Abstract
The aim of this study was to evaluate the effect of the colonic fermentation of unavailable carbohydrates from unripe banana (mass - UBM - and starch - UBS) over parameters related to glucose and insulin response in rats. Wistar male rats were fed either a control diet, a UBM diet (5 % resistant starch - RS) or a UBS diet (10 % RS) for 28 days. In vivo (oral glucose tolerance test) and in vitro (cecum fecal fermentation, pancreatic islet insulin secretion) analyses were performed. The consumption of UBM and UBS diets by Wistar rats for 28 days improved insulin/glucose ratio. Also, pancreatic islets isolated from the test groups presented significant lower insulin secretion compared to the control group, when the same in vitro glucose stimulation was done. Total short chain fatty acids produced were higher in both experimental groups in relation to the control group. These findings suggest that UBM and UBS diets promote colonic fermentation and can influence glycemic control, improving insulin sensitivity in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unavailable carbohydrates provide moderate blood glucose and insulin responses after the intake of carbohydrate-rich meals, allowing the glucose to enter the blood stream in low rate and extent [1]. These meals have also shown efficacy on the control of satiety, insulin resistance, blood levels of glucose, insulin and lipids [2].
Clinical, laboratorial and observational studies have already proposed that the post-prandial blood glucose should be minimized even in cases of normal levels of fasting glucose concentration [3, 4]. The association between the risk of cardiovascular diseases and hyperglycemia is stronger in relation to the post-prandial blood glucose of 1–2 h than to the fasting glucose levels, both in subjects glucose-tolerant or intolerant [5]. Diets that rapidly increase blood glucose levels not only lead to glycemic and insulin fluctuation but also stimulate the production of several contra-regulatory hormones such as norepinephrine, which causes an impact over insulin sensitivity that depends on time and on the stage of autonomic imbalance [6]. In this context, both quantity and quality of carbohydrates in the diet have been studied, aiming the preservation of beta cells function [7].
The cumulative effect of diets that are source of unavailable carbohydrates may produce low blood glucose response, decreased insulin secretion on the acute post-prandial phase, as well as stimulate the production of short chain fatty acids (SCFA) in the colon. The SCFA produced by the colonic fermentation of unavailable carbohydrates are related to systemic effects on the glucose and lipids metabolisms [8].
Dose–response studies, carried in humans, showed that resistant starch (RS), which is an unavailable carbohydrate, may reduce glucose and/or insulin levels [9]. A study developed by Robertson et al. [10] observed that the consumption of RS for 4 weeks caused a decrease in insulin levels and it suggests that these effects may be related to changes in the SCFA peripheral metabolism. In an in vitro model, the SCFA activated the FFAR2 (free fat acid receptor 2), which promote the release of GLP1 (glucagon like-peptide 1), a hormone involved in insulin metabolism [11].
Unripe banana is source of unavailable carbohydrates, especially resistant starch. In a previous study, the unripe banana presented high in vitro fermentability (expressed by total SCFA) and low glycemic response in humans [12].
The aim of the present work was to evaluate the effect of the colonic fermentation of unavailable carbohydrates from unripe banana (mass and isolated starch) over parameters related to glucose and insulin response in rats.
Material and Methods
Sources of Unavailable Carbohydrates
The unripe banana mass (UBM) and the unripe banana starch (UBS) were the sources of unavailable carbohydrates for the rat diets. UBM and UBS were produced according to the method described by Menezes et al. [12].
Diets
Four rat diets were used along the experiment. The adaptation diet was a commercial standard diet (Nuvital®) for rats. The control diet was formulated according to the AIN-93G recommendations [13] and the two test diets were made based on the same recommendations, but with a different profile of unavailable carbohydrates. Total carbohydrate (~64 g/100 g) and energy (~1557 kJ/100 g) were similar for all experimental diets, including the energy provided by dietary fiber, according to FAO recommendations [14]. The RS contents were: 5.5 g/100 g for UBM diet, 10.6 g/100 g for UBS diet and 1.9 g/100 g for Control diet (Table S1, Supplementary material). The RS was supplemented to the test diets at the expense of common corn starch.
Carbohydrate Analysis of the Diets
The resistant starch and total starch analysis were done based on the method described by McCleary et al. [15]. Glucose was quantified on the supernatants with GOD/POD/ABTS mixture. Dietary fiber was quantified according to Lee et al. [16], with modifications proposed by McCleary and Rossiter [17], in order not to consider the RS content.
In Vitro Fermentation Diet
In vitro fermentation of the indigestible fraction of unripe banana mass (UBM), unripe banana starch (UBS) and control diets was performed as previously described by Menezes et al. [12] (Table S1, Supplementary material).
Experimental Design
The experimental procedure was approved by the Committee of Ethics in Animal Research and Experimentation of the Faculty of Pharmaceutical Sciences of the University of Sao Paulo (Protocol n. 54, CEEA – FCF – USP, Brazil).
The experiment was performed with male Wistar rats (n = 48) weighing approximately 100 g each at the start of the experiment. Rats were individually housed in wire cages in a controlled ventilation and temperature room, and with the dark period from 7 pm to 7 am. During 1 week, all rats received the adaptation diet (adaptation period). At the end of this period, rats were divided into three groups of 16 rats each, all of the groups having the same body weight mean. One group received the control diet while the other two groups were given the test diets containing either 5 % RS (UBM diet) or 10 % RS (UBS diet). All rats were fed the experimental diets for 28 days and were named “Control group”, “UBM group” or “UBS group”, according to the diet. Diet and tap water were offered ad libitum to the animals. Diet intake was measured daily and body weight was measured each 2 or 3 days. Stool was collected daily on the first 4 days of every week, in pools from each group, for moisture and dry weight evaluation along the experiment. At the end of the experimental period, rats were sacrificed in order to collect blood, cecum and pancreas for analysis.
Analytical procedures with cecum samples
For the evaluation of the in vivo fermentation, at the end of each assay, 8 rats of each group were anaesthetized with 2:1 cetamine (Vetaset®) and xilazine (Coopazine®) (75 μL/100 g rat) and then had their cecum isolated and removed. Each whole cecum (content and cecum wall) was weighed and its content had the pH immediately measured with an UP-10 pHmeter (Denver Instrument, USA). Triplicates of 500 mg of each sample of cecum content were individually mixed with 400 μL of 0.1 % HgCl2 and 105 μL of 5 % H3PO4, immediately frozen in liquid nitrogen and stored at −20 °C for further SCFA analyses. Another 500 mg of cecum content was collected, frozen and stored at the same conditions for moisture determination. The stored cecum contents were purified for SCFA analyses as follows: samples were homogenized at ambient temperature on a Vortex mixer for 5 min and transferred to 25 mL centrifuge tubes with 6 mL of deionized water. The tubes were capped and centrifuged (7600 × g, 4 °C, 50 min), the supernatants were removed with a needle adapted to a 5 mL syringe and then filtered on a membrane of 0.8 μm pore diameter (Minisarts, Sartorius, Germany).
SCFA Analyses by Gas Chromatography (GC)
The SCFA analyses by GC were performed based on the method described by Schäfer [18]. Samples of 0.5 mL of purified cecum solution, containing SCFA, were mixed with 0.4 mL internal standard (2-metil-valeric acid) and 0.1 mL HClO4. The mixtures were centrifuged (4 °C, 7600 × g, 15 min) and supernatants were transferred to GC vials. The analyses were performed as previously described by Menezes et al. [12]. The analyzed SCFA were expressed as molarity/g of caecum content.
Glucose Tolerance Test (GTT)
The GTT was done according to the method proposed by Machado et al. [19], with modifications. All animals (8/group) had a 3 to 5-hour food restriction before the procedure, and then they were anesthetized intraperitoneally with chloral hydrate (400 mg/kg body weight).
A cannula was surgically fitted with a catheter in a jugular vein of each rat for both glucose administration and blood collection. The first blood sample was taken at 0 min, which was right before glucose solution (0.75 g/kg body weight) administration, to evaluate basal values of plasma glucose and insulin. Then, samples were routinely taken at 5, 10, 15, 20, 30 and 60 min after glucose administration. Blood samples (0.4 mL) were collected into microcentrifuge tubes containing sodic heparin (10 U/mL), centrifuged at 1500 × g for 15 min at 4 °C. Plasma was then stored at −20 °C until analyses of glucose and insulin. Glucose concentrations were determined by the glucose oxidase colorimetric method (kit Labtest Diagnóstica SA, Lagoa Santa, MG, BRA) and plasma insulin was analyzed by radioimmunoassay (Rat Insulin RIA Kit, Linco Research Inc., St Charles, MO, USA). Blood glucose and insulin levels were plotted versus time and the incremental area under the curves (AUC) (considering fasting line) was calculated.
Evaluation of the Insulin Secretion in Isolated Pancreatic Islets
Pancreatic islets were isolated at the Laboratory of Physiology of Insulin Secretion of the Institute of Biomedical Sciences of the University of Sao Paulo (ICB/USP), according to the method described by Ximenes et al. [20]. The aim of the procedure was to evaluate the insulin secretion in islets isolated from rats treated 28 days with the experimental diets.
At the end of the experimental treatment, 3 rats/group were sacrificed by decapitation and had their Langerhans islets isolated from the pancreas by perfusion and incubation. After decapitation, 15–20 mL of physiologic solution was injected in the pancreas, which was then removed. Each pancreas was homogenized and incubated with enzymes at 37 °C for 25 min to be digested. After incubation, the islets were isolated under electronic microscopy (about 10 islets/rat). Glucose solutions of different concentrations (5.6 and 16.7 mM) were injected in the islets medium, one after the other, and the insulin response production was determined by radioimmunoassay.
Statistical Analyses
The results were expressed as mean ± standard error (SEM) or ± standard deviation (SD). The D’Agostino-Peason normality test was done. Statistical analysis was carried out by one-way analyis of variance ANOVA/Tukey’s for parametric data or Kruskal-Wallis for non-parametric data, using the Statistica 10.0 software (StatSoft Inc., Tulsa, OK, USA. Differences were considered significant when P < 0.05.
Results and Discussion
Diets containing UBM (5 % RS) and UBS (10 % RS) presented high in vivo fermentability and lower insulin release, for similar blood glucose levels in rats.
Diet consumption was daily measured and both test groups (UBM and UBS group) had their diet consumption similar to the Control group. On the first experimental week, the mean of the daily diet intake of the groups was 16.0 ± 0.5 g/rat. On the fourth and last week of the experiment, the mean daily diet intake of the groups was approximately 18.8 ± 0.4 g/rat (Fig.S1, Supplementary material). Rats from the UBM group had their growth rate similar to the Control group. The UBS group presented a slight decrease in mean body weight from day 9 until the end of the experiment, even keeping the same diet intake as the other groups. The average weight of the rats was 277 ± 18 g in the Control group, 286 ± 22 g in the UBM group and 260 ± 28 g in the UBS group (Fig.S1, Supplementary material).
Short Chain Fatty Acids Profile
During the in vitro fermentation, it was possible to observe that the experimental diets presented higher SCFA production (UBM 2.4 and UBS 4.1 mmol/g of subtrate) compared to the Control diet (1.7 mmol/g of subtrate), which was proportional to the content of RS (Table S1).
According to Table 1, the cecal total SCFA was higher in both experimental groups compared to the Control group and the pH of the cecum content of the rats was inversely proportional to the total SCFA concentration in the cecum. The availability of substrates for the intestinal microbiota is quantitatively and qualitatively influenced by the diet composition. In addition to the substrate supply, other characteristics of the gut environment, whereas pH plays a major role, are responsible for the gut metabolic outputs [21]. In addition to being an important factor tending to avoid certain pathogens in the intestine, the pH of the cecum content is an excellent indicative of SCFA presence and concentration [22].
Both UBM and UBS groups presented a decrease in the cecum pH value in relation to the control group (P < 0.01) (Table 1). Some authors have indicated that the pH of rat cecum usually varies from 6.1 to 8.2 depending on the consumed diet, i.e., rats fed fermentable carbohydrates might have their cecal pH reduced in up to 2 units [21]. In the present study, the pH of the cecum content varied approximately 0.5 units in the UBM group and 1.0 unit in the UBS group, both in relation to the control group (Table 1). In fermentor systems, a pH 5.5 result in higher butyrate production and a pH 6.5 result in more propiogenic fermentation [22], which can be observed on Table 1.
During the adaptation week, all three groups presented similar mean stool moisture. On the first week of the experiment, it was already clear that the mean stool moisture from the UBM group (40 %, n = 16) and UBS group (40 %, n = 16) became higher than the one from the control group (33 %, n = 16) (P < 0.05, for both comparisons). In addition, there was an increase in the stool dry weight from the UBS group in relation to the control group (P < 0.05) during the last 3 weeks of the experiment (Table S2, Supplementary material). The higher mean stool moisture of both UBM and UBS groups could be a consequence of the ability of diets containing RS to cause higher water retention in the colon [23] and, consequently, to increase the total stool weight, which can accelerate the intestinal transit time. Such results might facilitate the elimination of metabolites that can be harmful to the intestinal health.
At the end of the experiment, the UBM group presented higher cecum moisture content and the UBS group presented higher dry weight of the cecum content, both compared to the control group (P < 0.01) (Table S2, Supplementary material). Comparing weights of the dry stool and cecum content, data seems coherent with the fact that in both results the UBS group presents the highest dry weight, which might indicate increase in the bacterial mass. The different types of RS and their sources can act in several ways and intensities over the intestinal lumen, and hence over the bowel function, as well as the different fiber components [21, 24].
The fermentation rate of the RS is variable according to the RS type (1, 2, 3 or 4). Some types are almost completely utilized within 24 h of in vitro fermentation and produce elevated concentrations of SCFA. Others are more resistant to the bacterial fermentation, not being entirely fermented even after 24 h. Some important consequences of RS reaching the large intestine as unavailable carbohydrates are the increase of fecal bulk, fecal pH changes and also a butyrogenic effect on the colonic microbiota [21]. These effects have great impact over dysfunctions such as constipation, diverticulitis, hemorrhoids and even colon cancer [25].
The colonic microbiota ferments the RS and the non-starch polysaccharides producing mainly SCFA (acetate, propionate and butyrate). According to the SCFA production, the UBM diet (RS = 5 %) and the UBS diet (RS = 10 %) were, respectively, 1.4- and 2.4-fold more fermentable in vitro (Table S1, Supplementary material) and 1.5- and 2.1-fold more fermentable in vivo, when compared to the control diet (Table 1), showing a correlation between in vitro and in vivo fermentation.
The in vivo colonic fermentation of RS resulted in high butyrate production (Table 1). Butyrate is the main energy source for colonocytes and is involved in the protection against colitis and colorectal cancer. Epidemiological data suggest that RS plays a more important role on reducing the risk of these diseases than other unavailable carbohydrates [21, 23].
Acetate is involved in the growth and proliferation of normal cells of the colonic epithelium, where it is absorbed and transported to the liver for peripheral utilization as energy source and having structural functions on the SCFA synthesis [26, 27].
Propionate is the SCFA which has the greatest systemic impact over lipid metabolism and insulin and glycemic control. The blood concentration of propionate produced during the colonic fermentation has been shown to modulate glucose tolerance and suppress free fatty acid levels [28].
Glucose Tolerance Test (GTT)
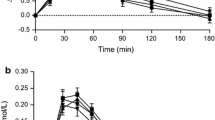
When the glucose and insulin AUC were studied, it was possible to observe lower insulin release (UBS 90.4 ng/mL.min; UBM 82.8 ng/mL.min), for the same blood glucose level, when compared to the Control group (148.3 ng/mL.min). An insulin/glucose ratio of 2.43 was observed for the Control group, 1.14 for the diet containing 5 % RS and 0.78 for the diet containing 10 % RS, which indicates a better insulin sensitivity after the consumption of the latter diet, for 28 days (Fig. 1, Table 2).
The higher concentration of propionate found in the cecum content of the UBS group can reinforce the hypothesis of propionate improving insulin sensitivity [29]. It was also shown that the intake of 30 g of RS per day, for 4 weeks (healthy humans), improves insulin sensitivity, and SCFA concentrations (acetate and propionate) were higher than in the control group [10]. In addition to a possible increase in insulin sensitivity, Menezes et al. [12], in a previous trial with healthy volunteers, observed that the increase in the area under the curve for glucose after ingestion of UBM and UBS was 90 and 40 % lower than the reference food, respectively, which should promote a decreased insulin release.
Data from Table 2 presents reduced in vitro insulin production by isolated pancreatic islets from the UBM and UBS group, in response to similar glucose doses. It suggests that the diets containing 5 and 10 % of RS from unripe banana have a suppressing effect over insulin secretion in the medium term. Ximenes et al. [20] have presented evidences that propionate inhibits glucose-induced insulin secretion in incubated and perfused pancreatic islets.
The pancreatic islets isolated from the test groups showed significant lower insulin secretion compared to the control group (Table 2), and the insulin release response measured in this in vitro test was similar to the GTT.
A decrease in insulin parameters was observed both in vivo and in vitro, as well as an increase in the in vivo production of SCFA. The test of insulin secretion by the pancreatic islets of rats fed UBM and UBS diets was an important tool in order to demonstrate that the habitual consumption of diets rich in unavailable carbohydrates may influence insulin release, possibly due to the action of SCFA produced during fermentation. However, studies with humans have shown that the relationship between SCFA and glucose and insulin homeostasis may be indirect and still needs to be explained [30].
Digestibility and physicochemical characteristics of starch present in unconventional starch sources, such as banana, combined with the RS physiological properties, enable these ingredients to be used for development of new products with functional properties [12, 31]. Analyses of the unripe banana flour showed that this ingredient is rich in dietary fiber and resistant starch, has low energy and available carbohydrates, as well as moderate antioxidant activity [32]. The initial stage of maturation and the process which the unripe banana goes through are extremely important once they may determine its final RS content. The potential of unripe banana to be used in bakery products has been explored in bread produced with unripe banana flour, containing 17.5 % of RS, and a predicted glycemic index of 65.08 % (in vitro), which was significantly lower than control bread (81.88 %), made with wheat flour [33].
As a next step, it is necessary to evaluate such sources of unavailable carbohydrates in clinical assays with humans and the possibility of using these unripe banana products for the prevention of chronic diseases such as type 2 diabetes and metabolic syndrome.
Conclusion
The observation of lower insulin secretion after the consumption (for 28 days) of a diet rich in unavailable carbohydrates from unripe banana (UBM and UBS), evidenced both in blood and in isolated pancreatic islets of rats, is an indicative that this promising functional ingredient can be used in products aiming the management of glucose and insulin homeostasis.
Abbreviations
- AUC:
-
Area under the curve
- DF:
-
Dietary fiber
- GC:
-
Gas chromatography
- GTT:
-
Glucose tolerance test
- RS:
-
Resistant starch
- SCFA:
-
Short chain fatty acids
- UBM:
-
Unripe banana mass
- UBS:
-
Unripe banana starch
References
Cummings JH, Stephen AM (2007) Carbohydrate terminology and classification. Eur J Clin Nutr 61(Suppl 1):S5–S18
Jenkins DJ, Kendall CW, Augustin LS, Vuksan V (2002) High-complex carbohydrate or lente carbohydrate foods? Am J Med 113(Suppl 9B):30S–37S
American Diabetes Association (ADA) (2014) Standards of medical care in diabetes-2014. Diabetes Care 37(Suppl 1):S14–S80
Heine RJ, Balkau B, Ceriello A, Del Prato S, Horton ES, Taskinen MR (2004) What does postprandial hyperglycaemia mean? Diabet Med 21(3):208–213
The DECODE Study Group. Diabetes epidemiology: collaborative analysis of diagnostic criteria in Europe (1999) Glucose tolerance and mortality: comparison of WHO and American diabetic association diagnostic criteria. Lancet 354(9179):617–621
Frontoni S (2013) Comment on: Straznicky et al. neuroadrenergic dysfunction along the diabetes continuum: a comparative study in obese metabolic syndrome subjects. Diabetes 62(1):2506–2516
Brand-Miller JC (2004) Postprandial glycemia, glycemic index, and the prevention of type 2 diabetes. Am J Clin Nutr 80(2):243–244
Cummings JH, Macfarlane GT (1991) The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol 70(6):443–459
Robertson MD (2012) Dietary-resistant starch and glucose metabolism. Curr Opin Clin Nutr Metab Care 15(4):362–367
Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN (2005) Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr 82(3):559–567
Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E et al (2012) Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61(2):364–371
Menezes EW, Dan MC, Cardenette GH, Goñi I, Bello-Pérez LA, Lajolo FM (2010) In vitro colonic fermentation and glycemic response of different kinds of unripe banana flour. Plant Foods Hum Nutr 65(4):379–385
Reeves PG, Nielsen FH, Fahey GC (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123(11):1939–1951
FAO (2003) Food Energy- Methods of analysis and conversion factors. In: FAO Food and Nutrition Paper. Report of 330 a technical workshop. 77. Rome: FAO
McCleary BV, McNally M, Rossiter P (2002) Measurement of resistant starch by enzymatic digestion in starch and selected plant materials: collaborative study. J AOAC Int 85(5):1103–1111
Lee SC, Prosky L, Devries JW (1992) Determination of total, soluble and insoluble dietary fiber in foods. Enzymatic-gravimetric method, Mes-TRIS Buffer: collaborative study. J AOAC Int 75:395–416
McCleary BV, Rossiter P (2004) Measurement of novel dietary fibers. J AOAC Int 87(3):707–717
Schäfer K (1995) Analysis of short chain fatty acids from different intestinal samples by capillary gas chromatography. Chromatographia 40(9–10):550–556
Machado UF, Nogueira CR, Carpinelli AR (1992) Obesity is the major cause of alterations in insulin secretion and calcium fluxes by isolated islets from aged rats. Physiol Behav 52(4):717–721
Ximenes HM, Hirata AE, Rocha MS, Curi R, Carpinelli AR (2007) Propionate inhibits glucose-induced insulin secretion in isolated rat pancreatic islets. Cell Biochem Funct 25(2):173–178
Topping DL, Clifton PM (2001) Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81(3):1031–1064
Louis P, Scott KP, Duncan SH, Flint HJ (2007) Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol 102(5):1197–1208
Fuentes-Zaragoza E, Riquelme-Navarrete MJ, Sánchez-Zapata E, Pérez-Álvarez JA (2010) Resistant starch as functional ingredient: a review. Food Res Int 43(4):931–942
Elia M, Cummings JH (2007) Physiological aspects of energy metabolism and gastrointestinal effects of carbohydrates. Eur J Clin Nutr 61(Suppl 1):S40–S74
Gibson PR, Barrett JS, Muir JG (2013) Functional bowel symptoms and diet. Intern Med J 43:1067–1074. doi:10.1111/imj.12266
Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ (2002) The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett 217(2):133–139
Zeng H, Lazarova DL, Bordonaro M (2014) Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J Gastrointest Oncol 6(2):41–51
Nilsson A, Granfeldt Y, Ostman E, Preston T, Bjorck I (2006) Effects of GI and content of indigestible carbohydrates of cereal-based evening meals on glucose tolerance at a subsequent standardised breakfast. Eur J Clin Nutr 60:1092–1099
Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K (2010) Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta 1801(11):1175–1183. doi:10.1016/j.bbalip.2010.07.007
Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL (2013) Short chain fatty acids and their receptors: new metabolic targets. Transl Res 161(3):131–140
Bello-Pérez LA, Paredes-López O (2009) Starches of some food crops, changes during processing and their nutraceutical potential. Food Eng Rev 1(1):50–65
Menezes EW, Tadini CC, Tribess TB, Zuleta A, Binaghi J, Pak N, Vera G, Dan MC, Bertolini AC, Cordenunsi BR, Lajolo FM (2011) Chemical composition and nutritional value of unripe banana flour (Musa acuminata, var. Nanicão). Plant Foods Hum Nutr 66(3):231–237
Juarez-Garcia E, Agama-Acevedo E, Sáyago-Ayerdi SG, Rodríguez-Ambriz SL, Bello-Pérez LA (2006) Composition, digestibility and application in breadmaking of banana flour. Plant Foods Hum Nutr 61(3):131–137
Acknowledgments
This research was financially supported by CAPES and FAPESP. Also, the acknowledgements to the XI.18 and 106PI0297 Projects of International Cooperation CYTED/CNPq projects and to the Instituto de Pesquisas Tecnológicas (Sao Paulo, Brazil).
Conflict of Interest
The author(s) declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dan, M.C.T., Cardenette, G.H.L., Sardá, F.A.H. et al. Colonic Fermentation of Unavailable Carbohydrates from Unripe Banana and its Influence over Glycemic Control. Plant Foods Hum Nutr 70, 297–303 (2015). https://doi.org/10.1007/s11130-015-0493-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-015-0493-6