Abstract
Although there is an extensive literature on the properties and possible electron transfer pathways of cytochrome b-559, which is a prominent subunit of the multi-subunit photosystem II complex which functions in oxygenic photosynthesis, there is presently no consensus on the function of b-559 in the photosynthetic electron transport chain. The inability in earlier times to define a redox-linked function of this cytochrome was, to a large extent, a consequence of an absence of biochemical and structure information to complement an extensive array of spectrophotometric studies of the cytochrome in situ. Based on the location of hetero-dimeric b-559 in the photosystem II reaction center complex, derived from crystal crystallographic structure analysis, and the absence of a necessary redox function for the cytochrome in PSII, it is proposed that the main function of cytochrome b-559 is linked to its role as a structure component in the PSII reaction center complex. This function resides in the association of b-559 through its heme histidine residues in the trans-membrane domains of the PsbE and PsbF subunits of the PSII reaction center. These subunits, along with PsbJ, are inferred, from the analysis of structure, to define the intra-membrane portal in the PSII reaction center for plastoquinol (PQH2) export which, through the PSII complex, provides the redox link to the cytochrome b6f complex in the electron transfer chain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Properties of cytochrome b-559 intra-membrane redox protein associated with details of oxygenic photosynthesis localized to the photosystem II reaction center have been recently discussed to a significant extent (Müh and Zouni 2016; Chu and Chen 2016; Eerden et al. 2017; Pospisil 2011).
The goals of the present review/discussion are as follows: (I) to describe relevant aspects in the history of studies on the function of cytochrome b-559, for which the author, who participated in these studies, may be able to present additional perspective; (II) to consider inferences for structure-based function of b-559 which can be derived from its environment in the membrane and in the photosystem II (PSII) reaction center. (III) In contrast to the literature on structure–function of cytochrome b-559, it is proposed that the function of the cytochrome resides mainly in its contribution to the structure of the photosystem II reaction center complex.
Cytochrome environment and function
Although determination of a well-defined redox function of cytochrome b-559 has not been achieved, the position of the cytochrome in the multi-subunit photosystem II reaction center complex (‘PSII’), described in recent crystallographic structure analysis of the reaction center (Tae et al. 1993), suggests a function for b-559 in the structure of the photosystem II reaction center complex.
‘b-559,’ a misnomer?
It is noted that it might be appropriate to place quotation marks around b-559 because, in fact, spectrophotometric data in the literature document that the alpha-band peak of the redox difference spectrum of ‘b-559’ is, in fact, slightly closer to 560 nm than to 559 nm, as discussed in reference (Tae et al. 1993) and subsequently noted elsewhere, including reference (Müh and Zouni 2016).
Environment of cytochrome b-559
This review is intended to complement other recent reviews/discussions in the literature which concern the membrane/protein structure environment and function of cytochrome b-559. A relatively recent review (Müh and Zouni 2016), which has a role in the background of the suggestion presented below (sections “Cytochrome b-559; Topography in the PS II Reaction Center; Consequences for Function” and “Cytochrome b-559; a Major Role in the Structure of the Photosystem II Reaction Center Complex”) on the function(s) of cyt b-559, included perspectives on (i) the protein environment of the cytochrome in the PS II reaction center complex and, specifically, (ii) the position of b-559 heme and apoprotein relative to the proposed pathway of inter-complex plastoquinone/ol traffic; (ii) regarding this traffic a description of a surface ‘portal’ framed in the trans-membrane sector of the photosystem II reaction center complex utilized for the release of plastoquinol (PQH2) into the membrane as an electron and proton donor to the cytochrome b6f complex which bridges the photosystem I and II reaction center complexes.
Historical context
To explain the somewhat cryptic reference to b-559 jn the title of this manuscript, one can consider two phases in the history of studies on its function, (i) before, and (ii) after determination of its crystal structure, and the application of this knowledge to the structure of the photosystem II complex in which it resides.
Considering the history of studies on the position and function of cytochrome b-559 in the electron transport chain (ETC) of oxygenic photosynthesis, in the early studies, the models were based on a ‘mitochondrial perspective’ (Chance 1982), i. e., an arrangement of the electron transport carriers in the photosynthetic chain organized similarly to the classical textbook description of a ‘linear’ mitochondrial sequence of electron transporters. In retrospect, it can be said that this was a consequence in the post-World War II years (1950–1970) of the intellectual, and admirable, domination of the field of organelle bioenergetics by the concepts derived from studies on the mitochondrial electron transport chain and its energetics (e. g., (Chance 1982; Slater 1967; Crofts and Berry 1998)).
Mitochondrial and chloroplast electron transport chains
A summary is shown below (scheme i) of the mitochondrial electron transport (respiratory) chain (i) extending from ‘low potential’ (− 0.32 V at pH 7 on the electrochemical redox scale) of reduced pyridine nucleotide (NADH) to molecular oxygen (O2) via the hydrogen carrier, ubiquinone, UQ, and the cytochrome b-c heme proteins, to the terminal electron acceptor and the protein site, cytochrome a-a3 oxidase, of binding of molecular O2, and its reduction to H2O (standard redox potential + 0.81 V for the O2/H2O redox couple at pH 7.0).
With this biochemical perspective, a consensus ‘simple’ early model of the ‘non-cyclic’ ‘linear’ photosynthetic electron transport chain, initiated by the reduced quinone electron acceptor, plastoquinol, of the photosystem II reaction center, and including the electron transport chain connecting the two photosystems, I (PSI) and II (PSII), via the intra-membrane diffusing hydrogen carrier, plastoquinone/ol, PQ/PQH2, was, and is as follows:
It is noted that such models usually included ‘cyt b’ functioning in a linear chain connecting the two photosystems (Cramer and Butler 1967), as described above in reaction (ii) above, a model to which an author (WAC) of the present article contributed, and which turned out to be wrong.
Improved insight on structure–function of the intra-membrane electron transport carriers of oxygenic photosynthesis occurred as a consequence of the application of crystal structure determination to the intra-membrane multi-subunit photosynthetic redox proteins, information initially obtained for the bacterial photosynthetic reaction center (Cramer and Butler 1967). Also essential for these studies was the development and application of spectrophotometric instrumentation with the ability to measure sub-picosecond (< 10–12 s−1) kinetics for studies on the primary photochemistry, for light-dependent electron transfer activity, utilizing the light-induced small (ca.10–4) absorbance changes of optically turbid samples.
Early studies
Initial studies on in situ properties of a b-type cytochrome with a redox (reduced minus oxidized) difference spectrum characterized by an alpha-band wavelength maximum at approximately 559 nm as a component of a detergent-separated fraction of the electron transport chain of oxygenic photosynthesis were carried out by Lundegardh (Deisenhofer and Michel 1989), and, subsequently, by Boardman and Anderson (Lundegarth 1962). In these studies, spinach chloroplasts were separated by incubation with the detergent-like solvent, digitonin, and cytochrome difference spectra were recorded at 20 °C and 77°K. Measurement at the lower temperature, in liquid nitrogen, was used to obtain better defined spectra. This allowed resolution of the separate absorbance peaks associated with three cytochrome components. The three cytochrome components of the electron transport chain, separable by differences in spectra include (i) cytochromes f and b6, heme-containing subunits in the isolatable redox difference (reduced minus oxidized) spectra. (ii) The presence of a third cytochrome associated with the electron transport chain is inferred from the redox (hydroquinone or ascorbate as reductant minus ferricyanide as the oxidant) difference spectrum which has a reduced α-band peak at 559–560 nm when measured at 20 °C, and at 556–557 nm when measured at cryogenic temperatures (e. g., at liquid nitrogen temperature, ca. − 196 °C). The lower temperature of measurement is utilized to obtain a more narrow distribution of energy levels and, thereby, ‘sharper’ spectra with a more narrow band.
width and, thereby, with better defined wavelength maxima. Such spectra were subsequently used to define the spectrophotometric signature of cytochrome ‘b-559,’ an integral component of the photo-system II reaction center complex (e. g., reference (Pospisil 2011)) of plant chloroplast membranes, present in a molar ratio of 2.95:1 referenced to the content of cytochrome f (McKenzie et al. 2020).
The early reports on the function of the b-559, which include studies of ours (e. g., references (Böhme and Cramer 1971; Horton and Cramer 1975; Horton et al. 1976)) were carried out at a time which, in retrospect, can be considered an early stage in our understanding of the organization and structure–function of the three major integral membrane protein complexes, photosystems I and II, and the cytochrome b6f complex, which comprise the linear electron transport chain of oxygenic photosynthesis. Referring to the difficulty in determining the function of cytochrome b-559, the b-559 in these studies was characterized as ‘enigmatic’ (Cramer et al. 1993).
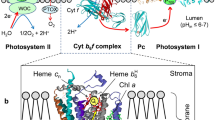
Some of the early interest in cytochrome b-559 can be attributed to an oxidation–reduction potential (midpoint potential, Em = + 350–400 mV) in native or undamaged membranes, which is unusually positive for a b-type cytochrome, approximately 0.3 V more positive than the potential of the b-cytochrome in a representative mitochondrial electron transport chain (reaction (i) above (Cramer and Knaff 1991), a general reference for oxidation–reduction reactions associated with biological energy transduction. Considering the significant effect of QB site inhibitors on the state of the b-559 (Horton et al. 1976; Kaminskaya et al. 2007), it can be inferred that the interaction between the QB quinone binding site and the cytochrome b-559 is exerted over a separation of approximately 25 Å between the QB site and the b-559 (Fig. 1).
Structure proximity of cyt b-559 to the prosthetic groups in the quinone transfer channels in the photosystem II reaction center complex. The arrangement of prosthetic groups is based on their presentation in reference (Müh and Zouni 2016), with distances between prosthetic groups in this version, based on information in references (Eerden et al. 2017) and (Kern and (40 co-authors), 2018), presented here to the nearest 0.5 Å
Relation of b-559 Function to its Em
The significance for function of the intra-membrane b-559 of the redox potential (Em = + 0.35- 0.40 V), which is unusually positive for energy-transducing b-type cytochromes, is not resolved in the present discussion. A central question regarding the function of cyt b-559 is associated with the absence of a proposal for its function, which (a) would be obligatory in the set of functions required for the structure and function of photosystem II, and (b) would accommodate its position in the structure of the multi-subunit PSII reaction center complex described in (1, 3, 5). A major change in perspective, based on structure considerations, i. e., the crystal structure of PSII (4; discussion of details in Müh and Zouni (2016)), results in the description reviewed here for the location of cyt b-559 in the PSII reaction center complex, inferred from its position in the structurally defined PSII reaction center complex (Figs. 2A–D), and the consequences of this location for its function.
Peripheral position of cytochrome b-559 in the PSII dimeric reaction center. (A) Schematic of cross-linking by heme of the PsbE and PsbF subunits of the PSII complex. (B) Major trans-membrane helical subunits of the PSII reaction center Psb X, F, E, and J are displayed as ribbons. Helices of cytochrome b-559 (Psb E and F) are shown in orange and red, respectively, and the heme in green sticks. Picture of the reaction center monomer on the right side shows additional single helix proteins surrounding cytochrome b-559: PsbJ (grey), X (aquamarine), Y (yellow), and Z (pink). (C) View from the side, the intra-membrane domain where supplemental single helix subunits are shown. (D) Heme ligation in cytochrome b-559 through His23 of PsbE and His24 of PsbF
Cytochrome b-559; topography in the PSII reaction center; Consequences for function
The heme in cytochrome b-559 ligates and links its PsbE and PsbF subunits (molecular weights 9.16 and 4.27 kDa, respectively, of spinach cytochrome b-559 (Fig. 2A). As noted, relative to the water-splitting apparatus, the b-559 is located on the opposite, the ‘stromal’ side, of the chloroplast thylakoid membrane, as shown in a representation of the arrangement of the major prosthetic groups in the photosystem II reaction center (Fig. 1). Electron transfer events in which the b-559 has been observed to participate, irrespective of a demonstration of kinetic competence and functional relevance, are (i) photo-oxidation at cryogenic temperatures (Knaff and Arnon 1969), and (ii) photo- reduction via the QB plastoquinone binding/exchange site (Whitmarsh and Cramer 1978). Only the latter pathway is physiologically relevant, as the crystal structure data indicate that the electron transfer distances for the former render the former pathway (‘i’ above) too slow to be meaningful.
An intra-membrane location of the b-559 heme accessible to the stromal, electrochemically negative surface of the membrane, was inferred from its relative accessibility to the highly charged (tri-valent) polar oxidant, ferricyanide (Horton and Cramer 1974). This ‘stromal’ or electro-negative (n-side) position of the cytochrome in the membrane (Figs. 1, 2B-D) has been suggested to be relevant in the determination of the trajectory, or pathway, of plastoquinol/quinone transfer (Müh and Zouni 2016). The associated electron transfer pathway extends from the primary quinone binding and reduction site of quinol formation, QA, through QB to the surface QH2-release (and uptake) sites in the photosystem II reaction center complex (Fig. 1). Regarding its intra-membrane position, the b-559 heme is positioned In the PSII reaction center complex near the plastoquinol exit portal of the PSII complex (Figs. 2B-D) which, in the functional photosynthetic membrane, faces the second of the three integral membrane protein complexes, the cytochrome b6f complex, which is positioned in the membrane for electron transfer between the two reaction center complexes. Plastoquinol, exiting PSII, donates electrons to the b6f complex and is deprotonated in the process. Proton translocation across the membrane involving the b6f complex occurs by pathways and mechanisms for which details are not presented here.
Thus, the n-side proximal position in the membrane of cyt b-559 is considered to be relevant. In the context of the trajectory and proposed trafficking portal (exit/entrance) of plastoquinol/quinone transfer from the primary QA quinone binding site and reduction site of quinol formation, quinol binding sites from QA through QB to the surface QH2-release (and ‘uptake’) sites in the photosystem II reaction center complex (Fig. 1).
b-559 Topography
Although cytochrome ‘b-559’ is located in the photosystem II reaction center complex which also contains the Mn sub-structure responsible for water-splitting/O2 evolution (the ‘OEC complex’), its position in the structure relative to the water-splitting domain of the PSII complex, in a skeleton representation of the PSII structure, is diagonally opposite, in a corner of the large multi-subunit photosystem II complex, relative to the water-splitting domain of the complex (Fig. 1). This figure, derived from (1) shows a map of the major plastoquinone binding sites in the PSII reaction center complex. In this rendition, the ‘b-559’ heme is shown close to the stromal, n-(electrochemically negative) side of the reaction center domain, approximately 40 Å from the Mn-containing oxygen-evolving sub-complex in PSII, and close to the surface of the PSII complex which faces the cyt b6f complex.
A position of the ‘b-559’ heme close to the membrane surface facing the stromal compartment of the chloroplast was inferred from its relative accessibility, compared to cytochrome f, to the membrane-impermeable high potential charged oxidant, ferricyanide (Horton and Cramer 1974). The molecular structure of ‘b-559’ in the context of the structure of the photosystem II reaction center complex, characterized in the literature as ‘enigmatic’ (Cramer et al. 1993), was described in the high-resolution structure of the multi-subunit PSII reaction center complex (Kern et al. 2018).
Regarding electron transfer functions coupled to free energy storage in the photosynthetic membrane which have been attributed to ‘b-559,’ electron transfer from ‘b-559’ to the PSII reaction center P680 is now realized to be kinetically insufficient and not associated with meaningful function in PSII electron transfer and energy storage because of the large (ca. 40 Å) electron donor–acceptor distance from b-559 to P680, which spans the PSII complex and the membrane (Fig. 1). This caveat applies specifically to the proposal that b-559 could function in an electron transport cycle operating in a pathway connected to the P680 reaction center (Thompson and Brudvig 1988).
Cytochrome b-559; a major role in the structure of the photosystem II reaction center complex
An inference which can be derived from this discussion, is that after more than half a century of study, involving the efforts of many laboratories spanning the world, including that of the authorsof this article, it is concluded that a meaningful electron transfer function of the cytochrome b-559 heme protein in the context of the photosystem II reaction center in which it resides, has not been obtained.
One is then led to consider an alternative possibility for the role or function of cytochrome b-559 in the PS II reaction center complex (Fig. 2). A proposed perspective describes the structure of the plastoquinol exit portal in the PSII reaction center (Müh and Zouni 2016; Eerden et al. 2017), through ligation to histidine residues (Babcock et al. 1985), in this case His 23 and His 24, respectively, in the trans-membrane psbE and psbF helices (Fig. 2A).
The essential nature of both histidine residues was first implied by mutagenic replacement of each of these histidines by a Leu residue (Pakrasi et al. 1991). Introduction of these missense mutations in the transformable unicellular cyanobacterium, Synechocystis 6803, resulted in complete loss of PSII activity. The b-559 heme is thus seen to have a major role in determination of the structure of the plastoquinol exit in the PSII reaction center, and presumably a portal as well for entrance of oxidized plastoquinone.
In summary, it is suggested that the cytochrome b-559 does not have a physiologically significant electron transfer function in the energy-transducing electron transport chain of oxygenic photosynthesis. However, the cytochrome has a structure-based function in photosystem system II where it contributes, through its ligation to the trans-membrane subunits psbE and psbF, and nearest-neighbor interactions with psbX and psbJ (Figs. 2B–D) in the structure of a plastoquinone exit-trafficking channel.
This channel specifically allows transfer of plastoquinone, reduced to the -quinol in the Photosystem II reaction center complex to transfer electrons to the cytochrome b6f complex and, coupled to the electron transfer, translocation of protons across the thylakoid membrane.
Abbreviations
- ATP:
-
Adenosine; cyt, cytochrome
- mDa:
-
Megadalton
- ms:
-
Millisecond
- OEC:
-
Oxygen-evolving complex
- PQ:
-
Plastoquinone
- p, n :
-
Electrochemically positive and negative sides of the membrane
- PS:
-
Photosystem
- Psb:
-
An acronym for photosystem II
References
Babcock GT, Widger WR, Cramer WA, Oertling WA, Metz JG (1985) Axial ligands of cytochrome b-559: identification and requirement for a heme-cross-linked polypeptide structure. Biochemistry 24:3638–3645
Boardman NK, Anderson J (1967) Fractionation of the photochemical systems of photosynthesis. II. cytochrome and carotenoid contents of particles isolated from spinach chloroplasts. Biochim Biophys Acta 143:187–203
Böhme H, Cramer WA (1971) Plastoquinone mediates electron transport between cytochrome b-559 and cytochrome f in spinach chloroplasts. FEBS Lett 15:349–435
Chance B (1982) Structure and function of the redox site of cytochrome oxidase. Adv Exp Med Biol 148:95–109
Chu H-A, Chen Y-H (2016) The roles of cytochrome b-559 in assembly and photoprotection of photosystem II revealed by site-directed mutagenesis studies. Front Plant Sci 6:1261
Cramer WA, Butler WL (1967) Light induced absorption changes of twocytochrome b components in the electron transport chain of spinach chloroplasts. Biochim Biophys Acta 14:332–339
Cramer, W. A., and D. B. Knaff (1991) Oxidation-Reduction; Electron and Proton Transfer. Energy Transduction in Biological Membranes; Chapter 2; Springer Study Edition [paperback, ISBN 0–387–97533–0]; 579 pp.
Cramer WA, Tae G-S, Furbacher P, Bottger M (1993) The enigmatic cytochrome b-559 of oxygenic photosynthesis. Physiol Plant 88:705–711
Crofts AR, Berry EA (1998) Structure and function of the cytochrome bc1 complex of mitochondria and photosynthetic bacteria. Curr Opin Struct Biol 8:501–509
Deisenhofer J, Michel H (1989) The photosynthetic reaction center from the purple photosynthetic bacterium, Rhodopseudomonas viridis. Science 245:1463–1473
Van Eerden FJ, Melo MN, Frederix PWJM, Periole X, Marrink SJ (2017) Exchange pathways of plastoquinone and plastoquinol in the photosystem II complex. Nature Comm 8:15214–15221
Horton P, Cramer WA (1974) The accessibility of the chloroplast cytochromes f and b-559 to ferricyanide. Biochim Biophys Acta 368:348–360
Horton P, Cramer WA (1975) Acid-base induced redox changes of the chloroplast b-559. FEBS Lett 56:244–247
Horton P, Whitmarsh J, Cramer WA (1976) On the specific site of action of 3-(3,4 di-chlorophenyl)-1,1-dimethylurea in chloroplasts: Inhibition of a dark acid-induced decrease in midpoint potential of cytochrome b-559. Arch Biochem Biophys 17:519–524
Kaminskaya O, Shuvalov VA, Renger G (2007) Evidence for a novel quinone binding site in the photosystem II (PS II) complex that regulates the redox potential of cytochrome b559. Biochemistry 46:1091–1105
Kern J et al (2018) Structures of the intermediates of Kok’s photosynthetic water oxidation clock. Nature 563:421–425
Knaff DB, Arnon DI (1969) Light-induced photo-oxidation of a chloroplast b-type cytochrome at -189 C. Proc. Natl Acad Sci. U S 63:956–962
Lundegarth H (1962) Quantitative relations between chlorophyll and cytochromes in chloroplasts. Physiol Plantarum 15:390
McKenzie SD, Ibrahim IM, Aryal UK, Puthiyaveetil S (2020) Stoichiometry of protein complexes in plant photosynthetic membranes. Biochim Biophys Acta 2:148141
Müh F, Zouni A (2016) Cytochrome b559 in Photosystem II. In: Cramer WA, Kallas T (eds) Cytochrome complexes, evolution, structures, energy transduction, and signaling advances in photosynthesis and respiration, vol 41. Springer, Dordrecht, pp 143–175
Pakrasi HB, De Ciechi P, Whitmarsh J (1991) Site directed mutagenesis of the heme axial ligands of cytochrome b559 affects the stability of the photosystem II complex. The EMBO J 10:1619–1627
Pospisil P (2011) Enzymatic function of cytochrome b-559 in photosystem II. Photochem Photobiol B 104:141–147
Slater EC (1967) An evaluation of the Mitchell hypothesis of chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Eur J Biochem 1:317–326
Tae G-S, Everly RM, Cramer WA, Madgwick SA, Rich PR (1993) On the question of the identity of cytochrome b-560 in thylakoid stromal membranes. Photosyn Res 36:141–146
Thompson LK, Brudvig GW (1988) Cytochrome b-559 may function to protect photosystem II from photoinhibition. Biochemistry 27:6653–6658
Whitmarsh J, Cramer WA (1978) A pathway for the reduction of cytochrome b-559 by photosystem II in chloroplasts. Biochim Biophys Acta 501:83–93
Acknowledgements
The authors’ research on ‘Structure-Function of Cytochromes in Oxygenic Photosynthesis,’ was supported at different times by non-overlapping grants from the Fogarty Foundation (TW0-1235), the National Science Foundation (GB-26635), the National Institutes of Health (GM-18457), a Research Career Development Award from the NIH (1 KO4 GM-29735), and grants from the USDA (59-2182-1-1-683-0) and DOE (Dept. of Energy) grant DE-SC0018238. We thank R. Harding for assistance with the assembly of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cramer, W.A., Zakharov, S.D. Concerning the enigmatic cytochrome b-559 of oxygenic photosynthesis. Photosynth Res 153, 157–162 (2022). https://doi.org/10.1007/s11120-022-00936-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-022-00936-5