Abstract
Background/objectives
Consumption of whole vs. refined grain foods is recommended by nutrition or dietary guideline authorities of many countries, yet specific aspects of whole grains leading to health benefits are not well understood. Gastric emptying rate is an important consideration, as it is tied to nutrient delivery rate and influences glycemic response. Our objective was to explore two aspects of cooked rice related to gastric emptying, (1) whole grain brown vs. white rice and (2) potential effect of elevated levels of slowly digestible starch (SDS) and resistant starch (RS) from high-amylose rice.
Subjects/methods
Ten healthy adult participants were recruited for a crossover design study involving acute feeding and testing of 6 rice samples (50 g available carbohydrate). Gastric emptying rate was measured using a 13C-labeled octanoic acid breath test. A rice variety (Cocodrie) with high-amylose content was temperature-cycled to increase SDS and RS fractions.
Results
In vitro starch digestibility results showed incremental increase in RS in Cocodrie after two temperature cycles. For low-amylose varieties, SDS was higher in the brown rice form. In human subjects, low-amylose and high-amylose brown rice delayed gastric emptying compared to white rices regardless of amylose content or temperature-cycling (p < 0.05).
Conclusions
Whole grain brown rice had slower gastric emptying rate, which appears to be related to the physical presence of the bran layer. Extended gastric emptying of brown rice explains in part comparably low glycemic response observed for brown rice.
Similar content being viewed by others
Introduction
The 2015 Dietary Guidelines for Americans, as well as similar authorities in other countries, recommends increased consumption of whole grains [1]. Diets with whole grains, compared to refined grains, are associated with low glycemic response and decrease in risk of type-2 diabetes and cardiovascular disease [2,3,4]. Although the low glycemic response of whole grain foods is often attributed to the fiber component, the overall basis of its action is not clear [5]. Viscous fibers have been implicated [6], but not all whole grains have viscous fibers. For instance, whole grain brown rice does not contain viscous fiber and only contains around 2% total dietary fiber [7], but has been shown to have a lower glycemic index than white rice [8] and is associated with lower incidence of diabetes [9]. In another study [10], only one of three rice varieties tested (Pelde v.) had reduced glycemic index in the brown rice. Other factors that pertain to grains, whether whole or refined, can also influence glycemic response, such as particle size and structural components.
Glycemic response of whole grains is dependent on both rate of digestion of starch and how fast the meal is emptied from the stomach. When gastric emptying rate is slow, it is positively correlated with low glycemic response [11, 12]. There is a less clear, though sometimes reported, relationship between slow gastric emptying and appetite response [13, 14].
For whole grain brown rice, its low glycemic response compared to white rice could be related to a slow gastric emptying rate. Brown rice contains bran that is removed during milling to make white rice. Its physical form has multiple cell layers that give an integrity and toughness to the kernel that may reduce its rate of breakdown in the stomach, thus reducing gastric emptying rate. A pig study showed that brown rice takes longer than white rice to break down and that the bran layer separates and accumulates in distal stomach [15]. While this resulted in slower protein (bran) emptying from the stomach, emptying of starch and dry matter were the same as white rice, which suggests that the whole grain components are emptied differently in the stomach. Additionally, a low glycemic response of brown rice may be related to slow digestion of starch that could trigger the ileal brake, a feedback mechanism that controls gastric emptying rate. All macronutrients, including carbohydrates, have been shown to stimulate the ileal brake [16, 17]. Starchy foods that digest slow and into the ileum could thus affect gastric emptying through this mechanism.
Digestion rate of starch is related to a number of factors mostly related to access of digestive enzymes to substrate. It is, for instance, well known that the amylose component of starch retrogrades after gelatinization to form resistant starch (RS), as well as slowly digestible starch (SDS) [18]. Starchy foods with relatively high amylose contain greater amounts of slowly digestible and resistant starch [19, 20], and this is associated with moderated glycemic response and increased satiety [21]. To enhance this effect, temperature-cycling has been used to increase SDS and RS fractions by promoting retrogradation [22, 23], by augmenting starch crystallite formation through nucleation, propagation, and maturation steps combined with extended storage [23]. Retrogradation of amylopectin is associated with the formation of SDS, while retrogradation of amylose is a more rapid and is associated with RS [24].
In the present study, it was hypothesized that brown rice has a slower gastric emptying rate than white rice, and that high-amylose rice containing more SDS and RS would further delay gastric emptying rate. We investigated in human subjects two rice varieties in whole grain brown and milled white forms on gastric emptying, and further examined rice types of different amylose contents with varying amounts of SDS and RS to potentially affect gastric emptying rate.
Materials and methods
Materials
Two rice varieties, whole grain brown and polished white types, classified as low-amylose (Jazzman) or high-amylose (Cocodrie) were a gift from Mars, Inc. Nutritional information on these cultivars can be found in Table 1.
Participants
Ten healthy men and women [body mass index (BMI; in kg/m2) of 22.25 ± 2.15] with an average age of 30 were recruited using flyers placed around the Purdue University campus. All subjects were free of diabetes and any gastrointestinal diseases, through self-reporting. Informed consent was gathered from all participants.
This research was approved by Purdue University’s Institutional Review Board Committee. The trial is registered at Clinicaltrials.gov, identifier NCT03035981.
Design
A randomized, crossover design was used with seven treatments (six rice and one fructooligosaccharide breath hydrogen control) tested in acute feeds with a 1-week washout period in-between. The treatment order was randomly assigned to the participants. Rice test meals were prepared on the morning of each test day, and 13C-octanoic acid (100 mg) was added to each test meal.
Subjects arrived to the testing room at 8:00 a.m. on each test day after a 10 h fast. After collecting two baseline breath samples (1.5 L bags, Cambridge Isotope Laboratories, Tewksbury, MA, USA), subjects consumed the rice test meal (50 g available carbohydrate) for that day in 10 min, and breath samples were collected into 300 ml bags (Cambridge Isotope Laboratories, Tewksbury, MA, USA) every 15 min for 4 h. Additional breath samples were collected into bags (300 ml) at the same time points for breath hydrogen testing. Subjects were provided 100 ml of water with the rice, and were asked to consume all water and rice (200–240 g) given to them. No food or drink was allowed during the remainder of the test session.
Test meals
The six rice treatments were chosen based on differences in starch digestibilities as tested in vitro. They were as follows: Jazzman low-amylose white rice (low-AM white), Cocodrie high-AM white rice (high-AM white), Jazzman low-AM brown rice (low-AM brown), Cocodrie high-AM brown rice (high-AM brown), and two temperature-cycled variations of Cocodrie high-AM white rice (high-AM white C1, high-AM white C2). Cocodrie is classified as a high-amylose rice, though our value for amylose was somewhat lower than has been reported [25]. An additional test day included a solution containing fermentable fructooligosaccharide (7 g/150 ml water, Beneo, Orafti, Morris Plains, NJ, USA) to provide a reference for positive breath hydrogen production. Rice test portions were prepared individually using a conventional rice cooker (Sunbeam–Oster Company, Boca Raton, FL, USA). Rice grains were placed in 400 ml beakers, and washed three times with water. A 2:1 water to rice ratio was used to cook the rice in the beakers with a cooking time of ~24 min for white rice and ~48 min for brown rice [25].
Gastric emptying and breath hydrogen tests
Breath samples were analyzed the same day using a 13CO2 breath analyzer (POCone, Otsuka Electronics Co., Ltd., Osaka, Japan). A BreathTracker Digital Microlyzer® (Quintron Instrument Company, Milwaukee, WI, USA) was used for breath hydrogen analysis.
Calculation of gastric emptying rate parameters
Half-emptying time and lag phase are parameters used to describe the gastric emptying rate of a food [26, 27]. The 13CO2/ 12CO2 ratio of a gas collected after a test meal to the baseline breath value [13CO2 delta over baseline (DOB, %)] were used to calculate the percent dose [13C] recovery (PDR) per hour and cumulative PDR over time [27]. Each individual’s body surface area was used in the calculation [28]. Gastric emptying profiles were modeled using a macro program in Excel (Microsoft Corporation, Redmond, WA, USA). Half-emptying time, the time necessary for half of the 13C dose to be metabolized; and lag phase, the time required for the 13CO2 excretion rate to attend its maximal level or time it takes for a food to break down within the stomach [29] were calculated.
In vitro testing
Nutritional composition
Total starch content of rice samples was determined using the procedure described in the Total Starch Assay Kit (Megazyme, Bray, Ireland) and total dietary fiber was quantified using the Total Dietary Fiber Kit (Megazyme, Bray, Ireland) based on AOAC Method 985.29. Protein content was quantified by the Dumas method (N × 6.25) using a LECO TruMac nitrogen analyzer (LECO Corporation, St. Joseph, MI, USA), and fat content was determined at an external laboratory (A&L Great Lakes, Fort Wayne, IN, USA).
Amylose content
Amylose content of rice flours was determined using the Amylose/Amylopectin Assay Kit (Megazyme, Bray, Ireland).
Temperature-cycling treatment of white rice
White rice of the high-amylose Cocodrie variety were cooked as described above, and stored immediately in tightly sealed containers and temperature-cycled as described in Table 2.
In vitro starch digestibility of cooked rice
Rapidly digestible starch (RDS), SDS, and resistant starch (RS) were measured according to the Englyst method [30, 31]. The enzyme mixture was prepared with pancreatin from porcine pancreas (Sigma–Aldrich, St. Louis, MO, USA) and amyloglucosidase from Aspergillus niger (Sigma-Aldrich, St. Louis, MO, USA).
Rice was prepared as above using 5 g portions. Immediately upon completion of the cooking cycle, 2 g cooked rice was mixed with water (17.5 ml) in tubes using a high shear mixer (Kinematica, Bohemia, NY, USA) for 30 s [32]. A solution containing pepsin, HCl, and guar gum (pH = 2) was added to the tubes, to simulate gastric conditions and to standardize the viscosity of the treatments, and tubes were incubated in a 37 °C water bath for 30 min. Tubes were shaken at 160 r.p.m. with glass marbles and 0.1 M sodium acetate for 2 min, the enzyme mixture (5 ml) was added, and shaking continued for a total of 120 min. Aliquots (100 µl) were removed and added to 900 µl ethanol after 20 min and 120 min to quantify RDS, SDS, and RS. Total starch content was measured following in vitro digestion of cooked rice [31]. Glucose release was measured using the glucose oxidase/peroxidase (GOPOD) method and a conversion factor of 0.9 was used to calculate starch content at each timepoint [30].
All samples were completed in duplicate on two separate days.
Statistical analysis
The half-emptying times and lag phases of each treatment are presented as mean ± standard deviation (SD) of all participants. Sample size was determined using a power calculation for a crossover design based on previous work indicating within patient standard deviation of 0.1 h and a minimum detectable difference in means of half-emptying time of 0.3 h. One-way repeated measures ANOVA (two-tailed) and a post-hoc Tukey’s test for multiple comparisons of half-emptying time and lag phase across treatment groups were performed using a randomized complete block design. Statistical significance was considered at p < 0.05 and analyses were completed using JMP Statistical Discovery Software (JMP Version 12, SAS Institute, Cary, NC, USA).
Results
Amylose content
Values for amylose and classification of rice varieties are found in Table 3.
In vitro starch digestibility
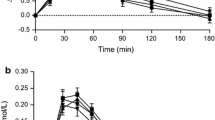
For white rice samples, the low-amylose and high-amylose varieties (Jazzman, and Cocodrie) contained the highest levels of RDS (88.3 and 84.7%), and lowest levels of SDS (11.3 and 12.8%) and RS (0.3 and 2.6%) (Fig. 1). For brown rice samples, SDS increased for the low-amylose variety to 17.5 (Jazzman), and RS increased for Cocodrie to 12.7.
The high-amylose Cocodrie was temperature-cycled with the largest effect on RS. After the first cycle, RS increased from 2.6 to 6.9% and, after the second cycle, to 13.5%.
Gastric emptying test
Gastric half-emptying times were more delayed by the brown than white rice treatments (Fig. 2). This was independent of the SDS and RS amounts. Statistically significant (p < 0.05) differences were found between the brown and white rices in both the low-amylose and high-amylose varieties (Jazzman and Cocodrie). The longest delay in half-emptying time was observed in the Cocodrie brown rice treatment, ~48 min longer than the Jazzman white rice. Cocodrie white and brown rice trended (non-significantly) towards longer delay in gastric emptying times compared to Jazzman. Half-emptying times for the two temperature-cycled Cocodrie white rices were not significantly higher than the untreated Cocodrie white rice. Lag phase values showed no difference across treatments, averaging 66 min.
Half-emptying times and lag phases of the six low- (L-AM) and high-amylose (H-AM) white and brown rice and temperature-cycled white rice treatments in human study. Half-emptying time and lag phase values are expressed as mean ± SD (n = 10) and were compared following a randomized treatment order, *indicates significance at p < 0.05 following ANOVA analyses and post-hoc Tukey’s test
White, non-temperature-cycled rice trended to the highest PDR per hour, followed by the temperature-cycled white rice, and then the brown rice having the lowest percent dose recovery per hour (Fig. 3). Cumulative PDR was in the range of 35–40%, which is similar to that reported [33]. Cumulative recoveries were similar in all experiments, indicating that there was not selective binding of octanoic acid to particular components (i.e., bran).
No significant rise in breath hydrogen was detected following consumption of the rice treatments indicating full digestion of the starch in the small intestine (not shown). For the positive control, there was a large increase in breath hydrogen at about 75 min following ingestion of the fructooligosaccharide solution.
Discussion
Brown rice delayed gastric emptying more than white rice regardless of whether its amylose content was low or high, or if treated with temperature-cycling. Reasons for this could be related to the physical properties of brown rice and how these may affect digestion downstream. Two possible explanations for the slower gastric emptying rate of the brown rice treatments are: (1) brown rice has a physical form, namely the additional layer of bran, that takes longer to break down or reduce in size in the stomach, and (2) the resulting particles from the physical breakdown in the stomach are digested at a slower rate than white rice particles, to stimulate the ileal brake to slow gastric emptying. Because the Cocodrie brown rice had approximately the same amount of SDS + RS as the temperature-cycled white rices (Fig. 1), but had significantly longer gastric half-emptying time, it seems plausible that the physical property of the brown rice was the reason for its slower gastric emptying rate. Likewise, the low-amylose brown rice had significantly higher gastric half-emptying time than the high-amylose temperature-cycled rices, supporting the first possibility.
Gastric simulation models have shown longer breakdown of brown than white rice [34, 35], which supports our hypothesis that it is the different physical property of brown rice that decreases gastric emptying rate. It might be surmised that if the brown rice, in this study, had been consumed as a flour rather than in its whole grain form, gastric emptying rate may have been similar to white rice.
It is also noteworthy to point out that despite the differences seen in half-emptying times between brown and white rice, the lag phases for all of the rice treatments remained similar to each other, regardless of the differences in amylose content and in vitro starch digestion rates (Fig. 1). Because the lag phase is considered a representation of the time spent on the physical breakdown of a food before leaving the stomach [29], the consistency in lag phase times could be an indication that the differences observed in half-emptying times are due to a post-gastric effect. This would provide some support for the second possibility above. If delayed gastric emptying of brown rice was only due to factors causing longer breakdown in the stomach, the lag phases of the brown rice treatments should have been significantly longer than those seen with white rices.
Gastric emptying rate is the main regulator of food entering the small intestine to be digested and absorbed, and is important for controlling postprandial glycemic response. Recent studies using pharmaceutical approaches to improve glycemia in individuals with diabetes [36] or obesity [37] involve treatment using incretin analogs, such as liraglutide and exenatide. While exact mechanisms for the physiological effects of these analogs are still being elucidated, one finding has been that individuals receiving these treatments exhibit significantly slowed, or delayed, gastric emptying, which resulted in greater weight loss and improved postprandial glycemic response compared to control groups [38]. The delaying of gastric emptying and reduced food intake was also observed when individual macronutrients were infused into the distal small intestine to elicit the ileal brake inhibitory feedback control system [16]. From a dietary perspective, identifying or developing foods with slow gastric emptying rates could increase satiety which could be important in addressing the obesity epidemic.
Conclusions
Brown rice displayed a slower gastric emptying rate than white rice regardless of the variation in amylose content and in vitro starch digestion rates. Understanding the mechanisms by which brown rice and other whole grains influence physiological parameters, including gastric emptying, to have a beneficial impact on health will aid in the development of whole grain processed foods.
References
Dietary Guidelines Advisory Committee, US Department of Agriculture. Scientific report of the 2015, Dietary Guidelines Advisory Committee; 2015.
Kristensen M, Toubro S, Jensen MG, Ross AB, Riboldi G, Petronio M, et al. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J Nutr. 2012;142:710–6.
Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr. 2012;142:1304–13.
Tighe P, Duthie G, Vaughan N, Brittenden J, Simpson WG, Duthie S, et al. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: A randomized controlled trial. Am J Clin Nutr. 2010;72:733–40.
Lattimer J, Haub M. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2:1266–89.
Hallfrisch J, Behall KM. Mechanisms of the effects of grains on insulin and glucose responses. J Am Coll Nutr. 2000;19:320S–325S.
Champagne ET, (ed). Rice: chemistry and technology. 3rd edn. St. Paul, MN: American Association of Cereal Chemists; 2004.
Foster-Powell, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56.
Sun Q, Spiegelman D, van Dam RM, Holmes MD, Malik VS, Willett WC, et al. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med. 2010;170(11):961–9.
Brand-Miller J, Pang E, Bramall L. Rice: a high or low glycemic index food? Am J Clin Nutr. 1992;56:1034–6.
Horowitz M, O’Donovan D, Jones KL, Feinle C, Rayner CK, Samsom M. Gastric emptying in diabetes: clinical significance and treatment. Diabet Med. 2002;19:177–94.
Marathe CS, Rayner CK, Jones KL, Horowitz M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care. 2013;36(5):1396–405.
Clegg ME, Ranawana V, Shafat A, Henry CJ. Soups increase satiety through delayed gastric emptying yet increased glycaemic response. Eur J Clin Nutr. 2013;67(1):8–11.
Geliebter A, Grillot CL, Aviram-Friedman R, Haq S, Yahav E, Hashim SA. Effects of oatmeal and corn flakes cereal breakfasts on satiety, gastric emptying, glucose, and appetite-related hormones. Ann Nutr Metab. 2015;66:93–103.
Bornhorst GM, Chang LQ, Rutherfurd SM, Moughan PJ, Singh RP. Gastric emptying rate and chyme characteristics for cooked brown and white rice meals in vivo. J Sci Food Agric. 2013;23:2900–8.
van Avesaat M, Troost FJ, Ripken D, Hendriks HF, Masclee AAM. Ileal brake activation: macronutrient-specific effects on eating behavior? Int J Obes. 2015;39(2):235–43.
Siegle ML, Schmid HR, Ehrlein HJ. Effects of ileal infusions of nutrients on motor patterns of canine small intestine. Am J Physiol. 1990;259:G78–G85.
Haralampu S. Resistant starch—a review of the physical properties and biological impact of RS3. Carbohydr Polym. 2000;41(3):285–92.
Kerberg AÅ, Liljeberg H, Bjö I. Effects of amylose/amylopectin ratio and baking conditions on resistant starch formation and glycaemic indices. J Cereal Sci. 1998;28:71–80.
Zhu L, Gu M, Meng X, Cheung SCK, Yu H, Huang J, et al. High-amylose rice improves indices of animal health in normal and diabetic rats. Plant Biotechnol J. 2012;10:353–62.
Sparti A, Milon H, Di Vetta V, Schneiter P, Tappy L, Jéquier E, et al. Effect of diets high or low in unavailable and slowly digestible carbohydrates on the pattern of 24-h substrate oxidation and feelings of hunger in humans. Am J Clin Nutr. 2000;72:1461–8.
Goodfellow BJ, Wilson RH. A fourier transform IR study of the gelation of amylose and amylopectin. Biopolymers. 1990;30:1183–9.
Zhou X, Baik B-K, Wang R, Lim S-T. Retrogradation of waxy and normal corn starch gels by temperature cycling. J Cereal Sci. 2010;51(1):57–65.
Zhang G, Sofyan M, Hamaker BR. Slowly digestible state of starch: mechanism of slow digestion property of gelatinized maize starch. J Agric Food Chem. 2008;56(12):4695–702.
Patindol JA, Guraya HS, Champagne ET, McClung AM. Nutritionally important starch fractions of rice cultivars grown in southern United States. J Food Sci. 2010;75:H137–144.
Sanaka M, Yamamoto T, Nakayama S, Nagasawa K, Kuyama Y. Reliability of the time to maximal [13CO2] excretion and the half-[13CO2] excretion time as a gastric emptying parameter: assessments using the Wagner-Nelson method. J Smooth Muscle Res. 2007;43:201–9.
Sanaka M, Nakada K. Stable isotope breath tests for assessing gastric emptying: a comprehensive review. J Smooth Muscle Res. 2010;46:267–80.
Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–66.
Perri F, Pastore MA, Annese V. 13C-octanoic acid breath test for measuring gastric emptying of solids. Eur Rev Med Pharmacol Sci. 2005;9:3–8.
Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46:S33–S50.
Englyst KN, Englyst HN, Hudson GJ, Cole TJ, Cummings JH. Rapidly available glucose in foods: an in vitro measurement that reflects the glycemic response. Am J Clin Nutr. 1999;69:448–54.
Reed M, Ai Y, Leutcher J, Jane JL. Effects of cooking methods and starch structures on starch hydrolysis rates of rice. J Food Sci. 2013;78:H1076–H1080.
Sanaka M, Yamamoto T, Kuyama Y. Retention, fixation, and loss of the [13C] label: A review for the understanding of gastric emptying breath tests. Dig Dis Sci. 2008;53:1747–56.
Wang Z, Ichikawa S, Kozu H, Neves MA, Nakajima M, Uemura K, et al. Direct observation and evaluation of cooked white and brown rice digestion by gastric digestion simulator provided with peristaltic function. Food Res Int. 2015;71:16–22.
Kong F, Oztop MH, Singh RP, McCarthy MJ. Physical changes in white and brown rice during simulated gastric digestion. J Food Sci. 2011;76(6):E450–7.
Linnebjerg H, Park S, Kothare PA, Trautmann ME, Mace K, Fineman M, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept. 2008;151(1):123–9.
Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22.
Van Can J, Sloth B, Jensen C, Flint A, Blaak E, Saris W. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes. 2013;38:784–93.
Acknowledgements
We would like to acknowledge the Ingestive Behavior Research Center, the Industry Fellows Program in the Department of Food Science, and the Whistler Center for Carbohydrate Research for their financial support in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Pletsch, E.A., Hamaker, B.R. Brown rice compared to white rice slows gastric emptying in humans. Eur J Clin Nutr 72, 367–373 (2018). https://doi.org/10.1038/s41430-017-0003-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-017-0003-z
- Springer Nature Limited
This article is cited by
-
A perspective on the benefits of consumption of parboiled rice over brown rice for glycaemic control
European Journal of Nutrition (2022)
-
Activation of gastrointestinal ileal brake response with dietary slowly digestible carbohydrates, with no observed effect on subjective appetite, in an acute randomized, double-blind, crossover trial
European Journal of Nutrition (2022)