratio and 3 wt% of SBA-15–Pr–SO3H under solvent-free conditions at 120 °C with 97% yield within 10 min (Table 2, entry 4).
Synthesis of highly substituted imidazoles
In order to make the process even more environmentally benign, it was thought of interest to make use of MW irradiation using solvent-free condition.
To extend the scope of the reactions and to generalize the procedure, a comparison was made between the results obtained by using solvent-free MW irradiation and thermal solvent-free conditions (Table 3). For the same, the reactions were performed with solvent-free MW irradiation under varied power (100, 300, 450, 600 and 800 W). The successful results were obtained with 1:4:1 (benzil: NH4OAc: benzaldehyde) molar ratio and 3 wt% of SBA-15–Pr–SO3H under MW solvent-free irradiation conditions at 600 W with 96% yield within 3 min for 3-CR (Table 3, entry 4a). In addition, the synthesis of 4-CR has also been carried out and the best result was obtained with 1:4:1:1 (benzil: NH4OAc: benzaldehyde: amine) molar ratio and 3 wt% of SBA-15–Pr–SO3H under MW solvent-free irradiation conditions at 600 W with 99% yield within 3 min (Table 3, entry 5a). It has been observed that MW irradiation technique has shown high % yields with very short reaction time as compared to the thermal solvent-free conditions.
The reactions were also investigated using a variety of substituted aldehydes to synthesize a wide range of multi-substituted imidazole derivatives in the presence of SBA-15–Pr–SO3H under both the optimized reaction conditions (entry 4a–4h and 5a–5g). The aromatic and aliphatic aldehydes were converted to the corresponding products in good yields within shorter reaction time. The time for maximum conversion into products and the % yields of the products are listed in Table 3. Also, it is observed that the presence of electron-withdrawing substituents such as, –Cl, –Br and –NO2 on the aromatic rings of the aldehydes underwent condensation in short reaction times with excellent % yields in both conditions (Table 3, entry 4b–4d, 5c, 5e) as compared to the electron-releasing substituents such as –CH3, –OCH3 and –OH (Table 3, entry 5b, 5d, 5g). The procedure worked well for all the aromatic substituted aldehyde derivatives for both the reactions without the formation of any side products such as oxidized products of anilines or aldehydes, which are usually observed under the influence of strong acids. Moreover, the aliphatic aldehyde substituents gave poor yields as compared to aromatic aldehydes substituents (Table 3, entry 4g, 4h, 5f, 5g).
Reusability of SBA-15–Pr–SO3H catalyst for the synthesis of imidazole scaffolds
The recyclability study of the catalyst is a significant feature to satisfy the green chemistry principle. In order to investigate the reusability of SBA-15–Pr–SO3H catalyst for the 3-CR of the model compound, the reaction was carried out under optimized reaction conditions using solvent-free MW irradiation at 600 W for 3 min. The catalyst was recovered from the reaction mixture by the addition of ethanol followed by vacuum filtration. The separated recovered catalyst was dried overnight at R. T. and then heated at 100 °C for 2 h for next use. The process was repeated up to five catalytic runs and the results are depicted in Fig. 7. No significant decrease in the activity and not much change in the yield of 2, 4, 5-triphenyl-1(H)-imidazole is observed. The SBA-15–Pr–SO3H catalyzed one-pot, 3-CR for the synthesis of tri-substituted imidazole afforded 96%, 96%, 96%, 95% and 95% yield, respectively, for five consecutive runs. Similar studies for the 4-CR in the synthesis of tetra-imidazoles was also carried out and led to the conclusion that the catalyst is reusable without considerable loss in its activity. The yield for the tetra-imidazoles was found to be 99%, 99%, 98%, 97% and 96%, respectively, for five runs and is shown in Fig. 7. In addition, to prove the catalyst activity, the SAXS patterns of fresh and spent catalyst (after five runs) show that the catalyst preserved its mesoporosity throughout the reaction and hence can be reused.
Experimental
Materials and method
All reagents were used of AR grade. Tetraethyl orthosilicate (TEOS, 98%), P-123, MPTMS (95%) and substituted aldehydes were procured from Sigma and Aldrich (Surat, India) chemical companies. H2O2 (30%), benzil, NH4OAc, amines and solvents were procured from Finar and Merck (Surat, India) chemical companies in the purest form and were used without further purification. MW irradiation was performed in a domestic MW oven (Samsung, model CE73JD, 800-watt output power). Reactions were performed by precoated Merck silica gel 60F254 TLC, using ethyl acetate: n-hexane as developing solvents, and the spot was detected by UV light absorption by placing in an iodine chamber. The organic products were characterized after purification by spectral analysis (M.P., UV, MASS, FT-IR, 1H and 13C NMR and elemental analysis (C, H, N). M. P. was determined using open capillary tubes and are uncorrected. UV measurements were recorded on a Cary 50 Varian UV–vis spectrophotometer at R. T. using quartz cells with 1.0 cm path length in ethanol medium. MASS spectra were recorded at an ionization potential of 10–30 eV on Waters Micromass ZQ™ 400 mass spectrometer. FT-IR spectrums were recorded on a DRS (8400-S-Shimadzu) FT-IR spectrophotometer using KBr pellets and 30 scans were acquired for each spectrum. 1H and 13C NMR spectra were recorded on a Brucker-400 III Avance spectrometer in DMSO solvent at 400 MHz and 100 MHz, respectively, using trimethyl saline (TMS) as an internal standard. C, H, N analysis was carried out on Elementar, Vario Micro Cube CHN analyzer.
Catalyst preparation
Synthesis of SBA-15
The siliceous SBA-15 material was synthesized as per reported method by one-pot co-condensation using P-123 as a structure-directing agent and TEOS as a source of silica under acidic conditions [30]. In the one-pot synthesis, 4 g of P-123 tri-block copolymer surfactant was dissolved in 125 g of 1.9 M HCl at R. T. with continuous stirring. The resultant solution was subsequently heated to 313 K, and then TEOS was added drop-wise with continuously stirring for 24 h at 313 K. Thereafter, the material was aged in a tightly capped Teflon coated autoclave reactor for 24 h at 403 K under static conditions. The product was filtered and dried at 120 °C for 3 h. P-123 was removed by performing soxhlet extraction using ethanol as a solvent for 24 h. The resultant products were collected and dried at 120 °C for 4 h and stored in vacuum desiccators in order to avoid contact with moisture.
Synthesis of SBA-15–Pr–SO3H
The SBA-15–Pr–SO3H was synthesized as per reported method by grafting it with the addition of a propyl sulfur group onto the surface of SBA-15 material via a one-pot co-condensation [30]. In a typical one-pot synthesis, 4 gm of P-123 tri-block copolymer surfactant was dissolved in 125 gm of 1.9 M HCl at R. T. with continuous stirring. The resultant solution was consequently heated to 313 K. Then TEOS was added drop-wise and allowed to pre-hydrolyze for approximately 45 min prior to addition of MPTMS-H2O2 solution. The molar composition of the resulting mixture was 0.0369TEOS:0.0062MPTMS:0.0554H2O2. It was continuously stirred for 24 h at 313 K and subsequently aged in a tightly capped Teflon coated autoclave reactor for 24 h at 403 K under static conditions, aiming to obtain, functionalized mesoporous silica with a larger pore width without the addition of the swelling agent. The resultant products were collected and subjected to ethanol reflux under soxhlet extraction for 24 h in order to remove surfactant which is used as a template. The resultant material was filtered, washed with hot ethanol and dried at 373 K for 6 h and stored in vacuum desiccators in order to avoid contact with moisture.
Catalyst characterization and instrumentation
The hexagonal mesoporosity of the catalysts was determined by SAXS on Xeuss (Model C HP100fm) instrument (scan range = 0.5°–5°) and phase of the catalysts was determined by WXRD recorded on Rigaku Miniflex (scan range = 7°–80°) with Cu-Kα radiation (λ = 1.5418 Å) using nickel filter. The BET surface area and BJH pore size distributions were measured using Micromeritics ASAP 2020 model with the multipoint N2 adsorption–desorption method at liquid nitrogen temperature (− 196 °C). Catalyst samples were degassed at 200 °C for 12 h prior to measurement of nitrogen sorption. Acidity was measured by the simple titration method using NaCl. TG–DTA was carried out on a Universal V4.5A TA instruments (TG–DTA Q500 V20.10 Build 36) using thermogravimetric analyzer with a nitrogen flow of 50 mL min−1 and heating speed of 10 °C/min from R. T. to 900 °C. FT-IR spectrum was recorded on a Shimadzu FT-IR-8400-S spectrometer within the spectral range of 4000–400 cm−1 and 4 cm−1 resolution, 30 scans were acquired for each spectrum. The pyridine FT-IR technique was used to find Lewis and Bronsted acidity of the samples and was measured in the region 1400–1550 cm−1 after pyridine adsorption on samples at 110 °C. The spectrums were recorded on the same FT-IR instrument. A morphological study and particular elemental composition of the catalysts has been determined by using SEM–EDX (Hitachi make Model S-3400N). Samples were coated with gold prior analyzing. TEM microphotographs were obtained using a JEOL, JEM 2100 microscope with an acceleration voltage of 200 kV.
General procedure for the 3-CR and 4-CR of imidazole synthesis
Solvent-free synthesis of tri-imidazole and tetra-imidazole scaffolds
The catalysts were activated by heating at 100 °C for 2 h prior to using them. All the reactions were carried out in a 50 mL of round bottom flask (RBF) attached to a condenser and equipped with a magnetic stirrer under heating in an oil bath for solvent-free reactions.
In a typical method of 3-CR synthesis, a mixture of benzil (1 mmol), NH4OAc (4 mmol), substituted aldehydes (1 mmol) and catalyst (3 wt%) were heated at 120 °C with continuous stirring for 10 min, in solvent-free conditions. The reaction mixture was melted initially and suddenly after a few seconds, it began to solidify. The completion of the reaction was monitored by TLC. Upon completion of the reaction, the solid product was allowed to cool at R. T., followed by addition of ethanol. The spent catalyst was collected from the residual solution by filtration under reduced pressure and then washed with ethanol. The crude imidazole solid product was recovered by evaporating the solvent and further purified via recrystallization with hot ethanol to obtain the pure imidazole product having M.P. 272–274 °C (reported M.P. 274–276 °C) in 95% yield for spectral analysis.
For the 4-CR, the same procedure was followed, except benzylamine (1 mmol) was used as an additional fourth component. The pure imidazoles product exhibits M.P. 160–162 °C (reported M.P. 160–165 °C) in 97% yield for spectral analysis.
MW irradiated solvent-free synthesis of tri-imidazoles and tetra-imidazoles
The catalysts were activated by heating at 100 °C for 2 h prior to its use and the reactions were carried out in a beaker (10 ml) for MW irradiation in solvent-free conditions.
In a typical synthesis of imidazoles, initially a mixture [3-CR: benzil (1 mmol), NH4OAc (4 mmol), substituted aldehydes (1 mmol), catalyst (3 wt%) and 4-CR: benzil (1 mmol), NH4OAc (4 mmol), substituted aldehydes (1 mmol), benzylamine (1 mmol), catalyst (3 wt%)] was gently mixed with the help of glass-rod and then the mixture was irradiated for a period of 30 s at a time. The reaction mixture is intermittently shaken between each irradiation. The total period of MW irradiation was 3 min in domestic MW oven at 600 W. The completion of the reaction was monitored by TLC. Upon completion of the reaction, the same procedure was followed as per “Solvent-free synthesis of tri-and tetra-imidazole scaffolds” section. The 3-CR and 4-CR having 96% and 99% yield, respectively. The derived both, 3-CR and 4-CR of imidazoles derivatives (4a and 5a) were confirmed by comparison of their physical and spectral data with those of authentic samples [15, 31, 38,39,40]. Reusability of the catalyst was carried out for each recycles by separating the spent catalyst from the reaction mixture by filtration under reduced pressure, followed by washing with ethanol, drying overnight and at 100 °C for 2 h.
Conclusion
In summary, an attempt has been made to synthesizing SBA-15–Pr–SO3H as an efficient solid acid catalyst and their catalytic applications in the organic transformations. The results demonstrated that the hexagonal structure, large pore volume, pore diameter, wall thickness, acidity and thermal stability are significant properties of this catalyst. The catalyst is stable and highly active to show an environmentally benign character. SBA-15–Pr–SO3H with their promising catalytic properties has shown excellent activity towards the one-pot synthesis of biologically active 3-CR and 4-CR derivatives under a simple and mild solvent-free MW irradiation conditions within shorter reaction time and excellent % yield via MCRs. The aldehyde substitution effects were also investigated and found that electron withdrawing substitution gives better performances in the designing of imidazole derivatives than electron releasing substitution. The key advantages of the presented green methodology are MW technique, clean reaction profiles, environmentally benign procedures, short reaction times, excellent yields, simple purification and crystallization method without the use of column chromatography, high catalytic activity and excellent recyclability and reusability of the catalyst without loss in its activity. The presented combination of multiple green approaches (MAOS, catalyst and MCRs) can be a promising synthetic pathway to afford other industrially important biologically active complex drug-like molecules.
References
J.P. Tierney, P. Lidstrom, B. Wathey, J. Westman, Tetrahedron 57, 9225 (2005)
D.M. D’Souza, T.J.J. Müller, Chem. Soc. Rev. 36, 1095 (2007)
A. Rivera-Hernández, I.S. López-Jimeno, G.A. Carmona-Reyes, R. Alfredo-Toscano, J.G. Penieres-Carrillo, C. Álvarez-Toledano, Tetrahedron Lett. 56, 4829 (2015)
J.J. Gabla, S.R. Mistry, K.C. Maheria, Catal. Sci. Technol. 7, 5154 (2017)
A.R. Karimi, Z. Alimohammadi, M.M. Amini, Mol. Divers. 14, 635 (2010)
B. Radziszewski, Chem. Ber. 15, 1493 (1882)
F. Japp, H. Robenson, Chem. Ber. 15, 1268 (1882)
G. Drefahl, H. Herma, Chem. Ber. 93, 486 (1960)
I. Lantos, W.Y. Zhang, X. Shui, D.S. Eggleston, J. Org. Chem. 58, 7092 (1993)
C. Zhang, E.J. Moran, T.F. Woiwode, K.M. Short, A.M.M. Mjalli, Tetrahedron Lett. 37, 751 (1996)
H. B. Lee, S. Balasubramanian, Org. Lett. 2, 323 (2000)
C.F. Claiborne, N.J. Liverton, K.T. Nguyen, Tetrahedron Lett. 39, 8939 (1998)
J.J. Gabla, S.R. Mistry, K.C. Maheria, J. Catal. Catal. 4, 20 (2017)
S. Balalaie, A. Arabanian, Green Chem. 2, 274 (2000)
M.C. Road, S. Nagar, M.L. Limited, A. Industrial, B. Village, J. Mandal, Rasayan J. Chem. 3, 335 (2010)
H.R. Shaterian, M. Ranjbar, J. Mol. Liq. 160, 40 (2011)
S.A. Siddiqui, U.C. Narkhede, S.S. Palimkar, T. Daniel, R.J. Lahoti, K.V. Srinivasan, Tetrahedron 61, 3539 (2005)
J. Safari, S. Gandomi-ravandi, Z. Akbari, J. Adv. Res. 4, 509 (2013)
J. Wang, R. Mason, D. VanDerveer, K. Feng, X.R. Bu, J. Org. Chem. 68, 5415 (2003)
A. Shaabani, A. Rahmati, J. Mol. Catal. A Chem. 249, 246 (2006)
D. Morawala, A. Dalai, K.C. Maheria,J. Por. Mater. 26, (2018)
S.R. Mistry, K.C. Maheria, J. Catal. Catal. 1, 1 (2014)
S.R. Mistry, K.C. Maheria, J. Mol. Catal. A Chem. 355, 210 (2012)
S.R. Mistry, R.S. Joshi, K.C. Maheria, J. Chem. Sci. 123, 427 (2011)
S.R. Mistry, R.S. Joshi, S.K. Sahoo, K.C. Maheria, Catal. Lett. 141, 1541 (2011)
H. Chaudhuri, S. Dash, A. Sarkar, Ind. Eng. Chem. Res. 56, 2943 (2017)
L. Cao, Z. Jia, S. Ji, J. Hu, J. Nat. Gas Chem. 20, 377 (2011)
V.F. Vavsari, G. Mohammadi Ziarani, A. Badiei, RSC Adv. 5, 91686 (2015)
R.H. Vekariya, H.D. Patel, Synth. Commun. 45, 1031 (2015)
K.A. Shah, J.K. Parikh, K.C. Maheria, Catal. Today 237, 29 (2014)
A. Teimouri, A.N. Chermahini, J. Mol. Catal. A Chem. 346, 39 (2011)
S. Samai, G.C. Nandi, P. Singh, M.S. Singh, Tetrahedron 65, 10155 (2009)
B. Maleki, H. Keshvari Shirvan, F. Taimazi, E. Akbarzadeh, Int. J. Org. Chem. 2, 93 (2012)
K. Babu, V. Surendhar, Hetero. Lett. 4, 235 (2014)
B.F. Mirjalili, A.H. Bamoniri, L. Zamani, Sci. Iran. 19, 565 (2012)
R. Yan, L. Ming, Z. Zong-Ze, Molecules 20, 20286 (2015)
N.M. Kalkhorani, M.M. Heravi,J. Chem. 1 (2013)
H.T. Najmeh Zahedi, A. Javid, M. Kazem Mohammadi, Bull. Chem. Soc. Ethiop. 32, 157 (2018)
K.F. Shelke, S.B. Sapkal, G.K. Kakade, B.B. Shingate, M.S. Shingare, Green Chem. Lett. Rev. 3, 27 (2010)
G.K. Sharma, N.K. Sharma, D. Pathak, Ind. J. Chem. 52B, 266 (2013)
Acknowledgements
The authors are grateful to the director, SVNIT, Surat, for providing research and financial assistance. This research was supported by a research grant for assistant professor No: Dean (R&C)/1503/2013-14 from Sardar Vallabhbhai National Institute of Technology, Surat (SVNIT, Surat). The authors would also like to thank IISER-Mohali, India, for SAXS characterization facility.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gabla, J.J., Lathiya, D.R., Revawala, A.A. et al. Propyl–SO3H functionalized SBA-15: Microwave-mediated green synthesis of biologically active multi-substituted imidazole scaffolds. Res Chem Intermed 45, 1863–1881 (2019). https://doi.org/10.1007/s11164-018-3707-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3707-3