Abstract
Purpose
β-Glucans (βG) and resistant starch (RS) are known for their effects on the improvement of glucose tolerance and enhancement of insulin sensitivity. Enrichment of bread with βG or RS was performed to examine potential postprandial benefits regarding gastrointestinal hormone responses.
Methods
Ten healthy normoglycaemic adults participated in the study and were provided with either a glucose solution (reference food, GS) or bread enriched with β-glucans (βGB) (3.6 g/30 g available CHO) or bread enriched with resistant starch (RSB) (15% of total starch), with 1-week intervals in amounts that yielded 50 g of available carbohydrates. Venous blood samples were collected before consumption and at 15, 30, 45, 60, 90, 120 and 180 min postprandially. Glucose, insulin, ghrelin, glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) responses as well as glycaemic index (GI) and subjective appetite ratings were evaluated.
Results
Ingestion of βGB and RSB elicited lower incremental area under the curve (iAUC) for glycaemic response compared to GS (P < 0.05). Both breads demonstrated a low GI (βGB: 48, RSB: 40). There were no significant differences in insulin response, ghrelin, GLP-1 or PYY between the two breads. A significantly lower desire to eat and higher fullness were detected 15 min after βGB and RSB consumption and until 180 min (P < 0.05 compared to GS).
Conclusion
Enrichment of bread with either βG or RS produced a low GI product but the two breads were not significantly different in relation to insulin, ghrelin, GLP-1 and PYY responses. The development of bread products which cause improved metabolic effects is of great importance for the promotion of public health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postprandial glycaemia is found to be directly associated with chronic non-communicable diseases, such as type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD), even several types of cancer, according to epidemiological evidence [1,2,3]. Carbohydrates, which represent the highest percentage of energy intake in humans’ diet, typically accounting for 45–70%, mainly determine the postprandial glucose levels [4].
Bread is a staple food and a primary source of carbohydrates in most European countries. Specifically, the average bread consumption in Europe is about 170 g per day per capita [5]. Among starchy foods, white bread prepared with wheat flour is the most frequently consumed cereal product, due to its palatability and great sensory characteristics [6]. Nonetheless, its porous matrix and high gelatinized starch content, classify it as a high glycaemic index (GI) food [7].
Lowering the GI of bread is of scientific interest considering the potential benefits of a low glycaemic diet on the risk and the management of T2DM and heart disease [8]. Among successful strategies, are included the addition of soluble dietary fibers such as β-glucans (βG) concentrates originating from oats or barley or the addition of resistant starch (RS) [9, 10].
Consumption of meals rich in βG is associated with the improved postprandial metabolic profile. The potential mechanism that can explain this beneficial effect seems to be their ability to increase the viscosity of the gut digesta, and thus the delay of the carbohydrate absorption from the gut [11]. This health claim was approved by European Food Safety Authority (EFSA) in 2011. Particularly, a food that contains at least 4 g of βG from oats or barley for each 30 g of available carbohydrates in a quantified portion as part of the meal can ameliorate the postprandial glucose rise [12, 13]. In fact, it has been shown that the natural content of βG in oats and barley flour is insufficient to meet the requirements of the health claim, in comparison with the addition of high-βG containing flour in bread, which seems to be able to reduce the glucose response [9].
Despite viscous fibers, improved postprandial glycaemia is also documented by non-viscous fibers, such as RS [14]. RS is defined as a starch fraction, or the products of its degradation, which is not digested in the upper gastrointestinal tract but fermented by the colonic microbiota [15,16,17]. There is increasing interest from the food industry about the development of RS containing products, not only due to its beneficial effects on glucose and lipid metabolism but also because of its sensory characteristics. More specifically, RS has white color, neutral flavor and small particle size, characteristics that may offer to fortified products, palatability and relevant tolerance in the usual gastrointestinal symptoms that follow the fiber consumption [18]. In parallel to the health claim for βG, EFSA, respectively, accepted as health claim that replacing digestible starches with RS in a meal contributes to a reduction in the postprandial blood glucose rise. The final content of RS must be at least 14% of the total starch in the meal [19].
The aim of the present study was to compare the postprandial metabolic effects of two different functional breads enriched either with βG (βGB) or resistant starch (RSB) in amounts suggested by the EFSA health claims. Glycaemic and insulinaemic responses, as well as ghrelin, GLP-1 and PYY responses were examined and were accompanied by estimation of subjective appetite ratings. Sensory characteristics of the enriched products were also evaluated.
Materials and methods
Subjects
A randomized, single-blind, crossover-designed trial was conducted at the 1st Department of Propaedeutic Internal Medicine, Laiko General Hospital and the Laboratory of Experimental Surgery and Surgery Research, Athens University Medical School, in collaboration with Laboratory of Chemistry, Biochemistry and Physical Chemistry of Foods. Ten apparently healthy subjects (five men and five women) aged between 23 and 35 years (mean 27 years; SD 3.9) with a normal body mass index (mean 24.5 kg m−2; SD 2.8) were recruited for the study by means of poster and electronic advertisements. They had a stable weight for at least 3 months before enrollment and normal exercise, eating and drinking habits, as ascertained by nutritional assessment interview at screening. According to ISO 26642 for the determination of the GI ten healthy subjects are required.
Exclusion criteria were an age younger than 18 years old or older than 60 years old, pregnancy and lactation, chronic medical illness (diabetes mellitus, cardiovascular disease, chronic liver, kidney or untreated thyroid disease), use of nutritional supplements which could interfere with the results, a history of drug and/or alcohol abuse or psychiatric disease prohibiting adherence to the protocol. Eligible subjects were enrolled in the study after being informed in detail about its nature and all procedures and giving their written consent for participation. The protocols were reviewed and approved by both the Institutional Review Board/Ethics Committee of Laiko Hospital and the Harokopio University of Athens.
Study design
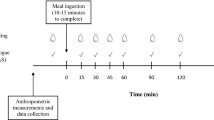
The study was designed as an acute, randomized controlled cross-over study with two types of bread and a solution containing 50 g of glucose, as the reference food. After meeting inclusion criteria, subjects were invited to visit the Diabetes Laboratory of the 1st Department of Propaedeutic Internal Medicine, Laiko General Hospital, four times in total, at 9:00 a.m. after a 12-h overnight fast. These four testing sessions, which were separated by intervals of one week, included consumption of the following: wheat bread enriched with β-glucans (βGΒ), wheat bread enriched with resistant starch (RSB) in random order and the glucose solution (GS), which was consumed twice, at the first and the last session respectively, according to ISO 26642.
Volunteers who participated in the study were advised not to drink alcohol or perform exercise on the day before each trial, as well as to maintain the same dietary habits and physical activity level until the completion of the study. Bodyweight, body fat, height and blood pressure measurements were performed at the first session, at the beginning of the study. Particularly, body weight was measured with light clothing on a scale (TANITA WB-110MA, Japan) and body fat by bioelectrical impedance analysis (Tanita BC-418, Tokyo, Japan). Height was measured using a stadiometer (Seca Mode 220, Hamburg, Germany) with subjects not wearing shoes, their shoulders in a relaxed position and their arms hanging freely. Waist circumference was determined at the midpoint between the lower margin of the least palpable rib and the top of the iliac crest in a standing position at the end of gentle expiration. Hip circumference measurement was taken around the widest portion of the buttocks. Energy intake and expenditure data were obtained by a dietician with the use of a 24-h dietary recall and a physical activity questionnaire respectively, at each one of the four sessions.
After 10 min of rest, an intravenous catheter was placed in a forearm vein and baseline samples were drawn (time 0). Then, a test meal of either a type of bread in an amount corresponding to 50 g of available carbohydrates or a solution containing 50 g of glucose diluted in 250 mL of water was served and subjects were advised to consume it within 10 min. Blood samples were collected before food ingestion and 15, 30, 45, 60, 90, 120 and 180 min postprandially. At the end of the intervention, the catheter was removed and the participants were evaluated for wellbeing prior to leaving Laiko Hospital.
Test breads
Meals designed for the study included a wheat bread enriched with β-glucans (βGΒ) and a wheat bread enriched with resistant starch (RSB), high amylose waxy maize starch. Breads were prepared by adding β-glucans or RS in the dough mixture. Τhe dough was rested for almost 3 min at room temperature, divided into 900 g portions, placed in baking tins and then put in proofing chamber for 60 min at 35 °C and relative humidity 80%. Tins with bread dough were baked in horizontal continuous flow belt oven with temperature regime between 205 and 275 °C for 28–30 min.
The nutritional composition of the two tested breads is presented in Table 1. Nitrogen (protein: Nx6.25) was measured by Kjeldahl (ISO 1871) and fat by Soxhlet procedures. Total dietary fibers were determined by the AOAC method 991.43, βG by the AOAC method 995.16 and RS by the AOAC method 2002.02 using a Megazyme Kit (Megazyme International Ireland Ltd, Wicklow, Ireland). On Table 1, total starch represents the total amount of starch including the RS.
Blood analysis
Blood samples were collected in pre-cooled vacutainers with K3EDTA as anticoagulant and centrifuged immediately (3000 rpm for 10 min at 4 °C) for plasma separation. For serum, blood was collected in plain vacutainers, allowed to clot at room temperature for 30 min and then centrifuged (3000 rpm for 10 min at 4 °C). After isolation, plasma and serum were stored at − 80 °C until analysis.
For total ghrelin determination, plasma was pretreated according to the procedure described in a previous study [20]. Ghrelin at 0, 30, 60, 120 and 180 min was assayed by a sandwich ELISA method on a microtiter plate using a commercially available human ghrelin kit [Human Ghrelin (Total) ELISA kit; Millipore]. Insulin was similarly detected by a sandwich ELISA method using a commercially available human insulin kit (Human Insulin ELISA kit; Millipore) at 0, 15, 30, 45, 60, 90, 120 and 180 min, in serum. Total GLP-1 and PYY at 0, 15, 30, 60, 90, 120 and 180 min were also assayed by a sandwich ELISA method using a commercially available human GLP-1 kit (Human Total Glucagon-Like Peptide-1 kit; Millipore) and a human PYY kit [Human PYY (Total) ELISA kit; Millipore], respectively. Glucose concentration at 0, 15, 30, 45, 60, 90, 120 and 180 min was measured in plasma by YSI 2300 STAT Plus Glucose Lactate Analyzer. At the beginning of the study, basal biochemical measurements including the total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triacylglycerols (TAG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (γ-GT), urea, creatinine, uric acid and total proteins were performed in serum, automatically, by an automated biochemical analyzer (Medilyzer), using commercially available diagnostic kits.
Subjective appetite
Subject’s appetite ratings, specifically, hunger, fullness and desire to eat after the consumption of the four test meals were assessed at the times 0, 15, 30, 45, 60, 90, 120 and 180 with the use of visual analogue scales (VAS). Three main questions were included, “How hungry do you feel”, “How full do you feel” and “How great is your desire to eat”. Subjects were asked to rate these three parameters on a 10-cm line scale ranging from 0 (“not at all”) to 10 (“extremely”), with labels at the extremities indicating the most positive and negative ratings. Subjects were not allowed to discuss their ratings with each other throughout the sessions.
Sensory evaluation
The subjects who participated in the study were asked to evaluate the sensory characteristics of the two types of bread (βGB, RSB). Palatability of the breads in terms of texture, color, aroma, taste and overall acceptability on a 9-point hedonic scale (1 = extremely dislike, 5 = neither like nor dislike, 9 = extremely like) were evaluated. The hedonic scale is a standard tool for measuring food acceptability, that is, how much a consumer likes or dislikes a product.
Statistical analysis
Descriptive statistics are presented as mean ± SD and the results as mean ± SEM. The incremental area under the curve (iAUC) was calculated for glycaemic and insulinaemic responses, ghrelin, GLP-1 and PYY applying the trapezoidal rule. Accordingly, iAUCs for VAS were estimated. The GI was determined as the mean individual ratios resulting from iAUC for glucose over a period of 120 min after ingestion of βGB and RSB relative to that after ingestion of the glucose solution. ANOVA for repeated measures, followed by the post hoc Bonferroni’s test, was used to identify significant differences regarding postprandial concentrations of plasma glucose, subjective appetite ratings and the respective iAUCs between treatments. A paired sample Students t-test was used to compare insulin, ghrelin, GLP-1 and PYY postprandial levels at specific time points as well as iAUCs between βGB and RSB. Students t-test was also performed to estimate the differences for the sensory properties. P < 0.05 was considered statistically significant. The SPSS 21.0 statistical software package was used for the analyses.
Results
The anthropometric and basal biochemical characteristics of the 10 subjects who completed successfully the four sessions of the study are presented on Table 2.
The GI of the two types of bread was found to be lower than 55 i.e. for βGB, 48 ± 10 and for RSB 40 ± 8. The mean individual ratios of iAUC resulting from iAUC over a period of 120 min for each of the test meals compared with that of the reference meal of the glucose solution were used for the calculation of GI.
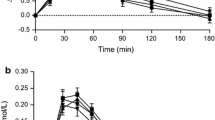
The glycaemic response to βGB, RSB and reference meal (GS) was determined over a 3 h period after the consumption of each test meal and is presented in Fig. 1a. Both breads ameliorated postprandial glycaemic response. The consumption of the reference meal caused the highest iAUC, i.e. 2818 ± 498 mg dL−1 × 180 min. iAUCs for RSB and βGB are presented in Table 3. The difference was statistically significant for the two breads compared to GS (P < 0.05). Mean peak glucose concentration was higher after GS followed by RSB and βGB (142.2 ± 7.6 compared to 113.8 ± 3.9 and 115.0 ± 4.3 mg/dL, respectively, P < 0.05). No statistically significant difference was noticed between the two types of bread.
Insulin response to the two different types of bread is illustrated in Fig. 1b. The iAUC did not differ between the two types of bread (P = 0.507). The peak insulin value was observed at 45 min postprandially after the consumption of βGB and RSB and no significant difference was detected between them (52.2 ± 10.7 and 57.6 ± 13.8 μU/mL, respectively, P = 0.668).
Total ghrelin response expressed as a decrease from preprandial values is presented in Fig. 1c. The iAUC did not differ between the two types of bread (P = 0.449). Moreover, both breads caused similar ghrelin suppression at the individual time points without any statistically significant differences. However, βGB caused the maximum suppression at time point 120 min while RSB at 30 min. At the time point 120 min there was a marginally statistically significant difference (P = 0.1) with the βGB leading to the larger decline of ghrelin secretion compared to RSB (− 292.52 ± 72.52 and − 130.11 ± 82.39 pg/mL, respectively).
Plasma GLP-1 and PYY concentrations expressed as an increase from preprandial values are illustrated in Fig. 1d, e respectively. There were no statistically significant differences between the iAUC of the two breads neither for GLP-1 nor for PYY (P = 0.190, P = 0.283, respectively). The plasma GLP-1 concentrations were higher during the first 60 min after the consumption of βGB than RSB. The difference was marginally significant (P = 0.1 at the time point 60 min).
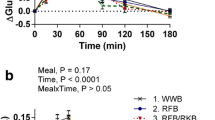
Figure 2 shows the subjective appetite rating differences from preprandial values over the 180 min after the consumption of the test breads. Perceived appetite for hunger, fullness and desire to eat was similar in response to the two breads (P > 0.5). iAUC values did not indicate any significant differences between the test breads (Table 4). However, significantly lower hunger, lower desire to eat and higher fullness were observed at all time points, after the consumption of the two breads in comparison to GS (P < 0.05).
Regarding sensory evaluation, both breads scored similarly and achieved high overall acceptance scores (Table 5). More specifically, βGB had an overall score of 6.5 ± 0.5 and RSB of 6.9 ± 0.5 (P = 0.1) which showed no statistical significance, but a trend that RSB tends to have better palatability than βGB.
Discussion
A rapid increase in the incidence of chronic metabolic disorders [21, 22] has created an urgent need of finding strategies that aim to their prevention and treatment. In conjunction with growing scientific evidence that associate mild postprandial glycaemia with beneficial metabolic outcomes [23,24,25], the development of low GI products arises as a potential useful practice. Techniques that seem to have promising results are the enrichment of foods with dietary fibers either soluble or insoluble.
White bread is reported as a high GI food (75 ± 2) [26]. In the present study, both breads are found to have a low GI, below 55 as it was expected since the content of β-glucans and resistant starch were close or exactly (respectively) the ones suggested by EFSA. The determination of GI was accomplished according to ISO 26642 and glucose solution was selected as a reference food and was tested twice, so the classification could be made by using the GI scale.
More specifically, the GI of βGB was found to be 48 ± 10, with glucose as a reference food. βGB lowered the postprandial glycaemic response by 48%. These findings are in accordance with previous clinical trials, which have shown that enrichment of breads with βG results to milder glycaemic response [27,28,29,30,31]. The mechanism of action that explains the result is well established. βG is very high molecular weight polysaccharides that exhibit high viscosities at low concentrations. Consequently, the enrichment of foods with this kind of fiber slows the mixing of the meal with the digestive enzymes and delays the gastric emptying, and thus retards the absorption of glucose [12, 32]. The dose of βG that can achieve the attenuation of postprandial glucose is 4 g for 30–80 g of available carbohydrates [32]. In the present study, the βGB contains 3.6 g per 30 g, a sufficient amount that can explain the lower GI of the bread. Recent study by Ekstrӧm et al. reported that even the amount of 1.9 g of βG per 30 g of available carbohydrates gave significant lowering in postprandial glucose levels (GI = 64 ± 5) [31].
Second aim of the present clinical trial was the evaluation of the satiety caused by the two different fortified breads. To this purpose, except for the examination of visual analogue scales (VAS), gastrointestinal hormones such as ghrelin, PYY and GLP-1 were measured.
Ghrelin is a peripheral hormone, that is synthesized in the gastrointestinal tract, especially in the fundus of the stomach, and its concentration is directly related to appetite ratings. In normal-weight subjects, plasma ghrelin decreases after the consumption of a meal. On the other hand, PYY, a hormone derived mainly from the colon, is considered to have potent anorexigenic properties [33]. Another peptide that acts as a satiety signal and increases postprandially is the GLP-1. This hormone is produced by the L cells of the distal small intestine in response to a nutrient load and its effect on the modulation of appetite is orchestrated by a complex brain-gut relationship [34].
The relationship of βG and the postprandial hormonal concentrations is not fully clarified. Research from Vitaglione et al. suggests that βG enriched bread is able to modulate appetite ratings through the significant alteration of ghrelin and PYY levels [28]. Similar outcomes were observed even if the βG were incorporated in other meals, such as biscuits, rice or juices [35,36,37,38]. As previously referred, βG are a very viscous fiber, a characteristic that gives to the enriched meal prolonged transit time and the absorption rate of nutrients. The prolonged presence of nutrients in the GI tract raises the possibility of interaction between nutrients and the intestinal mucosa to stimulate the release of peptides involved in appetite regulation. On the other hand, Juntunen et al. [39] showed that the GLP-1 response did not differ between bread with βG and white bread. Another more recent clinical trial, also reported that consumption of barley bars containing 1.2 g of barley βG did not change appetite and energy intake when compared with oat bars containing 0.3 g of oat βG [40].
The iAUCs for hunger, fullness and desire to eat were statistically significant different between the bread and the reference food, however, no significant difference was observed between the two breads. The use of VAS to measure variability of appetite, despite of being a subjective method, has reproducibility and seems to be connected with the levels of satiety hormones [41, 42].
With regard to RS, thorough research has been conducted, in both humans and animal models, for its effects in postprandial glycaemic response. Particularly, it lowers glycaemia when it replaces the available carbohydrates portion of meal [15]. In accordance with these findings, in our study RSB had an advantage over the glucose solution, as it lowered the postprandial glycaemic response by 59%. A plethora of studies has examined RS role in improving a variety of metabolic features. Previous clinical trials conducted in healthy subjects reported great reductions in postprandial glucose response after the consumption of RS enriched breads or crackers in comparison with control of white wheat bread [43,44,45,46,47,48,49]. In some cases, there was even found an improvement in insulin sensitivity, when RS represented the 10.8% to 12.3% dry matter of bread, in comparison with approximately 2% in the reference food (white wheat bread) [48, 49].
Moreover, a few studies in which healthy participants consumed rice or plantains containing a high amount of RS have shown a correlation between RS content and milder glycaemic response [50,51,52,53]. Attempts have been made to understand how RS influences glycaemic control. Its beneficial effects may be achieved through colonic production of short-chain fatty acids (SCFAs), then subsequent absorption, by exerting anti-lipolytic activity and affecting the activity of gut hormones, such as GLP-1, which concentrations increase following the consumption of RS-containing foods and stimulate insulin secretion [54]. In our study βGB caused higher ghrelin suppression than RSB however, a significant difference in iAUC was not observed probably due to the small number of individuals. The two breads caused similar GLP-1 and PYY rise. A higher increase in GLP-1 was noticed after the consumption of βGB during the first 60 min of the postprandial state.
The GI of RSB was measured 40 ± 8, with glucose as a reference food. The dose of RS that should be used to achieve a milder postprandial glucose is 14% of the total starch [20]. In the present study, the RSB contains about 15%, an amount that can explain the lower GI of the bread.
Consumption of RSB leads to decrease of hunger and desire for the next meal and increase in fullness. Similar results have been found in other clinical studies in which participants consumed breads enriched with RS and the results were compared with control bread with low concentration in RS [44, 55,56,57]. An intervention study comparing a high RS with a control diet have shown a reduction in hunger and increase in subjects’ satiety after the consumption of the first diet [58].
Regarding GLP-1 and PYY there has been reported in previous studies a correlation between consumption of bread rich in RS and an increase in these hormones’ postprandial concentrations in plasma [44, 48, 49, 59]. Intervention studies have shown beneficial effects in GLP-1 or PYY levels of a high RS compared to a control diet [60, 61]. It is also noteworthy that in a study in a rodent population, RS appeared to stimulate the secretion of GLP-1 and PYY from the intestine [62].
Between the two breads no statistically significant differences in the secretion of gut hormones were observed. However, there was a trend of a larger decline of ghrelin secretion and a higher concentration of GLP-1 after the consumption of βGB compared with RSB at time points 120 min and 60 min, respectively. This can be explained by taking into consideration that RS, as a non- viscous fibre, does not delay gastric emptying in a similar way to that observed with viscous fibers [15, 40, 63].
The results indicated a great effect of both breads enriched with either βG or RS in postprandial glycaemia compared with the reference food. There was also statistical significant difference in the VAS analogue scales for the two breads compared to the reference food. There were found no statistically significant differences in glucose or insulin responses after the ingestion of βGB and RSB. The two breads showed similar sensory characteristics and had great overall acceptability from the participants. According to our knowledge, this is the first study to compare the effects of beta-glucans and RS when supplemented in amounts suggested by EFSA, on bread postprandial glycemic and hormonal responses.
In conclusion, the addition of βG or RS to wheat bread can lead to a low GI food which may induce satiety. The replacement of white wheat bread with any of these two types of bread can be a positive nutritional alteration which can provide metabolic benefits for the prevention and management of chronic diseases such as diabetes and obesity.
References
Augustin LS, Kendall CW, Jenkins DJ et al (2015) Glycaemic index, glycaemic load and glycaemic response: an international scientific consensus summit from the international carbohydrate quality consortium (ICQC). Nutr Metab Cardiovasc Dis 25(9):795–815
Levitan EB, Song Y, Ford ES, Liu S (2004) Is nondiabetic hyperglycaemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med 164:2147–2155
Sieri S, Krogh V (2017) Dietary glycaemic index, glycaemic load and cancer: An overview of the literature. Nutr Metab Cardiovasc Dis 27(1):18–31
Lafiandra D, Riccardi G, Shewry PR (2014) Improving cereal grain carbohydrates for diet and health. J Cereal Sci 59(3):312–326
Cust AE, Skilton MR, van Bakel MM et al (2009) Total dietary carbohydrate, sugar, starch and fiber intakes in the European prospective investigation into cancer and nutrition. Eur J Clin Nutr 63(Suppl 4):S37–60
Quilez J, Salas-Salvado J (2012) Salt in bread in Europe: potential benefits of reduction. Nutr Rev 70(11):666–678
Fardet A, Leenhardt F, Lioger D (2006) Parameters controlling the glycaemic response to breads. Nutr Res Rev 19(1):18–25
Livesey G, Taylor R, Hulshof T, Howlett J (2008) Glycaemic response and health—a systematic review and meta-analysis: relations between dietary glycaemic properties and health outcomes. Am J Clin Nutr 87(1):258s–268s
Scazzina F, Siebenhandl-Ehn S, Pellegrini N (2013) The effect of dietary fibre on reducing the glycaemic index of bread. Br J Nutr 109(7):1163–1174
Stamataki NS, Yanni AE, Karathanos VT (2016) Non-cereal ingredients for the attenuation of glycaemic response to bread: a review of the clinical evidence. Food Funct 7(7):2926–2936
Wang Q, Ellis PR (2014) Oat β-glucan: physico-chemical characteristics in relation to its blood-glucose and cholesterol-lowering properties. Br J Nutr 112(Suppl 2):S4–S13
European Commission (2012) Regulations Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off J Eur Union 136:1–40
EFSA (2011) Scientific opinion on health claims already evaluated (ID 215, 568, 674, 712, 1398, 1633, 1974, 4191, 4192, 4193, 4236, 4335, 4698, 4704) pursuant to article 13(1) of regulation (EC) no 1924/200. EFSA J 9:2203
Robertson MD (2012) Dietary-resistant starch and glucose metabolism. Curr Opin Clin Nutr Metab Care 15:362–367
Barros JHT, Telis VRN, Taboga S, Franco CML (2018) Resistant starch: effect on rheology, quality, and staling rate of white wheat bread. J Food Sci Technol 55(11):4578–4588
Englyst HN, Kingman SM, Cummings JH (1992) Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr 46(Suppl 2):S33–50
Higgins JA (2014) Resistant starch and energy balance: impact on weight loss and maintenance. Crit Rev Food Sci Nutr 54(9):1158–1166
Maziarz M, Sherrard M, Juma S (2013) Sensory characteristics of high-amylose maize-resistant starch in three food products. Food Sci Nutr 1(2):117–124
EFSA (2011) Scientific Opinion on the substantiation of health claims related to resistant starch and reduction of post-prandial glycaemic responses (ID 681), “digestive health benefits” (ID 682) and “favours a normal colon metabolism” (ID 783) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J 9:2024–2041
Yanni AE, Stamataki NS, Stoupaki M et al (2016) Cr-enriched yeast: beyond fibers for the management of postprandial glycemic response to bread. Eur J Nutr 56(4):1445–1453
International Diabetes Federation (2017) IDF Diabetes Atlas, 8th edn. Update, IDF
Blüher M (2019) Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 15:288–298
Bhupathiraju SN, Tobias DK, Malik VS et al (2014) Glycaemic index, glycaemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr 100(1):218–232
Rossi M, Turati F, Lagiou P (2013) Mediterranean diet and glycaemic load in relation to incidence of type 2 diabetes: results from the Greek cohort of the population-based European Prospective Investigation into Cancer and Nutrition (EPIC). Diabetologia 56(11):2405–2413
Mirrahimi A, Chiavaroli L, Srichaikul K et al (2014) The role of glycaemic index and glycaemic load in cardiovascular disease and its risk factors: a review of the recent literature. Curr Atheroscler Rep 16(1):381
Fiona S, Atkinson K-P, Brand-Miller JC (2008) International tables of glycaemic index and glycaemic load values. Diabetes Care 31(12):2281–2283
Finocchiaro F, Ferrari B, Gianinetti A et al (2012) Effects of barley β-glucan-enriched flour fractions on the glycaemic index of bread. Int J Food Sci Nutr 63(1):23–29
Vitaglione P, Lumaga RB, Stanzione A et al (2009) Beta-Glucan-enriched bread reduces energy intake and modifies plasma ghrelin and peptide YY concentrations in the short term. Appetite 53(3):338–344
HartvigsenML GS, Lærke HN et al (2014) Effects of concentrated arabinoxylan and β-glucan compared with refined wheat and whole grain rye on glucose and appetite in subjects with the metabolic syndrome: a randomized study. Eur J Clin Nutr 68(1):84–90
De Angelis M, Rizzello CG, Alfonsi G (2007) Use of sourdough lactobacilli and oat fibre to decrease the glycaemic index of white wheat bread. Br J Nutr 98(6):1196–1205
Ekström LM, Henningsson Bok EA, Sjöö ME, Östman EM (2017) Oat β-glucan containing bread increases the glycaemic profile. J Funct Foods 32:106–111
Tosh SM (2013) Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur J Clin Nutr 67(4):310–317
Horner K, Lee S (2015) Appetite-related peptides in childhood and adolescence: role of ghrelin, PYY, and GLP-1. Appl Physiol Nutr Metab 40(11):1089–1099
Meera S, Adrian V (2014) Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord 15(3):181–187
Weickert MO, Spranger J, Holst JJ et al (2006) Wheat-fibre-induced changes of postprandial peptide YY and ghrelin responses are not associated with acute alterations of satiety. Br J Nutr 96(5):795–798
Aoe S, Ikenaga T, Noguchi H et al (2014) Effect of cooked white rice with high β-glucan barley on appetite and energy intake in healthy Japanese subjects: a randomized controlled trial. Plant Foods Hum Nutr 69(4):325–330
Pentikäinen S, Karhunen L, Flander L et al (2014) Enrichment of biscuits and juice with oat β-glucan enhances postprandial satiety. Appetite 75:150–156
Juvonen KR, Purhonen AK, Salmenkallio-Marttila M et al (2009) Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J Nutr 139(3):461–466
Juntunen KS, Niskanen LK, Liukkonen KH et al (2002) Postprandial glucose, insulin, and incretin responses to grain products in healthy subjects. Am J Clin Nutr 75(2):254–262
Peters HP, Boers HM et al (2009) No effect of added β-glucan or of fructooligosaccharide on appetite or energy intake. Am J Clin Nutr 89(1):58–63
Horner KM, Byrne NM, King NA (2014) Reproducibility of subjective appetite ratings and ad libitum test meal energy intake in overweight and obese males. Appetite 81:116–122
de Graaf C, Blom WA, Smeets PA, Stafleu A, Hendriks HF (2004) Biomarkers of satiation and satiety. Am J Clin Nutr 79(6):946–961
Ekstrom LM, Bjorck IM, Ostman EM (2012) On the possibility to affect the course of glycaemia, insulinaemia and perceived hunger/ satiety to bread meals in healthy volunteers. Food Funct 4:522–529
Johansson EV, Nilsson AC, Ostman EM et al (2013) Effects of indigestible carbohydrates in barley on glucose metabolism, appetite and voluntary food intake over 16 h in healthy adults. Nutr J 12:46
Gower BA, Bergman R, Stefanovski D et al (2016) Baseline insulin sensitivity affects response to high-amylose maize resistant starch in women: a randomized, controlled trial. Nutr Metab 13:2
Hallström E, Sestili F, Lafiandra D et al (2011) A novel wheat variety with elevated content of amylose increases resistant starch formation and may beneficially influence glycaemia in healthy subjects. Food Nutr Res 55:7074. https://doi.org/10.3402/fnr.v55i0.7074
Nilsson A, Granfeldt Y, Ostman E et al (2006) Effects of GI and content of indigestible carbohydrates of cereal-based evening meals on glucose tolerance at a subsequent standardised breakfast. Eur J Clin Nutr 60:1092–1099
Nilsson AC, Johansson-Boll EV, Bjorck IM (2015) Increased gut hormones and insulin sensitivity index following a 3-d intervention with a barley kernel-based product: a randomised cross-over study in healthy middle-aged subjects. Br J Nutr 114:899–907
Sandberg JC, Bjorck IM, Nilsson AC (2016) Rye-based evening meals favorably affected glucose regulation and appetite variables at the following breakfast; a randomized controlled study in healthy subjects. PLoS ONE 11:e0151985
Jiménez-Domínguez G, Ble-Castillo JL, Aparicio-Trápala MA et al (2015) Effects of acute ingestion of native banana starch on glycaemic response evaluated by continuous glucose monitoring in obese and lean subjects. Int J Env Res Pub He 12:7491–7505
Oladele EO, Williamson G (2016) Impact of resistant starch in three plantain (Musa AAB) products on glycaemic response of healthy volunteers. Eur J Nutr 55:75–81
Karupaiah T, Aik CK, Heen TC et al (2011) A transgressive brown rice mediates favourableglycaemic and insulin responses. J Sci Food Agric 91:1951–1956
Li M, Piao J-H, Tian Y et al (2010) Postprandial glycaemic and insulinaemic responses to GM-resistant starch-enriched rice and the production of fermentation-related H 2 in healthy Chinese adults. Br J Nutr 103:1029–1034
Lockyer S, Nugent AP (2017) Health effects of resistant starch. Nutr Bull 42:1
Chiu Y-T, Stewart ML (2013) Effect of variety and cooking method on resistant starch content of white rice and subsequent postprandial glucose response and appetite in humans. Asia Pac J Clinnutr 22:372–379
Monsivais P, Carter BE, Christiansen M et al (2013) Soluble fiber dextrin enhances the satiating power of beverages. Appetite 56:9–14
Nilsson A, Johansson E, Ekstrom L et al (2013) Effects of a brown beans evening meal on metabolic risk markers and appetite regulating hormones at a subsequent standardized breakfast: a randomized cross-over study. PLoS ONE 8:e59985
Sarda F, Giuntini EB, Gomez ML et al (2016) Impact of resistant starch from unripe banana flour on hunger, satiety and glucose homeostasis in healthy volunteers. J Funct Foods 24:63–74
Nilsson AC, Östman EM, Holst JJ et al (2008) Including indigestible carbohydrates in the evening meal of healthy subjects improves glucose tolerance, lowers inflammatory markers, and increases satiety after a subsequent standardized breakfast. J Nutr 138:732–739
García-Rodríguez CE, Mesa MD, Olza J et al (2013) Postprandial glucose, insulin and gastrointestinal hormones in healthy and diabetic subjects fed a fructose-free and resistant starch type IV-enriched enteral formula. Eur J Nutr 52:1569–1578
Robertson MD, Bickerton AS, Dennis AL et al (2005) Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr 82:559–567
Zhou J, Martin RJ, Tulley RT et al (2008) Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab 295(5):E1160–E1166
Marciani L, Pritchard SE, Hellier-Woods C et al (2013) (2013) Delayed gastric emptying and reduced postprandial small bowel water content of equicaloric whole meal bread versus rice meals in healthy subjects: novel MRI insights. Eur J Clin Nutr 67(7):754–758
Acknowledgements
Authors are grateful to the volunteers who participated in the study and all the persons involved in the conduct of the study. This work was supported by a research grant from ELBISCO S.A. (Grant no. K439) and was awarded to Dr. Amalia Yanni.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study.
Rights and permissions
About this article
Cite this article
Binou, P., Yanni, A.E., Stergiou, A. et al. Enrichment of bread with beta-glucans or resistant starch induces similar glucose, insulin and appetite hormone responses in healthy adults. Eur J Nutr 60, 455–464 (2021). https://doi.org/10.1007/s00394-020-02265-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02265-6