Abstract
Water deficit is one of the key factors that limits the carbon (C) assimilation and productivity of plants. The effect of variable water deficit on recently root-derived bicarbonate assimilation in Camptotheca acuminate seedlings was investigated. Three-month-old seedlings were subjected to three water regimes, well-watered (WW), moderate stress (MS), and severe stress (SS) induced by polyethyleneglycol, in conjunction with relatively high (H) and low (L) natural 13C-abundance of NaHCO3-labeled treatments in hydroponics for 14 days. The δ13C of the newly expanded leaves in H were generally more enriched in heavy isotopes than were those in L, indicative of the involvement of bicarbonate in aboveground tissues. The C isotope fractionation of newly expanded leaves relative to air (∆13Cair-leaves) ranged from 17.78 to 21.78‰ among the treatments. The ∆13Cair-leaves under the MS and SS treatments in H were both more negative than was that in L. A linear regression between Ci/Ca and ∆13Cair-leaves in both L and H were different from the theoretical regression. On the basis of the two end-member mixing model, the proportion of fixed CO2 supplied from bicarbonate contributing to the total photosynthetically inorganic C assimilation were 10.34, 20.05 and 16.60% under the WW, MS, and SS treatments, respectively. These results indicated that the increase in water deficit decreased the atmospheric CO2 gain but triggered a compensatory use of bicarbonate in C. acuminate seedlings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water deficit is one of the key factors that limits the carbon (C) assimilation and productivity of plants (Chaves and Oliveira. 2004; Ramírez et al. 2012; Sapeta et al. 2013). These have become important issues worldwide since the global climate has undergone much change in recent decades. The shortage of water was believed to be increased in many areas around the earth due to frequently extreme precipitation events (IPCC 2014). The effects of water deficit on plants include the routine modulation of morpho-anatomical characteristics (Pang et al. 2011; De Micco and Aronne 2012; Jung et al. 2014), biochemical (Ancillotti et al. 2015; Kumar et al. 2015; Wang et al. 2015) and photochemical (Zivcak et al. 2013; Lauteri et al. 2014) processes, proteomics and gene expression (Krasensky and Jonak 2012) at different regulatory levels. To diminish water loss via transpiration, leaf gas exchange is regulated by stomatal closure, which often leads to the remarkable reduction of atmospheric C gain. Overall, a critical trade-off between water retention and C acquisition is mediated in response to water deficit.
Higher plants can acquire various C sources for survival. The majority of inorganic C is sequestered from atmospheric CO2 by terrestrial plants via photosynthesis. As has been stated already, this process is almost always hindered by environmental stress, such as drought, heat, chilling, salinity and other biotic factors and their combinations (Suzuki et al. 2014; Martínez-Lüscher et al. 2015). In addition, plants can also fix a minor amount of C internally from respiration of stem tissues, roots and microbes (Stringer and Kimmerer 1993; Ford et al. 2007; Grossiord et al. 2012). Early studies revealed that dissolved inorganic carbon (DIC) in root xylem contributed to aboveground C gain (Teskey and McGuire 2007; Aubrey and Teskey 2009). Bloemen et al. (2016) reviewed that a large fraction of root-respired CO2 could be delivered to aboveground tissues by the transpiration stream. To our knowledge, the form of DIC (CO2, HCO3 −, and CO3 2−) was pH-dependent. Previous studies have shown that the pH of xylem sap ranges from 4.2 to 6.8 and varies with the species (Levy et al. 1999; Wegner and Zimmermann 2004). This may be one reason that many studies have focused on CO2 in the xylem rather than HCO3 −. Nevertheless, there is a trend that an increasing number of studies have seemed to shed light on the utilization of HCO3 − by higher plants. The formation of HCO3 − usually results from the hydrolyzation of carbonate. A low level of bicarbonate results in an increase in biomass or had no effect on it (Bialczyk and Lechowsk 1995; Covarrubias and Rombolà 2013), while a high concentration of bicarbonate inhibited the growth, net photosynthetic rate, and chlorophyll content (Yang et al. 2009; Wu and Xing 2012). Wegner and Zimmermann (2004) accurately detected bicarbonate-induced alkalization of the xylem sap pH using a novel xylem pH probe, which seemed to be beneficial to the transport of HCO3 −. Stringer and Kimerer (1993) observed that 14C-labeled bicarbonate was delivered to veins and then mostly incorporated into sugar, starch, and protein in the Populus leaves. Rombolà et al. (2005) used high 13C-abundance Ca13CO3 (20 atom % enriched 13C) to trace the fate of bicarbonate assimilation and found 3.81 and 1.09% of 13C abundance in the fine roots and leaf blades of sugar beet plants, respectively. All of these results demonstrated that plants could utilize bicarbonate as a way of C sequestration in response to bicarbonate. However, the relationship between atmospheric CO2 and root-derived bicarbonate with respect to photosynthetically inorganic C assimilation is still poorly understood.
Camptotheca acuminate is a perennial woody plant of the Nyssaceae family that is used for timber and camptothecin extraction (Yu et al. 2012; Ying et al. 2014). In addition, C. acuminate is native to China and adapted to the calcareous soil. The lime soil is characterized by high pH and high concentration of bicarbonate. The bicarbonate concentration was reported to increase with increasing soil water content and CaCO3 content (Zuo et al. 2007). In well-aerated soil, HCO3 − concentration was about 5 mM while more than 10 mM was observed in wet soil (Boxma 1972; Bloom and Inskeep 1986). During the growing season, plants usually exposed to variable water deficit. C. acuminate was described as drought tolerant by improving water retention, antioxidation, and membrane integrity (Ying et al. 2014). However, the research did not fully explain how this species adapted to calcareous soil with co-occurring water deficit and bicarbonate, which is very common under natural conditions (Deng et al. 2012; Hu et al. 2013). In this regard, we hypothesized that water deficit would enhance the development of the root system, helping to promote water uptake and bicarbonate utilization. We also hypothesized that bicarbonate might be a potentially important C source for photosynthesis under the circumstance of drought-induced stomatal closure. This paper is the first to explore the proportion of bicarbonate utilization under variable water deficit in C. acuminate.
Stable isotope techniques are used as a nonradioactive tracer to reveal the uptake and accumulation of isotope in plants (Dawson et al. 2002; Tang et al. 2016). To trace the fate of bicarbonate, we used the natural abundance 13C of NaHCO3 for labeling and examined the variation of the 13C signature in plant organs. H13CO3 − ions are passively absorbed, translocated from root to leaves, and catalyzed into CO2 by carbonic anhydrase (Moroney et al. 2001). Thus, atmospheric CO2 and the fixed CO2 supplied from root-derived bicarbonate are both assimilated in expanded leaves via photosynthesis. The new photosynthate is exported from the expanded leaves to the developing leaves and roots (Durand et al. 2016) as a result of the remobilization of storage material for growth (Masyagina et al. 2016). When plants are subjected to variable environmental stress, the response is usually reflected in the δ13C of newly assimilated carbohydrate. The rapid turnover C can significantly influence the overall δ13C of leaves (Cranswick et al. 1987; Brendel 2001). In addition, the δ13C of carbohydrate closely reflects the gas exchange parameters (Brugnoli et al. 1988; Göttlicher et al. 2006). The stable C isotope techniques, in combination with gas exchange measurement, might provide precise evidence to investigate bicarbonate assimilation under water deficit treatments.
In the present study, C. acuminate seedlings were exposed to different water regimes and NaH13CO3-labeled treatments. The following are our aims: (1) to study how C. acuminate responded to the labeling, bicarbonate, water deficit, and their interaction morphologically and physiologically; (2) to examine the C isotope composition and fractionation of newly expanded leaves; and (3) to quantify the proportions of fixed CO2 supplied from bicarbonate contribution to the total photosynthetically inorganic C assimilation under different water regimes in C. acuminate seedlings.
Methods
Plant materials
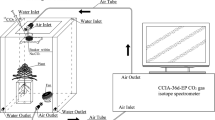
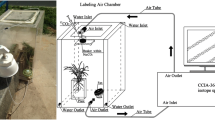
This work was conducted in the phytotron of the Institute of Geochemistry, Chinese Academy of Sciences (CAS), Guiyang, China. The phytotron was 10 × 5 × 4 m in length, width, and height, respectively. The light was provided by the metal halide lamps (HPI-T400W/645, Philips, the Netherlands) while the temperature was controlled by air-condition. Two small ventilation fans were installed to control the gas exchange at regular intervals. Seeds of C. acuminate were obtained from the nearby mother tree at the mature stage. Uniform seeds were selected and disinfected with 75% ethanol for 1 min and were then washed with distilled water three times. The seeds were germinated in trays with a moist bed covered with perlite for 2 weeks at a temperature of 25/19 °C in the light/dark and 55–60% relative humidity. Seedlings were then incubated in a photoperiod of 16/8 h of light/dark conditions, along with 500 ± 23 μmol m−2 s−1 of photosynthetic photon flux density. The seedlings were irrigated with 1/2 strength Hoagland nutrient solution every 3 days. After 76 days of growth, uniform seedlings were chosen and randomly transplanted to pots. The plastic pots were 19.5 × 14.5 × 5.6 cm in length, width, and height, respectively. With a tray beneath, each pot had several apertures for drainage. A soil mixture of perlite and vermiculite (1:3 v/v) was added to fix the plants in the pots.
Experimental design
We carried out a two-way factorial design with six treatments, which contained two labeled treatments in conjunction with three water regimes. Each treatment consisted of three replicates, and each seedling was treated as a replicate. The labeled treatments were separated into relatively high (H) and low (L) natural 13C-abundance of NaHCO3, with a δ13C of −9.76‰ in H and −26.78‰ in L. The H-labeled treatment was consistent with many studies which suggested that the 13C values of bicarbonate ranged from −7 to −14‰ in calcareous soil (Salomons and Mook 1986; Pan et al. 2002; Rovira and Vallejo 2008). Both of the bicarbonate labeled treatments contained three water regime sub-treatments: well-water (WW), moderate stress (MS), and severe stress (SS) treatments. Polyethylene glycol (PEG 6000) was used to induce water deficit (van der Weele et al. 2000; Ancillotti et al. 2015). The addition of PEG for WW, MS, and SS stress was 0, 100, and 200 g per liter in the solution, respectively. The values of the water potential (ψ w ) in the solution approximately equaled −0.01 Mpa (WW), −0.2 Mpa (MS), and −0.6 Mpa (SS), respectively. After the start of the experiment, the pots were irrigated with 250 ml modified solution, and the residual solution was collected and renewed every other day. The concentration of HCO3 − was determined by Aquamerck alkalinity test (MColortest, Merck, Germany). The modified 1/2 strength Hoagland nutrient solution contained 10 mM NaHCO3, 2 mM Ca(NO3)2, 2.5 mM KNO3, 0.5 mM NH4NO3, 0.125 mM KH2PO4, 1 mM MgSO4, and micronutrients. The solution pH was adjusted to 8.3 ± 0.2 at which the HCO3 − is the most abundant species among DIC (Millero 2003). The treatments lasted for 14 days.
Measurements of growth and gas exchange
Growth characteristics were monitored at the end of treatments by measuring the shoot length and leaf length (the second expanded leaf). All seedlings were harvested at the end of the experiment. The fresh weight of the whole plant and root length were determined. The dry weight of shoot and root were determined after drying the plant material at 70 °C for 3 days (until a constant weight was achieved). In the second fully expanded leaves, gas exchange measurements were performed with a portable leaf photosynthesis and fluorescence system Li-6400 (Li-Cor Inc, Lincoln, NE, USA). The air flow rate was set at 500 μmol s−1 and the CO2 concentration at 380 μmol m−2 s−1. The net photosynthetic rate (Pn), stomatal conductance(g s ), and the ratio of intercellular to ambient CO2 partial pressures (Ci/Ca) were measured in the leaf chamber by LI-6400-02B from 09:00 to 11:00 in the morning.
Measurement of carbon isotope signature
At the final harvest, newly expanded leaves were collected and soaked in 1 M HCl for 1 h to remove exogenous inorganic C. Samples were then dried at 70 °C for 3 days and ground for C isotope testing. Measurement of the δ13C value was performed by isotope ratio mass spectrometer (MAT252, Finnigan, Germany) at the Institute of Geochemistry, CAS. The δ13C was expressed as: δ13C (‰) = [(RSample/RStandard) − 1] × 1000, where RSample and RStandard are the 13C/12C ratio of the samples and the PDB, respectively. A small amount of atmospheric CO2 dissolved in the solution and mixed with the initially added NaH13CO3, resulting in the mixed bicarbonate solution. To determine the δ13C of bicarbonate, 15 ml of the solution was sampled at equal time intervals of 2 days. CO2 was generated in a reaction of the bicarbonate solution with phosphoric acid in a sealed and vacuumed bottle. CO2 was then purified and transferred to MAT252 for measurement of the δ13C values. The 13C composition of air (δa) was collected at the regular time points of different days during the treatments in this phytotron. The δa was determined by a trace gas analyzer (Isoprime, GV. Instruments, UK), with the value of −15.11 ± 0.37‰.
Estimation of the contribution of bicarbonate utilization to the total photosynthetically inorganic carbon assimilation
A simplified model as described by Farquhar et al. (1989) was used as follows to predict the instantaneous discrimination against 13C:
where a is the discrimination of diffusion (4.4‰) and b is the discrimination by RubisCO (27‰). To compare with Δ13Ci, the fractionation between air (source) and leaves (product) was determined by the following equation (Brugnoli et al. 1988):
In addition, a two end-member mixing model was designed to investigate the proportion of fixed CO2 supplied from bicarbonate contributing to the total photosynthetically inorganic C assimilation. In L treatment, the two end-member model was written as:
where δNL is the δ13C of newly expanded leaves, ɛ is the fractionation between source and sink during carbohydrate export, δSL is the mean δ13C of the bicarbonate in the solution, Δ1L is the fractionation from bicarbonate to carbohydrate, f L is the proportion of the fixed CO2 supplied from bicarbonate contributing to the total photosynthetically inorganic C assimilation, and Δ2L is the fractionation from atmospheric CO2 to carbohydrate and equals Δ13Ci. In contrast, the two end-member mixing model in H treatment was expressed as:
As suggested by Wu and Xing (2012), when the gas exchange and the solution absorption parameters were approximately equal under the same treatment in L and H labeling treatments, there were specific equations: Δ1L = Δ1H, Δ2L = Δ2H, f = f L = f H, which could be well demonstrated in the following text. Then Eqs. 3 and 4 were obtained:
Statistical analysis
A generalized linear model (GLM) was used for analysis of variance (ANOVA) of the treatments and replications, as well as the interactions. Significant differences between the mean values were calculated with Duncan’s Multiple Range Tests. Tests were considered significant at the p < 0.05 level and extremely significant at a level of p < 0.01. Mean values were expressed with the standard error (SE). All analyses were performed using the SAS 9.4 statistical package (SAS Institute Inc, NC, US).
Results
Solution absorption
Some alterations of the solution pH, the concentration, and 13C composition of bicarbonate were observed due to the continuing dissolution of atmospheric CO2 into the solution (Table 1). However, the pH values and the HCO3 − concentration showed no difference in all treatments (p > 0.05). The mean δ13C values of the bicarbonate were −23.35‰ in L and −15.31‰ in H. In L, the uptake rate of the solution was on average 103.6, 58.5 and 34.1 ml/day for WW L , MS L and SS L , respectively (p < 0.01). Similar results were observed in H. Furthermore, the evaporation was estimated to be approximately 5–7.5 ml/day in every pot (data not shown).
Growth and morphology
The growth and morphological traits of C. acuminate under all treatments are shown in Table 2. The results indicated that the biomass and shoot length were not affected by any treatments. The leaf length was nearly twice that of pre-trt under WW L and WW H treatments (p < 0.05). Approximately 62.14 and 65.05% of the leaf length increment was observed under MS L and MS H treatments in comparison to pre-trt, respectively (p < 0.01). Furthermore, the leaf length was totally inhibited when the SS L and SS H treatments were imposed. The bicarbonate, as a stress rather than labeling factor, had extremely significant influence on leaf length as compared to pre-trt (Tables 2, 3). In addition, water deficit and the interaction of bicarbonate and water deficit significantly affected the leaf length, while the labeling factor had no effect on it. Of all the growth indicators, the root length was the only one that increased under all treatments (p < 0.01). A general increase in the root length was observed with the increase in the water deficit. Bicarbonate, rather than water deficit or labeling factor, had an extremely significant effect on root length (Table 3).
Leaf gas exchange
Overall, Pn, g s , and Ci/Ca exhibited dramatic variation (Fig. 1). The tendency of Pn in L was consistent with that in H when the same water deficit treatment was imposed (Fig. 1a). Under WW treatment, the Pn gradually increased and reached the maximal values of 5.15 μmol m−2 s−1 for WW L and 5.34 μmol m−2 s−1 for WW H at the end of the experiment. When the MS treatment was imposed, the Pn was kept steady at approximately 4 μmol m−2 s−1 for both L and H during the first week and then declined in the second week, with the final values of 3.03 μmol m−2 s−1 for MS L and 2.26 μmol m−2 s−1 for MS H . Under SS treatment, the Pn declined sharply by 70.45 and 67.11% for SS L and SS H in the first 4 days, respectively. The effect of the water deficit on the Pn was extremely significant in the two labeled treatments (Table 3).
Parameters of leaf photosynthetic capacity under the three water deficits (WW, MS, and SS) in two labeled treatments (L, H). WW L : open rectangle; MS L : open circle; SS L : open triangle; WW H : closed rectangle; MS H : closed circle; SS H : closed triangle. a Pn in L and H; b g s in L and H; c Ci/Ca in L and H. Date was shown with mean ± SE, n = 3
The g s increased at first 4–6 days and then declined for WW L and WW H (Fig. 1b). MS treatments induced 60.72 to 64.29% reduction of g s in L and H during the 14 days. In addition, the g s decreased quickly to the level of 0.01 after SS treatment was imposed in L and H. The effect of bicarbonate, water deficit, and their interaction were extremely notable on g s (Table 3).
The Ci/Ca showed the same tendency in L and H (Fig. 1c). A slight decrease in Ci/Ca was observed under WW L and WW H treatments. Under the MS treatments, the Ci/Ca decreased dramatically within the first 2 days and then decreased slowly, with the final mean values of 0.27 and 0.31 for the MS L and MS H treatments, respectively. Similar results were found in the SS L and SS H treatments, in comparison with those in the MS L and MS H treatments. During the treatments, the mean values of Ci/Ca were 0.73, 0.36, and 0.32 in L under the WW, MS, and SS treatments, respectively, and those in the H were 0.69, 0.35, and 0.32, respectively. In addition, the effect of bicarbonate, water deficit, and their interaction were extremely notable on Ci/Ca (Table 3).
C isotope composition of newly expanded leaves and roots
The treatments led to a large range of δ13C of the newly expanded leaves, as compared to pre-trt (Fig. 2a). In L, the mean values of newly expanded leaves under the WW L , MS L , and SS L treatments were −36.89, −34.42, and −34.69‰, respectively. In contrast, the δ13C under the three water deficits in L were approximately 1–1.5‰ 13C-depleted compared to H (p < 0.01). Overall, the δ13C increased significantly from the WW to MS treatments and then decreased slightly from the MS to SS treatments in both L and H. An interaction analysis revealed that water deficit and labeling had an extremely significant effect on the δ13C of leaves (Table 3). Among the treatments, the δ13C of roots throughout the study ranged from −31.12 to −33.41‰ (Fig. 2b). A significant difference was observed as the water deficit increased from the MS to SS treatment, but it differed in the change pattern among L and H. Furthermore, the δ13C under the three water deficits in L were approximately 0.2–0.7‰ negative compared to H (p < 0.01). The water deficit and labeling had extremely significant effects on the δ13C values of roots (Table 3).
C isotope composition (δ13C, ‰) of different organs under three water regimes(WW, MS, and SS) in two labeled treatments (L, H). a δ13C of newly expanded leaves; b δ13C of roots. The bar chart was noted by different letters which were significantly different at p < 0.05. Date was shown with mean ± SE, n = 3
C isotope fractionation
Great C isotope fractionation was observed from air to leaves, mainly due to CO2 diffusion and Rubisco catalysis (Table 4). In all cases, the ∆13Cair-leaves ranged from 17.78 to 21.78‰. The ∆13Cair-leaves of the WW L and WW H treatments were not affected by the water deficit and bicarbonate as compared to the pre-trt. The MS treatment led to the lowest ∆13Cair-leaves among the three treatments in both L and H (p < 0.01). Moreover, the ∆13Cair-leaves of the MS H and SS H treatments were both more negative than were those of the MS L and SS L treatments, respectively (p < 0.01). The data showed that the water deficit and labeling had extremely significant effects on the ∆13Cair-leaves (Table 3). The ∆13Csolution-leaves showed pronounced differences between L and H (p < 0.01). The range of the ∆13Csolution-leaves observed (Table 4), 10.93–13.18‰ in L and 17.58–20.11‰ in H, was correlated with the mean δ13C of bicarbonate in the solution (Table 1). In comparison with the ∆13Csolution-leaves, the ∆13Cleaves-roots in H tended to be close to that of L. MS L , MS H , and SS H led to significant differences in comparison with pre-trt. Bicarbonate, water deficit, and labeling had significant effects on the ∆13Csolution-leaves values (Table 3).
There was a linear regression between Ci/Ca and ∆13Cair-leaves in both L and H (Fig. 3). The correlation coefficients revealed that 61% and 76% of the variation of ∆13Cair-leaves in L and H could be explained by Ci/Ca, respectively. In addition, The fitted equation in L (y = 5.43x + 17.64, R2 = 0.61) was similar with that in H (y = 7.57x + 15.49, R2 = 0.76). This result showed that the ∆13Cair-leaves in L were larger than that in H when the Ci/Ca was equal. However, these two equations were different from the theoretical regression equation (y = 22.6x + 4.4).
Quantification of bicarbonate utilization contributing to the total photosynthetically inorganic C assimilation
Under the WW, MS, and SS treatments, the proportions of fixed CO2 supplied from bicarbonate contributing to total photosynthesis were 10.34, 20.05, and 16.60% in both L and H, respectively, representing 0.55, 0.92, and 0.31 μmol m−2 s−1 of Pn, respectively (Fig. 4).
Discussion
Contribution of bicarbonate utilization to the total photosynthetically inorganic C assimilation
It was observed that 10.36 to 20.05% of fixed CO2 supplied from bicarbonate contribution to total photosynthesis. Under the water stress, there was a higher proportion of transported bicarbonate used by photosynthesis than when well-watered. It was clear that root-derived bicarbonate could enter the plants. The highest enrichment was always observed in the stem, veins, and petiole (Stringer and Kimerer 1993; Bloemen et al. 2015), whereas the portion in the leaves ranged from 0.8 to 8% (Enoch and Olesen 1993; Ford et al. 2007; Aubrey and Teskey 2009). Nevertheless, most studies did not quantify the proportion of fixed CO2 supplied from DIC via photosynthesis. It is known that about 40% of a plant’s dry mass was C which was fixed by photosynthesis (Lambers et al. 2008). The acquisition of atmospheric CO2 and the bicarbonate utilization both contributed to the plant’s C gain. In the present study, the bicarbonate contributed substantial proportion of the total C assimilation under WW treatment. The greater proportion (28%) of bicarbonate utilization was observed in paper mulberry (Broussonetia papyrifera) (Wu and Xing 2012). Hang and Wu (2016) suggested that the bicarbonate accounted for approximately 3.10 and 13.28% of the total C assimilation in two Brassicaceae plants, respectively. These results were, most likely, species-specific.
Water stress decreased the atmospheric CO2 gain but triggered a compensatory effect that maximized the bicarbonate utilization in C. acuminate seedlings. The water deficit induced stomatal closure was unfavorable to meet the continued demand for maintaining metabolism, leading eventually to C starvation (McDowell et al. 2008). Thus, it promoted the proportion of fixed CO2 supplied from bicarbonate utilization. Under the MS treatment, near 100% of increase in the proportion of bicarbonate utilization was observed over the WW treatment. Apparently, it was likely due to stomata closure limiting CO2 diffusion from atmosphere more than the constrain of transpiration (delivery of bicarbonate). For instance, as the water deficit increased from WW to MS, the mean g s values and uptake rate of solution decreased by approximately 64 and 40%, respectively. However, the results also showed that the decrease of Pn values were not proportionate to the decline of g s values. The mean Pn values only decreased about 17% in L and 28% in H when the water stress increased from WW to MS. It seemed that the stomatal conductance did not correlate with photosynthesis capacity under the water stress. Previous study showed that the activity of intercellular Carbonic anhydrase increased and catalyzed bicarbonate into CO2 quickly under the water stress (Pandey et al. 2000). Thus, we speculated that the carbonic anhydrase might have played an important role in promoting the proportion of bicarbonate utilization. Furthermore, MS treatment might be the turning point of bicarbonate utilization. The decline of the proportion revealed that the SS treatment exceeded the capacity of the maximum bicarbonate utilization in C. acuminate seedlings. The dramatic decline of g s and the total inhibition of growth, revealed that the plants suffered from great disadvantage. This adverse condition could not be totally compensated by the utilization of bicarbonate.
In this paper, the mixing model, which took bicarbonate and atmospheric CO2 as the two end-members, along with L and H labeling treatments, was used to calculate the relative contribution of root-derived bicarbonate utilization to the total photosynthesis. This simple model had ignored discrimination resulted from mesophyll conductance, respiration or photorespiration (O’Leary 1981; Gillon and Griffiths 1997), which might have caused the predicted discrimination differing from observed. Nevertheless, the specially designed L and H labeling played a vital role in interpreting the source and composition of isotope. The labeling factor only affected the isotope composition of newly expanded leaves and roots, and had no effect on the physiological process, like growth, gas exchange, and systematic discrimination. This phenomenon had been extensively observed among many plant materials in this and our previous work (Wu and Xing 2012; Hang and Wu 2016). We assumed that the discrimination resulted from mesophyll conductance, respiration, or photorespiration in L equaled to that in H under the same treatment. This ignored discrimination could be also eliminated by comparing Eq 3 and Eq 4. In addition, this approach differed from the traditional labeling (14C or high abundance of 13C), which might be underestimate due to respiration of 13C tracer from newly fixed carbohydrate. It was also distinguished from those methods calculating the transpiration ratio or the transport of CO2 in the xylem sap (Stringer and Kimerer 1993; Levy et al. 1999). Moreover, we had no idea about the measurement of bicarbonate transport in the xylem or leaves. The amount of fixed CO2 supplied from bicarbonate might be less than the amount of bicarbonate uptake. Some studies showed that a part of the bicarbonate was catalyzed by PEPC and produced malic acid in the roots and xylem sap or reversibly converted it into CO2 and then fixed it in the bark via corticular photosynthesis (Msilini et al. 2009; Cernusak and Hutley 2011; Covarrubias and Rombolà 2013). Altogether, the enhanced water deficit triggered a compensatory effect that maximized bicarbonate utilization. The water deficit highlighted the critical role of bicarbonate as a part of the global C budget and soil C sequestration. Field experiments must be conducted for the purpose of investigating the proportion of bicarbonate utilization under natural conditions.
Indication of C isotope signature
The influence of L- and H-labeled treatments on seedlings was faithfully recorded in the C isotope composition of newly expanded leaves and roots. The range of δ13C in newly expanded leaves reflected the different degree of mixing between atmospheric CO2 and bicarbonate assimilation under various water deficits. In the bicarbonate end-member, the different δ13C of photosynthate between L and H was attributed to the mean δ13C of the solution (Table 1). The proportion of bicarbonate assimilation was correlated with the solution uptake rate, which was approximately equal in L and H. In the atmospheric CO2 end-member, the Pn and ∆13Ci in L were both equal to that in H under the same water regimes. Thus, it could explain the phenomenon that the δ13C of newly expanded leaves in L showed 13C-depletion in heavy isotopes as compared to H. Furthermore, the ∆13Cleaves-roots ranged from −1.92 to −3.30‰, which was in good agreement with some studies (Badeck et al. 2005; Lamade et al. 2016). It suggested that the δ13C of roots was mostly influenced by the new photosynthate rather than the labeled solution remaining in the roots. Additionally, water deficit also enhances the export of C to the roots (Durand et al. 2016). All of the above observations indicated the involvement of bicarbonate assimilation in the short time scale.
The bicarbonate, water deficit, and their combination could impose osmotic stress on the plants (Ahmed et al. 2013), which results in a rapid gas exchange response like g s and Ci/Ca alteration. Ci/Ca has a close correlation with the yield and δ13C and ∆13C (Yasir et al. 2013; Lauteri et al. 2014). The alteration of Ci has an effect on the relative accumulation of the 13CO2 reaction with Rubisco and has therefore influenced the isotope composition (Farquhar et al. 1989). However, the plot of Ci/Ca versus ∆13Cair-leaves in Fig. 3 showed a different pattern from the predictive model (Brugnoli et al. 1988). Obviously, Ci/Ca affected discrimination values in the simple predictive model. However, there was still a large difference under MS and SS treatments where the most root-derived bicarbonate was assimilated. The different isotopic composition of this internal source was not included in the simple predictive model. It was not in line with previous study which observed a significant linear regression between ∆13Ci and ∆13Cair-leaves (Brendel 2001). Initially, the Ci in the predictive model was under the circumstance without considering the exogenous DIC addition. The root-derived bicarbonate could contribute to the amount of Ci due to its reversible conversion to CO2 (Moroney et al. 2001). The fixed CO2 supplied from bicarbonate involved in photosynthesis and subsequently affected C isotope discrimination. The underestimated amount of Ci explained that ∆13Ci was less than ∆13Cair-leaves (Fig. 3). In addition, ∆13Ci only represented the atmospheric CO2 end-member assimilation. In our experiment, the proportion of fixed CO2 supplied from bicarbonate also influenced the overall δ13C. As a indirect substrate for photosynthesis, the mean δ13C of bicarbonate was negative than that of atmospheric CO2 in both L and H. The 13C-depleted photosynthate in the bicarbonate end-member was mixed with a relatively 13C-enriched photosynthate in the atmospheric CO2 end-member at different proportions, resulting in a ∆13Ci that was less than ∆13Cair-leaves. Moreover, the Pn and Ci/Ca were maintained at a relatively high level in the first week but a low level in the second week under MS and SS treatments (Fig. 1). This process therefore led to both the yield of photosynthesis and ∆13Ci in the first week that was larger than that in the second week. The time-integrated Ci/Ca probably made it the third reason that ∆13Ci was less than ∆13Cair-leaves.
Conclusion
Water deficit has always led to a reduction in leaf gas exchange while constraining the atmospheric CO2 gain in C. acuminate seedlings. Nevertheless, bicarbonate stimulates increases in the root length and the formation of fine roots, helping with the uptake of water as well as with HCO3 − utilization. The bicarbonate was transported to leaves and then reversibly converted to CO2 as a substrate for photosynthesis. The different mixing proportions of CO2 and bicarbonate led to the differentiation of δ13C in newly expanded leaves and its fractionation relative to air and solution in two labeled treatments. Evidence from the natural 13C-abundance of NaHCO3-labeled treatments supported the hypothesis that with the enhancement of water deficit, the proportion of fixed CO2 supplied from bicarbonate contributing to the total photosynthesis increased to some extent. The role of bicarbonate was so important that it contributed greatly to the total C assimilation, which had been ignored or underestimated in past decades.
References
Ahmed IM, Cao F, Zhang M, Chen X, Zhang G, Wu F (2013) Difference in yield and physiological features in response to drought and salinity combined stress during anthesis in Tibetan wild and cultivated barleys. PLoS ONE 8(10):e77869
Ancillotti C, Bogani P, Biricolti S, Calistri E, Checchini L, Ciofi L, Gonnelli C, Del Bubba M (2015) Changes in polyphenol and sugar concentrations in wild type and genetically modified Nicotiana langsdorffii Weinmann in response to water and heat stress. Plant Physiol Biochem 97:52–61
Aubrey DP, Teskey RO (2009) Root-derived CO2 efflux via xylem stream rivals soil CO2 efflux. New Phytol 184(1):35–40
Badeck FW, Tcherkez G, Nogues S, Piel C, Ghashghaie J (2005) Post-photosynthetic fractionation of stable carbon isotopes between plant organs—a widespread phenomenon. Rapid Commun Mass Spectrom 19(11):1381–1391
Bialczyk J, Lechowsk Z (1995) Chemical composition of xylem sap of tomato grown on bicarbonate containing medium. J Plant Nutr 18(10):2005–2021
Bloemen J, Bauweraerts I, De Vos F, Vanhove C, Vandenberghe S, Boeckx P, Steppe K (2015) Fate of xylem-transported 11C- and 13C-labeled CO2 in leaves of poplar. Physiol Plant 153(4):555–564
Bloemen J, Teskey RO, McGuire MA, Aubrey DP, Steppe K (2016) Root xylem CO2 flux: an important but unaccounted-for component of root respiration. Trees 30(2):343–352
Bloom PR, Inskeep WP (1986) Factors affecting bicarbonate chemistry and iron chlorosis in soils. J Plant Nutr 9(3–7):215–228
Boxma R (1972) Bicarbonate as the most important soil factor in lime-induced chlorosis in the Netherlands. Plant Soil 37(2):233–243
Brendel O (2001) Does bulk-needle δ13C reflect short-term discrimination? Ann For Sci 58(2):135–141
Brugnoli E, Hubick KT, von Caemmerer S, Wong SC, Farquhar GD (1988) Correlation between the carbon isotope discrimination in leaf starch and sugars of C3 plants and the ratio of intercellular and atmospheric partial pressures of carbon dioxide. Plant Physiol 88(4):1418–1424
Cernusak LA, Hutley LB (2011) Stable isotopes reveal the contribution of corticular photosynthesis to growth in branches of Eucalyptus miniata. Plant Physiol 155(1):515–523
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55(407):2365–2384
Covarrubias JI, Rombolà AD (2013) Physiological and biochemical responses of the iron chlorosis tolerant grapevine rootstock 140 Ruggeri to iron deficiency and bicarbonate. Plant soil 370(1–2):305–315
Cranswick AM, Rook DA, Zabkiewicz JA (1987) Seasonal changes in carbohydrate concentration and composition of different tissue types of Pinus radiata trees. N Z J For Sci 17:229–245
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33:507–559
De Micco V, Aronne G (2012) Morpho-anatomical traits for plant adaptation to drought. In: Plant responses to drought stress, Springer Berlin Heidelberg, p 37–61
Deng Y, Jiang Z, Qin X (2012) Water source partitioning among trees growing on carbonate rock in a subtropical region of Guangxi, China. Environ Earth Sci 66(2): 635–640
Durand M, Porcheron B, Hennion N, Maurousset L, Lemoine R, Pourtau N (2016) Water deficit enhances C export to the roots in Arabidopsis thaliana plants with contribution of sucrose transporters in both shoot and roots. Plant Physiol 170(3):1460–1479
Enoch HZ, Olesen JM (1993) Plant response to irrigation with water enriched with carbon dioxide. New Phytol 125(2):249–258
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Biol 40(1):503–537
Ford CR, Wurzburger N, Hendrick RL, Teskey RO (2007) Soil DIC uptake and fixation in Pinus taeda seedlings and its C contribution to plant tissues and ectomycorrhizal fungi. Tree Physiol 27(3):375–383
Gillon JS, Griffiths H (1997) The influence of (photo)respiration on carbon isotope discrimination in plants. Plant Cell Environ 20(10):1217–1230
Göttlicher S, Knohl A, Wanek W, Buchmann N, Richter A (2006) Short-term changes in carbon isotope composition of soluble carbohydrates and starch: from canopy leaves to the root system. Rapid Commun Mass Spectrom 20(4):653–660
Grossiord C, Mareschal L, Epron D (2012) Transpiration alters the contribution of autotrophic and heterotrophic components of soil CO2 efflux. New Phytol 194(3):647–653
Hang H, Wu Y (2016) Quantification of photosynthetic inorganic carbon utilisation via a bidirectional stable carbon isotope tracer. Acta Geochimica 35(2):130–137
Hu B, Simon J, Rennenberg H (2013) Drought and air warming affect the species-specific levels of stress-related foliar metabolites of three oak species on acidic and calcareous soil. Tree Physiol 33(5):489–504
IPCC (2014) Climate Change 2014: Synthesis Report. In: Core Writing Team, Pachauri RK, Meyer LA (eds) Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland, pp 19–29
Jung V, Albert CH, Violle C, Kunstler G, Loucougaray G, Spiegelberger T (2014) Intraspecific trait variability mediates the response of subalpine grassland communities to extreme drought events. J Ecol 102(1):45–53
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63(4):1593–1608
Kumar MS, Ali K, Dahuja A, Tyagi A (2015) Role of phytosterols in drought stress tolerance in rice. Plant Physiol Biochem 96:83–89
Lamade E, Tcherkez G, Darlan NH, Rodrigues RL, Fresneau C, Mauve C, Lamothe-Sibold M, Sketriené D, Ghashghaie J (2016) Natural 13C distribution in oil palm (Elaeis guineensis Jacq.) and consequences for allocation pattern. Plant cell Environ 39(1):199–212
Lambers H, Chapin III FS, Pons TL (2008) Photosynthesis, respiration, and long-distance transport. In: Plant physiological ecology. Springer, New York, pp 11–99
Lauteri M, Haworth M, Serraj R, Monteverdi MC, Centritto M (2014) Photosynthetic diffusional constraints affect yield in drought stressed rice cultivars during flowering. PloS ONE 9(10):e109054
Levy PE, Meir P, Allen SJ, Jarvis PG (1999) The effect of aqueous transport of CO2 in xylem sap on gas exchange in woody plants. Tree Physiol 19(1):53–58
Martínez-Lüscher J, Morales F, Sánchez-Díaz M, Delrot S, Aguirreolea J, Gomès E, Pascual I (2015) Climate change conditions (elevated CO2 and temperature) and UV-B radiation affect grapevine (Vitis vinifera cv. Tempranillo) leaf carbon assimilation, altering fruit ripening rates. Plant Sci 236:168–176
Masyagina O, Prokushkin A, Kirdyanov A, Artyukhov A, Udalova T, Senchenkov S, Rublev A (2016) Intraseasonal carbon sequestration and allocation in larch trees growing on permafrost in Siberia after 13C labeling (two seasons of 2013–2014 observation). Photosynth Res 131(1):267–274
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist 178(4):719–739
Millero FJ (2003) Physicochemical controls on seawater. Treatise Geochem 6:1–21
Moroney JV, Bartlett SG, Samuelsson G (2001) Carbonic anhydrases in plants and algae. Plant Cell Environ 24(2):141–153
Msilini N, Attia H, Bouraoui N, M’rah S, Ksouri R, Lachaâl M, Ouerghi Z (2009) Responses of Arabidopsis thaliana to bicarbonate-induced iron deficiency. Acta Physiol Plant 31(4):849–853
O’Leary MH (1981) Carbon isotope fractionation in plants. Phytochemistry 20(4):553–567
Pan G, He S, Cao J, Tao Y, Sun Y (2002) Variation of δ13C in karst soil in Yaji Karst Experiment Site, Guilin. Chin Sci Bull 47(6):500–503
Pandey DM, Goswami CL, Kumar B, Jain S (2000) Hormonal regulation of photosynthetic enzymes in cotton under water stress. Photosynthetica 38(3):403–407
Pang J, Yang J, Ward P, Siddique KH, Lambers H, Tibbett M, Ryan M (2011) Contrasting responses to drought stress in herbaceous perennial legumes. Plant Soil 348(1–2):299–314
Ramírez DA, Parra A, de Dios VR, Moreno JM (2012) Differences in morpho-physiological leaf traits reflect the response of growth to drought in a seeder but not in a resprouter Mediterranean species. Funct Plant Biol 39(4):332–341
Rombolà AD, Gogorcena Y, Larbi A, Morales F, Baldi E, Marangoni B, Tagliavini M, Abadía J (2005) Iron deficiency-induced changes in carbon fixation and leaf elemental composition of sugar beet (Beta vulgaris) plants. Plant soil 271(1–2):39–45
Rovira P, Vallejo VR (2008) Changes in δ13C composition of soil carbonates driven by organic matter decomposition in a Mediterranean climate: a field incubation experiment. Geoderma 144(3):517–534
Salomons W, Mook WG (1986) Isotope geochemistry of carbonates in the weathering zone. Handb Environ Isotope Geochem 2:239–269
Sapeta H, Costa JM, Lourenco T, Maroco J, Van der Linde P, Oliveira MM (2013) Drought stress response in Jatropha curcas: growth and physiology. Environ Exp Bot 85:76–84
Stringer JW, Kimmerer TW (1993) Refixation of xylem sap CO2 in Populus deltoides. Physiol Plant 89(2):243–251
Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R (2014) Abiotic and biotic stress combinations. New Phytol 203(1):32–43
Tang YT, Cloquet C, Deng THB, Sterckeman T, Echevarria G, Yang WJ, Morel JL, Qiu RL (2016) Zinc isotope fractionation in the hyperaccumulator Noccaea caerulescens and the nonaccumulating plant Thlaspi arvense at low and high Zn supply. Environ Sci Technol 50(15):8020–8027
Teskey RO, McGuire MA (2007) Measurement of stem respiration of sycamore (Platanus occidentalis L.) trees involves internal and external fluxes of CO2 and possible transport of CO2 from roots. Plant Cell Environ 30(5):570–579
van der Weele CM, Spollen WG, Sharp RE, Baskin TI (2000) Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J Exp Bot 51(350):1555–1562
Wang Z, Xu Y, Chen T, Zhang H, Yang J, Zhang J (2015) Abscisic acid and the key enzymes and genes in sucrose-to-starch conversion in rice spikelets in response to soil drying during grain filling. Planta 241(5):1091–1107
Wegner LH, Zimmermann U (2004) Bicarbonate-induced alkalinization of the xylem sap in intact maize seedlings as measured in situ with a novel xylem pH probe. Plant Physiol 136(3):3469–3477
Wu YY, Xing DK (2012) Effect of bicarbonate treatment on photosynthetic assimilation of inorganic carbon in two plant species of Moraceae. Photosynthetica 50(4):587–594
Yang CW, Xu HH, Wang LL, Liu J, Shi DC, Wang DL (2009) Comparative effects of salt-stress and alkali-stress on the growth, photosynthesis, solute accumulation, and ion balance of barley plants. Photosynthetica 47(1):79–86
Yasir TA, Min D, Chen X, Condon AG, Hu YG (2013) The association of carbon isotope discrimination (∆) with gas exchange parameters and yield traits in Chinese bread wheat cultivars under two water regimes. Agric Water Manag 119:111–120
Ying YQ, Song LL, Jacobs DF, Mei L, Liu P, Jin SH, Wu JS (2014) Physiological response to drought stress in Camptotheca acuminata seedlings from two provenances. Front Plant Sci 6:361
Yu JH, Yuan SS, Tang ZH, Li DW, Zu YG (2012) Degradation and ecological functions of RubiscoLSU during severe drought stress leaves of Camptotheca acuminata. Adv Mater Res 518:5429–5435
Zivcak M, Brestic M, Balatova Z, Drevenakova P, Olsovska K, Kalaji HM, Yang X, Allakhverdiev SI (2013) Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth Res 117(1–3):529–546
Zuo Y, Ren L, Zhang F, Jiang RF (2007) Bicarbonate concentration as affected by soil water content controls iron nutrition of peanut plants in a calcareous soil. Plant Physiol Biochem 45(5):357–364
Acknowledgements
This work was supported by the National Key Basic Research Program of China (2013CB956701), the National Key Research and development Program of China (2016YFC0502602) and National Natural Science Foundation of China (U1612441). The authors wish to thank Yu Wang and Ning An for their technical assistance, and two anonymous reviewers and Rui Wang for valuable comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rao, S., Wu, Y. Root-derived bicarbonate assimilation in response to variable water deficit in Camptotheca acuminate seedlings. Photosynth Res 134, 59–70 (2017). https://doi.org/10.1007/s11120-017-0414-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-017-0414-7