Abstract
The mechanism that causes the difference in carbon (C) turnover rate in root populations is unclear. The carbon utilization strategy is assumed to be the main causal factor responsible for differences in root turnover rate. In this study, we determined the correlations between root turnover rate, production, and proportions of C allocated to roots using 13CO2 as a labeling gas in a 13C pulse labeling experiment. The proportions of δ13C were measured in various organs of the grass Bothriochloa ischaemum sampled 0, 6, 24, 48, 216, and 360 h after labeling in three treatments: control (CK), mild water stress (MS), and serious water stress (SS). We found that drought stress increased short-term C allocation to belowground. Fine roots have stronger C demand than coarse root under drought condition. The amount of 13C gradually decreased in leaves and increased in soil with time after 13C pulse labeling. Stem 13C increased with the level of stress and peaked at 24 h, while both fine- and coarse-root 13C peaked at 216 h. 13C distributed to fine roots in MS was significantly higher than in the other treatments at 216 h. The fine-root turnover rate in SS treatment was positively correlated with root biomass but not the amount of 13C. Larger C allocation to roots increased fine-root mass in MS, stimulated rapid fine-root turnover, and increased C input to both the rhizosphere and soil. The fine-root turnover in CK was significantly positively correlated with both 13C amount and biomass, which indicated that increasing short-term C input accelerated turnover in the fine-root pool. The C allocation difference between the fine roots and coarse roots may be a key cause of the different turnover rate in the root population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The production and turnover of fine roots in plants play important roles in terrestrial ecosystems because fine roots are crucial for the cycling of water, nutrients, and carbon (C). The acquisition of sufficient soil resources by fine roots ensures adequate supplies of water and nutrients for the plant’s photosynthesis and aboveground growth. Fine-root production is closely associated with the above- and belowground distribution of C in plants, and the pool of fine-root biomass is crucial for terrestrial biogeochemical cycles (Tefs and Gleixner 2012). It is evidential that temperate grasslands are C sinks of the terrestrial biosphere due to their rapid fine-root turnover and high root biomass content (Carrillo et al. 2014). Interactions between soil and plant roots in arid and semi-arid regions have been documented in a few studies (Clemmensen et al. 2013; Matamala et al. 2003; Phillips et al. 2012; Schmidt et al. 2011). These previous works demonstrated that soil moisture is a major factor concerning temperate grassland photo-assimilates and their allocation to roots, which thereby affects fine-root production and rate of turnover. In turn, fine-root turnover affects the dynamics of soil organic C input from roots to the soil in both short- and long-term pools by stimulating the microbial activities. The response of root turnover rate to drought stress remains controversial, as fine-root turnover rate may be either positively or negatively correlated with the degree of drought stress, depending on the time that fine roots are exposed to water stress (Reid and Crush 2013; Reid et al. 2015). The current obstacle for predicting the response of turnover to drought is, at least in part, due to a lack of mechanistic understanding of the methods used to estimate root lifespan, the C allocation from shoot to root, and the carbon structure in the root.
Differences between reported fine-root longevities and turnover rates have been associated with differences in research methods (Dirk et al. 2009; Finér et al. 2011; Metcalfe et al. 2007; Sah et al. 2011) and environmental conditions (Finér et al. 2011; Leppälammi-Kujansuu et al. 2014; Watson et al. 2000; Yuan and Chen 2012). Isotopes, ingrowth cores, minirhizotrons, and sequential cores have often been used for estimating fine-root longevities, turnover rates, and production (Brunner and Ostonen 2013; Finér et al. 2011; Hansson et al. 2013; Sah et al. 2011; Trumbore et al. 2001). Employing different estimation methods can yield different results for even a single species. The fine-root production of temperate Pinus taeda forest was estimated to be 130 g/m2 a using the minirhizotrons method (Pritchard et al. 2010), whereas the sequential coring methods found it to be 80 g/m2·a (Matamala et al. 2003; Matamala and Schlesinger 2010; Pritchard et al. 2010). This discrepancy may stem from the use of a single root pool to estimate the fine-root turnover rate and root production. The fine-root pool is usually considered as the first three orders of roots or all roots within fixed diameter ranges (e.g., < 2 mm)(Comas et al. 2002; Guo et al. 2008; Holdaway et al. 2011; Liu et al. 2018; Pregitzer et al. 2002; Wang et al. 2017). However, this definition lumps together two, functionally recognizable fine-root pools. The absorptive roots are the primary roots for the uptake of water and nutrient resources with faster root turnover rates (less than 1 year, “fast pool”), whereas the transport roots are the root of fundamental structural and transportation functions, with the turnover rates approaching one decade (“slow pool”) (Gaudinski et al. 2010a; McCormack et al. 2015). This “two-pool” theory has been reported to perform better than the methods treating fine roots as a single pool in the simulations and estimations of root turnover and production (Gaudinski et al. 2010b). Nevertheless, there is a lack of knowledge for the mechanism that explains how the two-pool theory supports root turnover and production under drought condition.
The two-pool theory resolved the problem that calculated the rate of root turnover but not the rate at which fine-root C was transferred to the soil system. Solly et al. (2018) studied the lifespan of fine roots in temperate, boreal, and sub-arctic forests. Using annual growth rings, they found that mean ages of fine roots ranged from < 1 to 12 years. The lifespan of the same fine roots, however, was estimated to be 10 ± 1 years (mean ± 1 SE) using an isotope-based method. This phenomenon might result from two reasons: a time lag between plant carbon assimilation and root production and/or internal carbon storage of roots. Fine-root C is usually classified as structural C (stored C) and non-structural C (short-term C). The heterogeneity of the presence of structural C in fine roots may also be due to multiple pools of C in the fine-root population that have different rates of turnover (Lynch et al. 2013). Fine-root heterogeneity in the turnover of C has recently been demonstrated using isotopic methods (Fahey et al. 2012; Gaudinski et al. 2010b; Keel et al. 2012; Riley et al. 2010; Trueman and Gonzalezmeler 2010), which show that fine-root C can be turned over in just a few months but the process can also take up to several years. Fine-root turnover has been classified into “fast” and “slow” pools for estimating C cycling in ecosystems (Gaudinski et al. 2010b). The difference in the rates of fine-root turnover for the “fast” and “slow” C pools due to the differential use of stored and short-term C, however, is not clear. In a study for investigating the impact of nutrition limitation on pine roots, Wang and Liu (2014) found that the demand for new carbohydrates was distinct between different orders of roots. Drought stress may elicit a similar response, whereby heterogeneity among different roots leads to variation in C demand, which in turn may affect root turnover and production, while this remains untested. Characterizing the heterogeneity of structural C in fine roots is key to improve the estimation accuracy for the contribution of fine-root productivity and root turnover to soil C. We hypothesized that the allocation of both stored and short-term fine-root C would be correlated with indices of root physiology and ecology, such as fine-root longevity, production, and “fast” and “slow” turnover pools. These relationships would provide a theoretical basis for estimating the contribution of fine-root productivity and root turnover to soil C in terrestrial ecosystems more accurately in arid and semi-arid regions. Previous researches suggested that drought promotes the transport of new carbohydrates to roots because roots exhibit the highest C demand under drought stress (Bahn et al. 2013; Poorter et al. 2011; Wei et al. 2005), but it is still unclear if increasing 13C allocation to the roots would affect root turnover and production rates.
The aims of the present study were to compare the differences in fine-root production, longevity, turnover rate, and C allocation, subsequently to analyze the effects of the distribution of stored and short-term C on fine-root production and turnover under water stress. We hypothesized that (1) the effect of water stress on longevity, turnover rate, and production volume differed between the “fast” and “slow” root pools, (2) fine roots have higher demands for short-term C than coarse roots under drought stress, and (3) C utilization strategy contributes to the difference in turnover rate and root production between different pools with the same root population.
Materials and Methods
Experimental Materials and Design
The experiment was carried out at Xi'an University of Technology. The C4 grass Bothriochloa ischaemum, which is an important species for the restoration of degraded grassland and has been widely grown in the arid and semi-arid regions of China, was selected for the experiment. Seeds of B. ischaemum for the test were harvested in October 2013 from natural grassland in Ansai, northern Shaanxi; harvested seeds were stored in paper bags.
Seventy-two transparent plexiglass cuboid pots were filled with soil (Calcic Cambisol; FAO-UNESCO 1977) on May 1, 2014 from the research site to a bulk density of approximately 1.2 g/cm3. Each pot was 19 cm long, 4 cm wide, and 27 cm tall. The soil had a total nitrogen (N) content of 0.69 g/kg, and its field capacity (FC) was 22 cm3/cm3. Five seeds were sown by scattering in each pot; the seedling that grew most vigorously in each group was retained after emergence. Seed holes lacking emerged seedlings were supplemental seeded by using the seeds that grew in the same experimental condition. Moisture control began after seedling thinning on July 1, 2014 using three soil-moisture levels: sufficient water supply (80% FC as a control, CK), mild water stress (60% FC, MS), and serious water stress (40% FC, SS). Each moisture treatment had 24 pots.
Pot soil moisture was controlled by weighing: the pots with B. ischaemum were first weighed for all moisture treatments as the background weights. The background weights for CK, WS, and SS were 3875, 3765, and 3655 g/pot, respectively. The pots were then weighed at 18:00 everyday and watered if the weight was lower than the background weight until the end of the experiment on November 11, 2014. The biomass of B. ischaemum in each pot was included in the background weight before the moisture-control treatments. The difference between the initial biomass of the seedlings (0.867 g) and the maximum wet-grass biomass of each pot (5.812 g) after the test was only 0.1% of the total weight of the overall moisture-controlled pot (3880 g). The biomass of B. ischaemum growth on the moisture-treatment control was neglected.
Experimental Methods and Measurement
13C Pulse-Labeling and Sampling
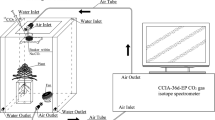
We used three airtight plexiglass chambers for labeling the potted plants with a stable C isotope (Fig. 1). Each chamber was 50 cm long, 50 cm wide, and 80 cm tall. The four sides and bottom of each chamber were hermetically sealed. The top of each chamber had an opening (30 × 30 cm) that could be closed when required with a lid of the same size and sealing strips.
Each moisture treatment contained 24 pots (18 labeled and 6 without labels). Six un-labeled pots in each treatment were selected randomly from the experimental field before labeling and evenly distributed into two groups. The two groups of un-labeled pots were harvested at two different dates: one simultaneously with labeled samples on 15 September (the last labeling day); the other one was harvested on 11 November. Labeling was conducted between 9:00 and 12:00 am on September 13–15, 2014. Six pots of seedlings were randomly selected from the 24 pots in each moisture treatment every labeling day and placed inside the air chambers. A CCIA-36d-EP CO2 isotope spectrometer (ABB—Los Gatos Research, San Jose, United States) was connected to the air chambers to monitor the CO2 concentration and δ13C. A plastic membrane was used to seal the shoot–root junction before labeling to partition the soil from the air. The initial CO2 concentration in the chamber was 450 μmol/mol, δ13C was 5000 ‰, and temperature was 27–28 °C. The 13CO2 with an abundance of 99.9% was injected into the air chamber with a syringe at the beginning of labeling, and then added to the air chamber repeatedly through a tube during labeling when δ13C fell below 5000‰ until the end of the experiment. An electric fan was used to accelerate the air movement in the chamber to quickly and evenly distribute the 13CO2. The labeling process lasted 120 min. The CO2 concentration in the air chamber at the end of labeling was approximately 450 μmol/mol, and δ13C was 5000‰.
13C-labeled samples (new leaves, old leaves, stems, fine roots, coarse roots, soil, and rhizospheres) were collected 0, 6, 24, 48, 216, and 360 h after 13C pulse-labeling (each comprising three replicate samples per water treatment). The aboveground parts of each plant were cut off and numbered. Then, the soil in each pot along with the root was put on a plastic cloth, and whole roots were then separated from the soil. Roots in the rhizospheres (the soil strongly adhering to roots and collected within the space exploited by the roots) and soil were collected with forceps, then numbered and rinsed with distilled water and blotted with filter paper. Roots were immediately dissected into two kinds of segments, fine roots (lateral roots) and coarse roots (main roots) and scanned for root morphology (see the scanning details in the next section).
Root Morphological Scanning and Sampling Root-Growth Dynamics in Pots
After sampling, the fine roots and coarse roots were, respectively, scanned with a scanner (Expression 4490, Epson China Co., Ltd., Beijing, China), and image files were saved in TIFF format with a resolution of 300 dpi. Root-image analyzing software (WinRHIZO Tron 2013, Regent Instruments Inc., Quebec City, Canada) was then used to analyze morphological indices such as root length, surface area, and diameter. Then, the roots and aboveground samples were dried at a constant temperature of 60 °C for 48 h, weighed dry (to the nearest 0.0001 g), pulverized, sieved through a 0.178 mm screen, and stored for C and N concentrations measurement. The rhizospheres and soil were sieved through a 0.149 mm screen and prepared to C and N concentrations measurement. SRL (specific root length) and SRA (specific root area) were calculated from the ratios of root length to biomass and surface area to biomass, respectively.
Root-growth dynamics were sampled from May 11 to November 11, 2014. Seven pots were randomly chosen every fifth day for root scanning when the plants were living (Fig. 2). Two sides of the pots (19 cm long and 27 cm tall) were scanned as described above. WinRHIZO Tron was then used to distinguish between the dead and living roots based on their colors and to analyze root length, surface area, and diameter in the images. Living and dead fine roots were defined as suggested by Wang et al. (2017) and Wells et al. (2002): living if they were white or brown and dead if they, or the epidermal folds, were black or if live roots had disappeared in the next scan. Root longevity was calculated as the number of days between the first appearance of a root in an image and its subsequent disappearance or death. The turnover rate of both fine and coarse roots was calculated as the inverse of longevity. All roots in the images were included in the analysis. A log-rank test was used to determine the difference in longevity between the three water treatments in SPSS (Johnson et al. 2000). The rate of root elongation was defined as the ratio of the difference in length of roots every 5 days and the number of intervening days. The rate of root thickening was defined as the ratio of average difference in diameter every 5 days and the number of intervening days. Root lengthening production and death during the observation days (185 days) were calculated following the method of Burton et al. (2000). This method estimates fine-root production based on the sum of the differences in root-length density obtained in each image:
where RLDp is the production of fine roots during the two adjacent sampling time intervals (mm/cm2 day), RLDn+1 and RLDn are the fine-root length density measured on n + 1th and nth observation days, respectively (mm/cm2), and T is two adjacent sampling time intervals (day, 5 days).
The survival analysis was performed using the Kaplan–Meier method (Lee and Wang 2003) in the statistical software SPSS21.0, and the median lifespan of the fine and coarse roots were calculated (the time is taken for the survival rate to reach 50%, Median root longevity, MRL).
Determination of δ13C and Calculation of the C-Allocation Ratio
The above plant and soil samples (after through screen) were burned to get the gas samples using a MultiN/C3100 analyzer (Analytik Jena, Jena, Germany). The sieved root samples of 0.005–0.006 g each or the sieved 0.1 g of soil sample (cleaned with 1 mol/L sulfuric acid to remove non-organic C in the soil and then oven-dried) were burned in a closed combustion chamber at 1050 °C to generate CO2. All the CO2 generated after complete combustion were collected in sealed aluminum foil gas sample bags to measure its isotopic value. The total organic C (g/kg) was also recorded simultaneously. δ13C of the roots was determined using Pee Dee Belemnite (PDB) as a standard, and the stable C isotopic ratio was calculated as
where (13C/12C) PDB is the 13C/12C ratio of the PDB and δ13C is the deviation (‰) of the 13C/12C ratio of the sample from that of PDB.
The natural abundance in soil or plant organs is indicated in most studies as δ13C, calculated by
where RSample = 13C/12CSample and RPDB = 13C/12CPDB = 0.0112372.
The 13C product fixed by photosynthesis that entered the leaves was transported to the roots for growth and transferred to the rhizosphere and soil after root death and decomposition. The abundance of each sample component, Fsample, and the abundance of unlabeled sample components, Ful (%), were calculated using RSample for each component:
13Csample (mg) of each component was calculated with the total C content in the above- and belowground samples, Csample (g/kg):
Measurement of C and N Concentrations in the Root and Soil Samples
The total organic C (g/kg) of plant and soil samples were recorded at the same time as gas samples collecting.
The root samples were sieved through a 0.178 mm screen and the air-dried soil samples were sieved through a 0.149 mm screen. Subsequently, the root and sand samples were heat-digested and diluted with water to constant volume. The total N concentrations (g/kg) of the root tissues and soil samples were measured using a Kjeldahl Auto Analyzer (Kjeltec 2300, Foss Tecator AB, Hilleroed, Denmark).
Data analysis method
All test data were plotted using SigmaPlot 10.0 (IBM Corporation, Chicago, USA) and processed using SPSS 21.0 (IBM Corporation, Armonk, USA). Correlation analyses were conducted on root morphological and growth characteristics, C and N concentrations of the roots and soil, and characteristics of the stable C isotope. Significance for all the above indicators was determined, using the one-way ANOVA and Duncan’s test to identify and compare significant differences between the above indicators and ratios (α = 0.05).
Results
Strategy of 13C Allocation in Three Water Treatments
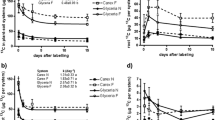
Allocations of 13C were mainly used for organ growth and respiration, and the remainder was accounted for by the soil organic C from root decomposition (Fig. 3). The pattern of allocation of 13C to new and old leaves in the three water treatments was often opposite that of the soil. The amounts of foliar 13C in all treatments decreased over time (Fig. 3a and b), and 13C distributed to leaves was significantly higher in CK than the other treatments. The amounts of soil 13C (Fig. 3e and f) increased for all sampling days after delivery of the 13C pulse. Rhizospheric 13C was higher in MS than CK and SS for 0–48 h, but 13C distributed to the rhizosphere was higher in CK than the other two treatments at 216 and 360 h, and peaked at 0.071 mg by 360 h.
Amounts of 13C in plant organs in the water treatments: a–g represent new leaves, old leaves, fine roots, coarse roots, soil, rhizospheres, and stems, respectively. Different lowercase letters represent significant difference at 0.05 level among different soil water treatments. CK, control, 80% FC; MS, mild water stress, 60% FC; SS, Serious water stress, 40% FC
13C distributed to shoots (Fig. 3g) increased with the level of stress and peaked at 36.77 mg by 24 h in SS and then decreased rapidly to about 21.92 mg by 360 h. 13C was distributed more to fine than coarse roots for 6–24 h in both water-stress treatments, SS and MS (Fig. 3a and d), but the amounts of 13C in fine and coarse roots differed by 216 and 360 h. 13C distributed to fine roots in MS was 16.85 mg by 216 h and 12.03 mg by 360 h, significantly higher than in the other treatments. More 13C was allocated to coarse than fine roots: 18.68 mg by 216 h and 16.04 mg by 360 h.
Characteristics of Root Morphology, Biomass and Nutrients, and Soil Nutrients
Total root length and total root surface area increased significantly in SS over time; total root length was 2.86- and 2.60-fold longer in SS than CK and MS, respectively, and total root surface area was about 2.7–3.0-fold larger in SS than CK and MS (Table 1). Specific root length was significantly longer in MS than the other two treatments, but specific root area did not differ significantly among three treatments. Overall root biomass did not differ significantly among the three moisture treatments, however, the taproot (coarse root) and lateral roots (fine roots) exhibited contrasting responses to soil moisture. As water stress increased, fine-root biomass increased while coarse-root biomass decreased. The ratio of fine to coarse roots was 6.123 in SS, which was 5.649- and 8.056-fold higher than in MS and CK, respectively. The fine-root N content increased with soil moisture (Table 2). The rhizospheric N content was highest in MS but not differed significantly among the treatments. Root and bulk-soil C contents remained unaffected by water stress, regardless of the degree of stress, but the C/N ratios of the fine roots and soil were higher in SS than CK.
Root Lengthening, Average Length, Turnover, and Longevity
The lengthening production during the observation days of fine roots was significantly higher in the two water-stress treatments than CK, but the lengthening production of coarse roots showed the opposite pattern (Table 3). Average production of fine and coarse roots was similar to the lengthening production. The rates of coarse-root turnover were 2.13–2.28-fold per year, with the highest observed in the MS treatment. The relative rates of fine-root turnover in SS, MS, and CK were 4.12, 5.32, and 5.15-fold per year, indicating that the rates were higher in fine than coarse roots and that moderate stress favored the turnover of fine roots. Fine- and coarse-root longevities were shortest in MS, at 70.4 and 147.54 days, respectively. Root longevity, however, was 1.34-fold longer for fine roots and 1.16-fold longer for coarse roots in SS than MS compared with CK. Comparison of three methods for calculating root turnover and longevity under three soil moisture conditions is shown in Supplementary Table 1. A log-rank test of the cumulative survival showed that there were significant differences between the three water treatments in fine-root lifespan, whereas the survivorship of the coarse root was similar (p < 0.05, Fig. 4). The median lifetimes of fine roots in the SS, MS, and CK treatments were 105, 75, and 55 days, respectively (Table 3).
Factors Affecting Root Length
The correlation analysis of the lengthening of fine roots indicated that the amount of 13C was positively correlated with fine-root lengthening in CK and MS, but not SS (Table 4). Fine-root biomass was positively correlated with root production in all treatments. The ratio of fine- to coarse-root biomass was positively correlated with root production in both water-stress treatments. Rhizospheric C content and C/N ratio were positively correlated with fine-root production in MS. Coarse-root lengthening was positively correlated with 13C in all treatments, but coarse-root biomass was positively correlated with 13C only in MS. Coarse-root production was correlated with root length and area negatively in MS but positively in CK.
Factors Affecting Root Turnover
The rates of turnover of both fine and coarse roots were most affected by 13C and root biomass (Table 5). The rates of turnover of fine and coarse roots which positively correlated with the amount of 13C in MS and CK, however, were not significantly correlated in SS. Fine-root biomass in all treatments and coarse-root biomass in MS were positively correlated with the rate of root turnover. Rhizospheric C content, however, was positively correlated with the rate of fine-root turnover in MS and SS.
Discussion
Strategy of Short-Term Allocation of C to Roots
The strategies of plant C allocation include belowground C input and relative allocation of assimilated C (Kollmann et al. 2004). Farrar and Jones developed four hypotheses for the mechanisms of controlling C acquisition by roots: the functional-equilibrium hypothesis and the “push,” “pull,” and “shared control” hypotheses (Farrar and Jones 2000; Pausch and Kuzyakov 2018). Our observations of the depletion of the 13C pool in new leaves, old leaves, and stems by 24 and 48 h after labeling support the “push” control hypothesis of root C allocation, at least during mid-September, a time when there was not high C demand to support rapidly growing aboveground tissues. Fine-root 13C peaked by 216 h in the three treatments, indicating that the most metabolically active fine roots had a “pull” component in all treatments. The high fine-root respiratory demand associated with water uptake or higher root production and turnover rate, or both, may be the physiological driver underlying this “pull” component of fine-root C allocation. The significant higher biomass ratio of fine to coarse roots in SS relative to MS and CK also supported this view (Table 1). The allocation of C to roots may therefore depend on the supply from both the fine-root demand (“pull” component) and the stem C pool (“push” component), which supported the “shared control” concept of root C allocation.
However, the driving force of the “pull” component may differ in both the fine- and coarse-root pools, depending on the availability of moisture. Regarding the “pull” hypothesis (root C demand), we found two interesting results: drought stress increased the root demand for short-term C, and the level of this C demand (the force of the “pull”) was also affected by root heterogeneity. Firstly, after the labeled C had been fully dispensed in the plant (216 h after labeling), the 13C content of the fine and coarse root in MS treatments were significantly higher than those in CK (Fig. 3). This suggested that drought stress increased root short-term C demand. Secondly, the labeled 13C was likely stored as non-structural carbohydrates, and its allocation was likely rapid (Pausch and Kuzyakov 2018). Labeled 13C generally fills a pool of soluble carbohydrates (Non-structural carbohydrates, NSC) in leaves at first, before being transferred to roots. The NSC in roots is typically used for metabolic activities (root respiration), creation of structural and storage compounds (SC, such as lignin and cellulose), or they are stored as soluble carbohydrate pools for remobilization. The C metabolized during root respiration in the first several hours will then be stored in sink organs as structural and stored compounds after 48–72 h (Carbone and Trumbore 2007; Subke et al. 2012; Wang and Liu 2014). The ratios of 13C in fine roots for both SS/CK and MS/CK treatments were significantly higher than those in the coarse roots after 48 h (Supplementary Table 2). The results suggested that the fine roots had higher demand for short-term C than coarse roots. After 48 h, the NSCs transferred to both roots might be used as SC in sink organs or reserved as soluble carbohydrates, which could be reutilized by the plants against various stresses. The ratio of 13C in fine roots under MS/CK treatment was still significantly higher than that in the coarse roots after 360 h labeling, indicating the use of NSCs for root growth (Supplementary Table 2). The fine/coarse-root biomass ratio (reflective of SC accumulation) in SS was found to be higher than that in CK after 360 h of labeling (Table 1), indicating an obvious difference in C demand between fine and coarse roots. Consequently, the “pull” force (C demand of root) was stronger in SS and MS than CK. These results are consistent with our initial hypothesis that fine roots have a higher demand for short-term C than coarse roots under drought stress.
New and Stored C in Roots and Root Production
Biomass results from the accumulation of photosynthetic products (both NSC and SC) in organs. Therefore, the labeled photosynthates (new C, reflecting the NSC used to plant growth) and biomass (reflecting storage C, including the SC accumulation and the NSC as remobilization form reserves). δ13C of fine and coarse roots in the three treatments increased during 0 h to 48 h and peaked at 216 h. This indicates that the labeled photosynthetic products were used for root growth (Fig. 3c and d), and is consistent with other studies that have found that new fine-root growth was largely from recent photosynthates (Gaudinski et al. 2009; Langley et al. 2002; Wang and Liu 2014). The sources of C for root production, however, remain controversial. The fine roots in a diverse, Swiss mixed forest (Bader et al. 2009) and the large-diameter fine roots of Pinus sylvestris (Sah et al. 2011) are largely composed of stored C rather than recently generated photosynthates. In contrast, Matamala et al. (2003) and Trueman and Gonzalez-Meler (2010) reported that little or no stored C was used for fine-root production. The strategies adopted by plants for C allocation include belowground C input and relative allocation of assimilated C (Farrar and Jones 2000; Kollmann et al. 2004). Plants change the balance of distributional strategies between short-term and stored C by changing features such as root morphology and nutrient supply, or by changing the percentage of photo-assimilates (new photosynthates) transported to stored C to adapt to different levels of soil moisture. These results are consistent with our assumption that water stress affects the strategy for the use of new and stored C by roots.
The increased C input to fine roots in SS was resulted from the accumulation of fine roots, which increased the biomass and proportion of fine roots (Table 4), meanwhile led to higher ratio of fine- to coarse-root biomass in SS when compared to other treatments. The increased production of fine roots, however, was not derived from recent photosynthetic products (short-term C), as neither fine-root production nor biomass accumulation was correlated with 13C. Short-term C was quickly transferred to structural and stored compounds, which were not immediately decomposed by rhizospheric microorganisms, thus prolonging the life of the fine roots (longevity was longest for fine roots, Table 3). Coarse-root production, however, showed the opposite response, which was significantly and positively correlated with 13C and biomass. The increase in coarse-root production was likely attributed to the added new C, because coarse-root biomass was significantly correlated with 13C.
Both fine- and coarse-root production in MS were supplied by short-term C, as both were significantly and positively correlated with 13C. Coarse- and fine-root 13C were also significantly positively correlated with biomass, indicating that the short-term C in the roots was also rapidly converted into, and stored as, structural C, supporting the accumulation of biomass. The new fine-root C increased the proportion of root C input. Fine-root longevity was shortest in this treatment, and the rate of root turnover was the highest. The rapid fine-root turnover also increased the C contents of the rhizosphere and soil. Previous studies have suggested that soil N content in this experimental area was low (Wang et al. 2017) and that the rhizospheric C/N ratio increased due to the higher C content.
Interestingly, root production in CK was significantly and positively correlated with fine- and coarse-root 13C, but only fine-root biomass increased with the accumulation of new fine-root C; indicating that both fine- and coarse-root short-term C were converted into and stored as structural C. Coarse-root biomass, however, accumulated stored C before new C, and fine-root biomass accumulated stored C transferred from new C. Such high heterogeneity in the allocation of short-term C to coarse and fine roots suggested that the main C pool for root growth consisted of new photosynthates, consistent with the results obtained by Langley et al. (2002) and Wang and Liu (2014). Plants can absorb more water by increasing the length of their fine roots and increasing the surface area of roots. Plants also supply more C to aboveground tissues by increasing the coarse-root length and root surface area under a sufficient water supply, and these responses demand greater allocation of assimilates to coarse roots (Liu et al. 2020, 2017). The positive correlations between coarse-root production and root length and surface area support this conclusion.
Effects of Root Morphology, Nutrients, and Short-Term C Allocation on Root Production and Turnover
Both isotopic approaches and theoretical modeling (Gaudinski et al. 2010b; Guo et al. 2008; Keel et al. 2012; Luo 2010; Riley et al. 2010; Trueman and Gonzalez-Meler 2010) have recently questioned the multiyear longevity of some root C (“slow” C pool) and the faster turnover of other C (on the order of months, “fast” C pool). The turnover rates of multiple C pools are important for quantifying the contribution of fine roots to total forest net primary productivity (NPP), because multiple replacements per year of even a small amount of fine-root biomass can have large consequences for fine-root NPP (Lynch et al. 2013).
The lengthening production during the growing period of fine roots in the SS treatment was significantly higher than CK. However, under SS treatment, this parameter of the coarse roots was lower than CK (Table 3). Combined with the results of coarse-root turnover rate (MS turnover was higher than CK), it is clear that coarse and fine roots responded to drought stress differently. Hence, the two-pool theory (fine roots for “fast” pool and coarse roots for “slow” pool) may perform better than the single pool theory when describing the response of root longevity, turnover, and production to drought stress, supporting our first hypothesis.
Previous studies have indicated that the difference in the root turnover rates between multiple C pools may be attributed to the heterogeneity in the C turnover in fine roots (Matamala et al. 2003; Matamala and Schlesinger 2010). Our observations strongly suggested that high rates of root turnover in SS increased root biomass. However, the increased biomass was not derived from short-term C (biomass was not significantly correlated with 13C), but from stored C. The increase in the proportion of stored C in fine roots would extend root longevity and reduce the turnover rate, suggesting that the fine roots transferred C to the “slow” pool before adapting to the severe drought. This analysis indicated that C storage increased fine-root biomass and accelerated the turnover rate in the “fast” pool under severe drought stress.
Though both being water-stress treatments, MS and SS had completely different strategies for using short-term C. 13C was highest in MS and was significantly correlated with biomass, indicating that short-term C affected structural C, and root growth consumed stored C with longer root longevity (“slow” pool). The increase in new C, however, also increased the rate of root turnover and the accumulation of biomass (rates of both fine- and coarse-root turnover were positively correlated with 13C and biomass). Fine-root longevity was shorter, but turnover rate was higher in MS than the other treatments, supporting the assumption of the use of new C in the “fast” pool.
The high rates of fine- and coarse-root turnover with a sufficient water supply accelerated biomass accumulation and increased the amount of new C. Fine-root 13C, however, was significantly positively correlated with biomass, but coarse-root 13C was not. These results indicated that new C was the C source of fine-root biomass and that short-term C accelerated turnover in the “fast” pool. In contrast, the stored C for the “slow” pool supported coarse-root turnover. The results were in favor of the better performance of the two-pool model for C turnover in roots than the one-pool model because the root C utilization strategy was different in those two pools, consequently supported our third hypothesis.
Conclusion
We assessed the amount of 13C between fine root and coarse root varying after C isotope labeling, which suggests that drought drives the increase in fine-root C demand. Our results also indicate that the root turnover rate was composed of multiple pools with different turnover rates. This result supports the statement that dual-pool theory performs better than the single pool when describing the response of root longevity, turnover, and production to drought stress. The high variability of C allocation in roots resulted from the differences in both structural C and water stress, which might contribute to the difference in “fast” and “slow” C pools. Under severe drought stress, the turnover rate in the “fast” pool was fueled by stored C since the stored C increases fine-root biomass. The turnover of the two pools in MS treatment was driven by new C. Under CK treatment, the new C was used for fast pool and stored C was used for slow pool. The NSC content of root was measured in this study, but the results still provided significant support to show that NSCs fueled root growth and production. Subsequent experiments will be necessary to differentiate among the relative contribution of the different NSC components in root turnover rate and root production.
References
Bader M, Hiltbrunner E, Körner C (2009) Fine root responses of mature deciduous forest trees to free air carbon dioxide enrichment (FACE). Funct Ecol 23(5):913–921
Bahn M, Lattanzi FA, Hasibeder R, Wild B, Koranda M, Danese V, Brüggemann N, Schmitt M, Siegwolf R, Richter A (2013) Responses of belowground carbon allocation dynamics to extended shading in mountain grassland. New Phytol 198(1):116–126
Brunner I, Ostonen I (2013) Fine-root turnover rates of European forests revisited: an analysis of data from sequential coring and ingrowth cores. Plant Soil 362(1–2):357–372
Burton AJ, Pregitzer KS, Hendrick RL (2000) Relationships between fine root dynamics and nitrogen availability in Michigan northern hardwood forests. Oecologia 125(3):389–399
Carbone M, Trumbore SE (2007) Contribution of new photosynthetic assimilates to respiration by perennial grasses and shrubs: residence times and allocation patterns. New Phytol 176(1):124–135
Carrillo Y, Dijkstra FA, Lecain D, Morgan JA, Blumenthal D, Waldron S, Pendall E (2014) Disentangling root responses to climate change in a semiarid grassland. Oecologia 175(2):699
Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, Stenlid J, Finlay RD, Wardle DA, Lindahl BD (2013) Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339(6127):1615–1618
Comas L, Bouma T, Eissenstat D (2002) Linking root traits to potential growth rate in six temperate tree species. Oecologia 132(1):34–43
Dirk G, Dietrich H, Christoph L (2009) Estimating fine root longevity in a temperate Norway spruce forest using three independent methods. Funct Plant Biol Fpb 36(1):11–19
Fahey TJ, Jacobs KR, Sherman RE (2012) Fine root turnover in sugar maple estimated by 13C isotope labeling. Can J For Res 42(10):1792–1795
Farrar JF, Jones DL (2000) The control of carbon acquisition by roots. New Phytol 147(1):43–53
Finér L, Ohashi M, Noguchi K, Hirano Y (2011) Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. For Ecol Manage 262(11):2008–2023
Gaudinski J, Torn M, Riley W, Swanston C, Trumbore S, Joslin JD (2010a) Use of stored carbon reserves in growth of temperate tree roots and leaf buds: analyses using radiocarbon measurements and modeling. Glob Change Biol 15(4):992–1014
Gaudinski JB, Torn MS, Riley WJ, Dawson TE, Joslin JD, Majdi H (2010b) Measuring and modeling the spectrum of fine-root turnover times in three forests using isotopes, minirhizotrons, and the Radix model. Global Biogeochem Cycles. https://doi.org/10.1029/2009GB003649
Gaudinski JB, Torn MS, Riley WJ, Swanston C, Trumbore SE, Joslin JD, Majdi H, Dawson TE, Hanson PJ (2009) Use of stored carbon reserves in growth of temperate tree roots and leaf buds: analyses using radiocarbon measurements and modeling. Glob Change Biol 15(4):992–1014
Guo D, Mitchell RJ, Han W, Hendricks JJ, Fahey TJ, Hendrick RL (2008) Fine root heterogeneity by branch order: exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods. New Phytol 177(2):443–456
Hansson K, Helmisaari HS, Sah SP, Lange H (2013) Fine root production and turnover of tree and understorey vegetation in Scots pine, silver birch and Norway spruce stands in SW Sweden. For Ecol Manage 309(12):58–65
Holdaway RJ, Richardson SJ, Dickie IA, Peltzer DA, Coomes DA (2011) Species- and community-level patterns in fine root traits along a 120 000-year soil chronosequence in temperate rain forest. J Ecol 99(4):954–963
Johnson MG, Phillips DL, Tingey DT, Storm MJ (2000) Effects of elevated CO2, N-fertilization, and season on survival of ponderosa pine fine roots. Can J For Res 30(2):220–228
Keel SG, Campbell CD, Högberg MN, Richter A, Wild B, Zhou X, Hurry V, Linder S, Näsholm T, Högberg P (2012) Allocation of carbon to fine root compounds and their residence times in a boreal forest depend on root size class and season. New Phytol 194(4):972–981
Kollmann J, Dietz H, Edwards PJ (2004) Allocation, plasticity and allometry. Perspectives in Plant Ecology Evolution & Systematics 6(4):205–206
Langley J, Drake B, Hungate B (2002) Extensive belowground carbon storage supports roots and mycorrhizae in regenerating scrub oaks. Oecologia 131(4):542–548
Lee ET, Wang JW (2003) Statistical methods for survival data analysis, 3rd edn. Wiley, New York, pp 64–77
Leppälammi-Kujansuu J, Salemaa M, Dan BK, Linder S, Helmisaari HS (2014) Fine root turnover and litter production of Norway spruce in a long-term temperature and nutrient manipulation experiment. Plant Soil 374(1–2):73–88
Liu Y, Li P, Wang T, Liu Q, Wang W (2020) Root respiration and belowground carbon allocation respond to drought stress in a perennial grass (Bothriochloa ischaemum). CATENA 188:104449
Liu Y, Li P, Xu GC, Xiao L, Ren ZP, Li ZB (2017) Growth, morphological, and physiological responses to drought stress in Bothriochloa ischaemum. Frontiers in Plant Science 8:230
Liu Y, Wang G, Yu K, Li P, Xiao L, Liu G (2018) A new method to optimize root order classification based on the diameter interval of fine root. Scientific Reports 8(1):2960
Luo Y (2010) Uncertainties in interpretation of isotope signals for estimation of fine root longevity: theoretical considerations. Glob Change Biol 9(7):1118–1129
Lynch DJ, Matamala R, Iversen CM, Norby RJ, Gonzalez-Meler MA (2013) Stored carbon partly fuels fine-root respiration but is not used for production of new fine roots. New Phytol 199(2):420–430
Matamala R, Gonzàlezmeler MA, Jastrow JD, Norby RJ, Schlesinger WH (2003) Impacts of fine root turnover on forest NPP and soil C sequestration potential. Science 302(5649):1385–1387
Matamala R, Schlesinger WH (2010) Effects of elevated atmospheric CO2 on fine root production and activity in an intact temperate forest ecosystem. Glob Change Biol 6(8):967–979
McCormack ML, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo D, Helmisaari HS, Hobbie EA, Iversen CM, Jackson RB (2015) Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol 207(3):505–518
Metcalfe DB, Meir P, Williams M (2007) A comparison of methods for converting rhizotron root length measurements into estimates of root mass production per unit ground area. Plant Soil 301(1/2):279–288
Pausch J, Kuzyakov Y (2018) Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem scale. Glob Change Biol. https://doi.org/10.1111/gcb.13850
Phillips RP, Meier IC, Bernhardt ES, Grandy AS, Wickings K, Finzi AC (2012) Roots and fungi accelerate carbon and nitrogen cycling in forests exposed to elevated CO2. Ecol Lett 15(9):1042–1049
Poorter H, Karl JN, Peter BR, Oleksyn J, Poot P, Mommer L (2011) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193(1):30–50
Pregitzer KS, Deforest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL (2002) Fine root architecture of nine North American trees. Ecol Monogr 72(2):293–309
Pritchard S, Strand A, Mccormack M, Davis M, Finz A, Jackson R, Matamala R, Hh O, R. (2010) Fine root dynamics in a loblolly pine forest are influenced by free-air-CO2-enrichment: a six-year-minirhizotron study. Glob Change Biol 14(3):588–602
Reid JB, Crush JR (2013) Root turnover in pasture species: perennial ryegrass (Lolium perenne L). Crop Pasture Sci 64(2):165
Reid JB, Gray RAJ, Springett JA, Crush J (2015) Root turnover in pasture species: chicory, lucerne, perennial ryegrass and white clover. Annals of Applied Biology. https://doi.org/10.1111/aab.12228
Riley WJ, Gaudinski JB, Torn MS, Joslin JD, Hanson PJ (2010) Fine-root mortality rates in a temperate forest: estimates using radiocarbon data and numerical modeling. New Phytol 184(2):387–398
Sah SP, Jungner H, Oinonen M, Kukkola M, Helmisaari HS (2011) Does the age of fine root carbon indicate the age of fine roots in boreal forests? Biogeochemistry 104(1/3):91–102
Schmidt MW, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kögelknabner I, Lehmann J, Manning DA (2011) Persistence of soil organic matter as an ecosystem property. Nature 478(7367):49–56
Solly E, Brunner I, Helmisaari H-S, Herzog C, Leppälammi-Kujansuu J, Schöning I, Schrumpf M, Schweingruber F, Trumbore S, Hagedorn F (2018) Unravelling the age of fine roots of temperate and boreal forests. Nature Communications 9(1):3006
Subke JA, Vallack HW, Leronni V, Baxter R, Ineson P (2012) Fast assimilate turnover revealed by in situ CO pulse-labelling in Subarctic tundra. Polar Biol 35(8):1209–1219
Tefs C, Gleixner G (2012) Importance of root derived carbon for soil organic matter storage in a temperate old-growth beech forest—evidence from C, N and 14 C content. For Ecol Manage 263:131–137
Trueman RJ, Gonzalezmeler MA (2010) Accelerated belowground C cycling in a managed agriforest ecosystem exposed to elevated carbon dioxide concentrations. Glob Change Biol 11(8):1258–1271
Trumbore SE, Davidson EA, Cook AC, Markewitz D, Richter DD (2001) The age of fine-root carbon in three forests of the Eastern United States measured by radiocarbon. Oecologia 129(3):420–429
Wang G, Liu F (2014) Carbon allocation of Chinese pine seedlings along a nitrogen addition gradient. For Ecol Manage 334:114–121
Wang G, Xue S, Liu F, Liu G (2017) Nitrogen addition increases the production and turnover of the lower-order roots but not of the higher-order roots of Bothriochloa ischaemum. Plant Soil 415(1–2):423–434
Watson CA, Ross JM, Bagnaresi U, Minotta GF, Roffi F, Atkinson D, Black KE, Hooker JE (2000) Environment-induced modifications to root longevity in Lolium perenne and Trifolium repens. Ann Bot 85(3):397–401
Wei L, Zhang X, Hou Z, Xu D, Yu X (2005) Effects of water stress on photosynthesis and carbon allocation in cunninghamia lanceolata seedlings. Acta Phytoecol Sin 29(3):394–402
Wells CE, Glenn DM, Eissenstat DM (2002) Changes in the risk of fine-root mortality with age: a case study in peach, Prunus persica (Rosaceae). Am J Bot 89(1):79–87
Yuan ZY, Chen HYH (2012) Indirect methods produce higher estimates of fine root production and turnover rates than direct methods. PLoS ONE 7(11):e48989
Acknowledgements
This research was funded by National key research and development program (Nos. 2016YFC0402407, 2016YFC0402404, and 2016ZZKT-13), National Natural Science Foundation of China (No. 41561144011), and the School foundation of Xi’an University of Technology (310–252071506).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Y., Li, P., Xiao, L. et al. High Heterogeneity of Root Carbon Allocation Affects Root Turnover Rate and Production of Bothriochloa ischaemum Under Drought Stress. J Plant Growth Regul 40, 226–239 (2021). https://doi.org/10.1007/s00344-020-10090-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-020-10090-8